Abstract
Background
Borrelia burgdorferi (sensu lato) and rickettsiae of the spotted fever group are zoonotic tick-borne pathogens. While small mammals are confirmed reservoirs for certain Borrelia spp., little is known about the reservoirs for tick-borne rickettsiae. Between 2012 and 2014, ticks were collected from the vegetation and small mammals which were trapped in Saxony, Germany. DNA extracted from ticks and the small mammals’ skin was analyzed for the presence of Rickettsia spp. and B. burgdorferi (s.l.) by qPCR targeting the gltA and p41 genes, respectively. Partial sequencing of the rickettsial ompB gene and an MLST of B. burgdorferi (s.l.) were conducted for species determination.
Results
In total, 673 small mammals belonging to eight species (Apodemus agrarius, n = 7; A. flavicollis, n = 214; Microtus arvalis, n = 8; Microtus agrestis, n = 1; Mustela nivalis, n = 2; Myodes glareolus, n = 435; Sorex araneus, n = 5; and Talpa europaea, n = 1) were collected and examined. In total, 916 questing ticks belonging to three species (Ixodes ricinus, n = 741; Dermacentor reticulatus, n = 174; and I. trianguliceps, n = 1) were collected. Of these, 474 ticks were further investigated. The prevalence for Rickettsia spp. and B. burgdorferi (s.l.) in the investigated small mammals was 25.3 and 31.2%, respectively. The chance of encountering Rickettsia spp. in M. glareolus was seven times higher for specimens infested with D. reticulatus than for those which were free of D. reticulatus (OR: 7.0; 95% CI: 3.3–14.7; P < 0.001). In total, 11.4% of questing I. ricinus and 70.5% of D. reticulatus were positive for Rickettsia spp. DNA of B. burgdorferi (s.l.) was detected only in I. ricinus (5.5%). Sequence analysis revealed 9 R. helvetica, 5 R. raoultii, and 1 R. felis obtained from 15 small mammal samples.
Conclusion
Small mammals may serve as reservoirs for Rickettsia spp. and B. burgdorferi (s.l.). While the prevalence for Rickettsia spp. in M. glareolus is most likely depending on the abundance of attached D. reticulatus, the prevalence for B. burgdorferi (s.l.) in small mammals is independent of tick abundance. Dermacentor reticulatus may be the main vector of certain Rickettsia spp. but not for Borrelia spp.
Similar content being viewed by others
Background
Tick-borne diseases require invertebrate vectors (ticks) and vertebrate hosts for the completion of their life-cycle [1, 2]. Two of the most common tick species in Europe - and at the same time the most important vectors - are the castor bean tick Ixodes ricinus and the meadow tick Dermacentor reticulatus. Their immature life stages (larvae and nymphs) parasitize mostly on small-sized birds and on small mammals. This is why small mammals are essential for the maintenance and distribution of ticks and thus tick-borne diseases [3–7].
Borrelia burgdorferi (sensu lato) is the causative agent of Lyme disease (LD) which is considered the most common tick-borne disease in Europe and North America [8, 9]. Borrelia burgdorferi (s.l.) is a complex of gram-negative bacteria belonging to at least 20 genospecies from which nine occur in Europe [10]: B. afzelii, B. bavariensis, B. bissetti, B. burgdorferi (sensu stricto), B. finlandensis, B. garinii, B. lusitaniae, B. spielmanii and B. valaisiana. Borrelia burgdorferi (s.l.) is mainly transmitted by I. ricinus ticks in which transovarial transmission was recorded for B. miyamotoi but not for genospecies belonging to the B. burgdorferi (s.l.) complex [11]. Over 40 vertebrate species, in particular small mammals, are considered reservoir hosts for B. burgdorferi (s.l.) [12, 13].
Rickettsia spp. are divided into four groups: the spotted fever group (SFG), the typhus group, the ancestral group and the transitional group [14, 15]. Tick-borne rickettsioses are caused by obligate intracellular gram-negative bacteria from the SFG. Ixodes ricinus, D. reticulatus and Rhipicephalus spp. are mainly involved in the circulation of pathogenic Rickettsia species in Europe (such as R. aeschlimannii, R. conorii, R. helvetica, R. massiliae, R. monacensis, R. raoultii, R. sibirica and R. slovaca). Transovarial and transstadial transmission has been observed in these tick species. DEBONEL (Dermacentor-borne necrosis erythema lymphadenopathy) also known as TIBOLA (tick-borne lymphadenopathy) syndrome is transmitted by D. reticulatus and associated with R. slovaca and R. raoultii [15–19]. Wild boars (Sus scrofa) and domestic ruminants are considered as potential reservoirs for R. slovaca. Additionally, sika deer (Cervus nippon), dogs (Canis lupus familiaris), common rabbits (Oryctolagus cuniculus) and lizards (Teira dugesii) are potential reservoirs for R. helvetica, R. conorii, R. massiliae and R. monacensis, respectively [15, 17, 20–24]. However, the reservoir of R. raoultii is still not established.
Prevalence rates for Borrelia spp. and Rickettsia spp. in I. ricinus ticks in Germany differ and can reach levels of 34 and 61%, respectively [25–31]. In Germany, the investigations of Rickettsia spp. in wild-living small mammals are scarce and were conducted mostly on Myodes glareolus, Apodemus flavicollis and Erinaceus europaeus [32–34]. Earlier, Borrelia spp. was detected in small animals such as Glis glis, E. europaeus, A. flavicollis and Mus musculus in Germany [35–37]. However, all studies previously published on Borrelia spp. in small mammals from Germany were focused on the detection of a single locus (ospA gene). In the present study, multi-locus sequence typing (MLST) of eight housekeeping genes was conducted in order to detect different sequence types of B. burgdorferi (s.l.) in small mammals.
The aims of this study were (i) detection of tick-borne rickettsiae and B. burgdorferi (s.l.) by qPCR in captured small mammals and in the questing ticks from selected suburban areas in Saxony, Germany; (ii) species identification of these pathogens by conventional PCR and MLST; and (iii) comparison of prevalence rates of B. burgdorferi (s.l.) and of tick-borne rickettsiae between the respective small mammals and tick species.
Methods
Study sites
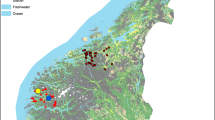
From 2012 to 2014, small mammals as well as questing ticks were collected at six different study sites in and near the city of Leipzig in Saxony, Germany. Previously, these study sites were described in detail and consecutively named from “E” to “I” (E: 51°16'27.6"N, 12°19'18.8"E; F: 51°17'13.0"N, 12°20'40.2"E; G: 51°16'20.3"N, 12°23'12.7"E, H1: 51°18'14.6"N, 12°24'41.4"E; H2: 51°17'35.5"N, 12°24'07.5"E, I: 51°18'01.2"N, 12°22'09.5"E) by our group [38]. Three of those six study locations (sites E, F and G) surround a lake which was artificially created from a former brown coal mining area and which is now often frequented by visitors for recreational activities. Site “H” is subdivided in two small areas located in a recreational city park which was created from a former waste disposal area. Site “I” is a part of one of the largest riparian forests in Middle Europe and is located near the city centre of Leipzig. Sites “I” as well as “G” were only investigated in 2012 due to financial restrictions (see complete sequence batches in Additional files 1 and 2).
Small mammals and their attached ticks
Small mammals were captured from March to October in 2012, from January to November in 2013, and from January to October in 2014. Each month, twenty Sherman© live animal traps (H. B. Sherman Traps, Inc., Tallahassee, Fla., USA) were baited with apple slices and placed at each study site for two consecutive nights. Captured small mammals were immediately anesthetized with CO2 and subsequently euthanized by cervical dislocation (local permit numbers: 36.11-36.45.12/4/10-026-MH, 364.60/2009-102-2). By the use of taxonomic keys, captured animals were morphologically identified [39]. For the present study, the ectoparasites (ticks in particular) were additionally collected from their bodies. Skin samples as well as ticks, which were morphologically identified [40] in advance, were stored at -80 °C until further processing.
Collection of questing ticks
Simultaneously to each rodent trapping action, questing ticks were collected monthly by the use of the flagging method at each study site. The ticks were morphologically identified and stored individually at -80 °C until further processing [40].
Tissue preparation and DNA extraction
Skin samples were taken individually and then 0.6 g of sterile steel beads (sized 2.8 mm, Peqlab Biotechnologie, Erlangen, Germany) as well as 600 μl phosphate buffered saline were added to each sample. Moreover, 0.6 g ceramic beads (sized 1.4 mm, Peqlab Biotechnologie) and 200 μl PBS were added to each engorged or questing tick. All samples were homogenized at 5700× rpm for 20 s in the Precellys®24 tissue homogenizer (Bertin Technologies). Subsequently, DNA was extracted from all samples with the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s recommendations for tissue DNA extraction. The quality and the quantity of the DNA samples were measured with a spectrophotometer (NanoDrop® 2000c, Peqlab Biotechnologie).
PCR methods
Initially, small mammal and tick DNA samples were screened for the presence of Rickettsia spp. and Borrelia burgdorferi (s.l.) by qPCR. Real-time PCR analysis targeting the citrate synthase gene (gltA, 70 bp) was performed for Rickettsia spp. as previously described [41]. The initial screening for Borrelia burgdorferi (s.l.) which is targeting the p41 flagellin gene (96 bp) was carried out following a previously published protocol [42].
All Rickettsia-positive samples yielding a cycle threshold value (CT) below 35 were further analysed by a conventional PCR targeting 811 bp of the outer membrane protein B gene (ompB) of SFG rickettsiae [43]. A 1.5% agarose gel was stained with Midori Green (NIPPON Genetics, Düren, Germany) and PCR products were analysed under UV illumination. Five randomly selected samples which were positive for B. burgdorferi (s.l.) by real-time PCR and yielded a CT value below 33 were further analysed by multi-locus sequence typing (MLST) targeting the following housekeeping genes: nifS, pyrG, clpX, pepX, uvrA, rplB, cplA and recG [44]. For all genes a semi-nested or a nested approach was performed as described, however with slight modifications. The first amplification step for the genes clpX, rplB, pepX as well as the second amplification step for the genes rplB, clpA and clpX were performed with a touchdown protocol with 11 cycles with annealing temperatures ranging down from 56 to 46 °C, and further 34 cycles with an annealing temperature of 46 °C. The first amplification step of the nifS gene was likewise a touchdown protocol with nine cycles with annealing temperatures ranging down from 51 to 43 °C, and further 36 cycles with an annealing temperature of 46 °C. The annealing temperature of the nifS gene in the second amplification step was 51 °C as for the uvrA gene in both amplification steps. The annealing temperature for the first amplification step of the recG gene and for the second amplification step of the pepX gene was 55 °C. The annealing temperature of the first amplification step of the pyrG gene and the clpA gene was 47 °C. The annealing temperature in the second amplification step was 49 °C for the pyrG gene and 50 °C for the recG gene.
Sequencing was performed commercially (Interdisziplinäres Zentrum für Klinische Forschung, Leipzig, Germany) for both, Rickettsia spp. and Borrelia spp. MLST, with forward and reverse primers of each gene used for PCR amplification. Results were analysed with the Bionumerics Software (Version 7.6.1. Applied Maths, Inc., Austin, TX USA). Sequences were aligned to available data in GenBank with BLASTn (http://blast.ncbi.nlm.nih.gov/Blast.cgi) Obtained MLST sequences were aligned and compared to sequences from the MLST database (http://pubmlst.org/borrelia).
Statistical analysis
Confidence intervals (95% CI) were determined for prevalences of Rickettsia spp. and B. burgdorferi (s.l.) in small mammals and in the questing ticks by the Clopper and Pearson method with the use of the Graph Pad Software (Graph Pad Software Inc., San Diego, Ca., USA). Pearson’s Chi-squared test was used with a type I error α of 0.05 to test the independence of compared prevalences. Fisher’s exact test was used for small sample sizes (n < 30) (Graph Pad Software). The odds ratio was calculated testing the association between the D. reticulatus ticks burden on Myodes glareolus and the prevalence of Rickettsia spp. in M. glareolus.
Results
Collection of small mammal samples
Altogether, 673 small mammals belonging to eight species (Apodemus agrarius, n = 7; A. flavicollis, n = 214; Microtus arvalis, n = 8; Microtus agrestis, n = 1; Mustela nivalis, n = 2; Myodes glareolus, n = 435; Sorex araneus, n = 5; Talpa europaea, n = 1) were collected. In 2012, a total of 454 small mammals were trapped: 306 M. glareolus; 127 A. flavicollis; 8 Mi. arvalis; 4 A. agrarius; 5 S. araneus; 2 Mu. nivalis; 1 Mi. agrestis; and 1 T. europaea. In 2013, only 90 small mammals were captured: 42 M. glareolus and 48 A. flavicollis. In 2014, a total of 129 small mammals were captured: 87 M. glareolus, 39 A. flavicollis and 3 A. agrarius.
Tick infestation on small mammals
Overall 3330 ticks were collected from 602 small mammals in the years 2012 (n = 1728), 2013 (n = 475) and 2014 (n = 1127). All small mammal species were infested with ticks except for Sorex araneus, Microtus agrestis and Talpa europaea. Totals of 310 D. reticulatus (159 larvae and 151 nymphs), 2802 I. ricinus (2583 larvae and 219 nymphs), 3 I. trianguliceps (3 nymphs), 208 Ixodes spp. (187 larvae and 21 nymphs), and seven ticks which could not be identified due to damage, were collected. Data on tick infestation per small mammal species are shown in Table 1.
Collection of questing ticks
Altogether 916 questing ticks were collected: 741 I. ricinus (79 females, 105 males, 504 nymphs and 53 larvae), 174 D. reticulatus (72 females and 102 males) and one I. trianguliceps (female). The breakdown of ticks by year and life-cycle stage is shown in Table 2.
PCR analysis for Rickettsia spp. and Borrelia burgdorferi (s.l.) in small mammals
In total, 210 out of 673 small mammals were positive for Borrelia burgdorferi (s.l.) (31.2%; 95% CI: 27.8–34.8). Of these, 140 out of 454 small mammals in 2012 (30.8%; 95% Cl: 26.9–35.5), 22 out of 90 (24.4%; 95% CI: 16.7–34.3) in 2013, and 48 out of 129 (36.7%; 95% Cl: 28.6–44.9) in 2014 were positive for B. burgdorferi (s.l.) detected by qPCR. Pairwise comparisons for the prevalence between the years revealed no significant differences. The prevalence in both dominant small mammal species was high, 32.9% (95% CI: 28.6–37.4) for M. glareolus and 25.4% (95% CI: 28.6–37.4) for A. flavicollis. Interestingly, these prevalence values did not differ significantly (P = 0.5302).
Due to financial restrictions, only five M. glareolus samples were tested by MLST. All sequenced samples were positive for B. afzelii. While four samples had the sequence type (ST) 165 (sample ID “321–324” in the Borrelia burgdorferi MLST database), one sample had the ST 559 (sample ID “1565”) (see complete sequence batches in Additional files 1 and 2).
Regarding the prevalence of Rickettsia spp., overall 170 of 673 small mammals (25.3%; 95% CI: 22.1–28.7) were positive. In 2012, a total of 134 out of 454 small mammals (29.7%; 95% CI: 22.1–28.7), in 2013, only 4 out of 90 (4.0%; 95% CI: 1.4–11.2) and in 2014, a total of 32 out of 129 small mammals (24.8%; 95% CI: 16.3–35.7) were positive for Rickettsia spp. detected by qPCR. The prevalence was significantly lower in 2013 compared to both of the other years (P < 0.0001). The chance of encountering Rickettsia spp. in M. glareolus was seven times higher for individuals infested with D. reticulatus than for specimens which were free of D. reticulatus (OR: 7.0; 95% CI: 3.3–14.7; P < 0.0001). In total, 17 samples (12 M. glareolus and 5 A. flavicollis) were sequenced. Altogether 15 records were available from these 17 samples. Two could not be further determined by sequencing. Nine samples were positive for R. helvetica (4 A. flavicollis and 5 M. glareolus), five for R. raoultii (all M. glareolus) and one for R. felis (A. flavicollis). All R. raoultii-positive M. glareolus were infested with D. reticulatus ticks. All R. helvetica-positive small mammals were infested with I. ricinus or had no tick at all, except for one M. glareolus which was simultaneously infested with I. ricinus and D. reticulatus. All sequences positive for R. helvetica showed 100% identity to a sequence in GenBank (KU310591) which was earlier obtained from an I. persulcatus tick from Russia (Katarshov et al. unpublished). All sequences positive for R. raoultii showed 100% identity to a sequence in GenBank (KU961542) which was earlier obtained from a D. marginatus tick from Russia (Katarshov et al. unpublished). The single R. felis sequence showed 100% identity to a sequence in GenBank (GU324467) which was also obtained from A. flavicollis in Germany [33]. The prevalence and the distribution of Borrelia spp. as well as Rickettsia spp. for all small mammal species are shown in Table 3.
PCR analysis for Rickettsia spp. and Borrelia burgdorferi (s.l.) in questing ticks
Altogether 4.2% (95% CI: 2.7–6.5) of the examined questing ticks were positive for Borrelia burgdorferi (s.l.). All positive ticks were I. ricinus (5.5%; 95% CI: 3.5–8.3); none of the 105 D. reticulatus (95% CI: 0.0–2.8) examined nor the single I. trianguliceps were positive for Borrelia burgdorferi (s.l.). The prevalences did not differ significantly between the years 2012–2014 (P = 0.298). The prevalence was significantly higher in I. ricinus than in D. reticulatus (P = 0.01). Furthermore, the prevalence of B. burgdorferi (s.l.) was significantly higher in small mammals than in questing I. ricinus (P < 0.0001).
Overall, 24.8% of all examined ticks were positive for Rickettsia spp. (95% CI: 21.1–28.8). The prevalence in ticks did not differ significantly between the years (P = 0.288). The prevalence was 11.4% (95% CI: 8.6–15.2) in I. ricinus and 70.5% in D. reticulatus (95% CI: 61.1–78.4). The single I. trianguliceps was positive for Rickettsia spp. Regarding both dominant tick species collected, the prevalence was significantly higher in D. reticulatus than in I. ricinus (χ 2 = 164.42, P < 0.0001). Overall, the prevalence in ticks compared to the small mammals did not differ significantly (χ 2 = 0.013, df = 1, P = 0.889). However, the prevalence in D. reticulatus ticks was significantly higher than in small mammals (χ 2 = 84.18, df = 1, P < 0.0001).
Discussion
This study was focussed on the detection of Borrelia burgdorferi (s.l.) and rickettsiae of the spotted fever group in wild-living small mammals and questing ticks from Germany. Borrelia burgdorferi (s.l.) is the causative agent of Lyme disease (LD) which is the most prevalent tick-borne disease in Europe and North America [8, 9]. LD may cause severe symptoms with manifestations in the skin, joints, nervous system and heart tissue in humans as well as in companion animals, especially in dogs [45–48]. Ixodes ricinus is known to be the main vector in Europe, whereas I. scapularis is the main vector in North America, and I. persulcatus in Eurasia [49–51]. The prevalences of B. burgdorferi (s.l.) in I. ricinus in Europe differ regionally. Studies from Europe, e.g. France [52, 53], the Netherlands [54], Slovakia [55] and Austria [56], show infection levels in I. ricinus ticks ranging from 3.3 to 22.5%. Earlier studies from Germany also showed high prevalence ranging from 11 to 36.2% in different regions of the country [57–59]. The present study confirms I. ricinus as the main vector for B. burgdorferi (s.l.), as the prevalence from this study was in line with previous studies from Europe [52–56]; however being lower than in previous studies from Germany (5.5%) [57–59]. The absence of Borrelia burgdorferi (s.l.) in questing I. ricinus larvae suggests a non-existent or insufficient transovarial transmission path [60]. However, transstadial transmission in ticks is verified [61]. Previous studies reported significantly higher prevalence for B. burgdorferi in adult I. ricinus ticks than in nymphs [52, 56, 59]. Our results are in contrast to these findings as I. ricinus nymphs were significantly more frequently infected than I. ricinus adults. Although in the past, spirochetes were detected in 11% of adult D. reticulatus ticks by immunofluorescence microscopy employing an antibody against B. burgdorferi [62], this non-specific method may likewise detect similar spirochetes such as B. miyamotoi [63]. Moreover, other study confirmed that D. reticulatus is not a suitable vector for B. burgdorferi (s.l.) [64, 65]. In our study, none of the D. reticulatus ticks examined tested positive for B. burgdorferi (s.l.); this supports the view that D. reticulatus is of minor importance in the natural life-cycle of this pathogen complex.
More than 40 vertebrate species, in particular birds and small mammals like rodents, are considered as reservoir hosts for B. burgdorferi (s.l.) in Europe [12, 13]. Previous studies from France, Ireland and Austria showed prevalence of B. burgdorferi (s.l.) in small mammal species ranging from 2.3 to 24% [66–68]. The infection level in small mammals in the current study was slightly higher than these obtained in earlier European studies (31.3%). In present research, each species belonging to the order Rodentia was positive and with high prevalence of B. burgdorferi (s.l.) (25.4–62.5%), whereas the insectivores (1 Talpa europaea and 5 Sorex araneus) and the carnivores (1 Mustela nivalis) were all negative. These findings are in line with a study from Austria where all rodent species were positive for B. burgdorferi (s.l.) and also with high prevalence (13.3–77.0%) [68]. The prevalence of spirochetes in rodents from this study was high and independent from their tick burden, and moreover significantly higher than in questing I. ricinus. These results therefore support the hypothesis that the rodent species studied are potential reservoirs for B. burgdorferi (s.l.). They are known to harbour B. japonica, B. afzelii, B. bissettii and the NT29 ribotype as well as the OspA serotype A of B. garinii [69].
Borrelia afzelii was found in all five small mammal samples. Studies from other European countries confirm that B. afzelii is a genospecies which is associated with rodents [70, 71]. In Europe, MLST was performed for the identification and genotyping of Borrelia spp. in rodents from central Slovenia [72], questing I. ricinus ticks from Norway [73] and the UK [74], and ticks and rodents from France [75, 76]. In Germany, the MLST method has thus far been used in research on phylogenetic relationships and global evolution of the B. burgdorferi (s.l.) species complex [77], and on the population structure and pathogenicity of B. afzelii and B. burgdorferi (s.s.) [78]. To our knowledge, this is the first study using MLST for the detection of allelic combinations of B. burgdorferi in small mammals from Germany. The analysis of the eight housekeeping genes, i.e. nifS, uvrA, clpA, clpX, rplB, recG, pyrG and pepX, revealed ST 165 and 559, both sequence types belonging to B. afzelii. These sequence types were described earlier in I. ricinus ticks from Latvia, Slovenia and France according to Borrelia spp. MLST database (http://pubmlst.org/bigsdb?db=pubmlst_borrelia_isolates&page=profiles).
Rickettsiae of the spotted fever group may cause a variety of clinical symptoms such as lymphadenopathia, fever and headache in humans [79]. In Europe, there are several different species of varying pathogenic potential (R. aeschlimannii, R. conorii, R. helvetica, R. massiliae, R. monacensis, R. raoultii, R. sibirica and R. slovaca) [15]. In the present study, Rickettsia spp. were detected in all collected tick species (I. ricinus, I. trianguliceps and D. reticulatus). Results from France, the Netherlands, Austria and Poland showed infection levels in I. ricinus ticks ranging from 1.4 to 41% [80–83]. The prevalence obtained in the present study is in line with these findings. High infection rates (11–50%) for Rickettsia spp. in D. reticulatus were detected in previous investigations from the UK, Slovakia and Croatia [84–86]. The infection level in the present research is higher (70.5%), though not as high as in a previous study by our group (85.6%) which was conducted in the same study sites [27]. Transovarial and transstadial transmission of Rickettsia spp. have been described in ticks. Moreover, horizontal transmission during feeding on a bacteriaemic host and co-feeding of Rickettsia-positive arthropods were also demonstrated [87, 88]. Dermacentor reticulatus is known to be the main vector of R. raoultii. As the prevalence in adult D. reticulatus ticks was very high but much lower in small mammals, it is probable that transovarial transmission is the main transmission path in D. reticulatus and that rodents are not of primary importance for the maintenance the natural circulation of R. raoultii.
The prevalence of Rickettsia spp. was significantly higher in D. reticulatus than in I. ricinus and in small mammals, pointing out that D. reticulatus-related rickettsiae are maintained independently from a vertebrate reservoir in nature, in contrast to I. ricinus-related rickettsiae. In Europe, there are very few studies about the maintaining and distribution of Rickettsia spp. in wild small mammals [32, 33, 81]. In Germany, two studies revealed the occurrence of R. helvetica in A. agrarius, A. flavicollis and M. glareolus [27, 32, 33]. In the present study, Rickettsia spp. was also found in these three rodent species. The study sites of the current research were earlier investigated for Rickettsia spp. by our group. These preliminary studies revealed high prevalences in D. reticulatus (56.7–85.6%), I. ricinus (13.4–17.5%), and small mammals (28.6%) [27, 38]. The prevalence rates for Rickettsia spp. in the present study are in line with earlier findings for D. reticulatus (70.5%), however slightly lower for I. ricinus (11.4%) and small mammals (25.3%). In previous investigations on small mammals from Germany, R. felis, R. helvetica, R. monacensis and R. raoultii were detected [27, 33]. Our results confirmed the occurrence of all mentioned Rickettsia spp. except for R. monacensis. All R. raoultii-positive rodents were infested with D. reticulatus, the main vector for R. raoultii. Interestingly, the D. reticulatus tick burden was positively correlated with the prevalence of Rickettsia spp. in M. glareolus. Myodes glareolus had a seven times higher chance of encountering Rickettsia infection while being infested with D. reticulatus in comparison to M. glareolus without D. reticulatus. Comparisons of the prevalence of Rickettsia spp. in small mammals between the years 2012–2014, revealed significantly lower infection rates in 2013 than in 2012 and 2014. Interestingly, none of the small mammals captured in 2013 was infested with D. reticulatus. This leads to the assumption that small mammals infected with D. reticulatus-related rickettsiae are rather incidental than potential reservoir hosts.
Conclusions
The prevalence for B. burgdorferi (s.l.) in small mammals was high (> 30%) and independent of tick abundance, suggesting small mammals as reservoirs. To our knowledge, this is the first detection of Borrelia spp. sequence types in small mammals from Germany, revealing ST 165 and ST 559 which belong to Borrelia genospecies B. afzelii. Small mammals may also serve as reservoirs for I. ricinus-transmitted Rickettsia spp. Bank voles (Myodes glareolus) had a seven times higher chance of encountering Rickettsia spp. infection while being infested with D. reticulatus in comparison to M. glareolus without D. reticulatus. As the prevalence in questing adult D. reticulatus was very high (> 70%) but much lower in rodents (c.25%), a potential reservoir function of bank voles is unlikely. The prevalence of R. raoultii in M. glareolus can be a result of infestation with infected D. reticulatus. We suggest that transstadial (and likely transovarial) transmission in D. reticulatus is the main mode of maintenance of R. raoultii natural life-cycle.
Abbreviations
- CI:
-
Confidence interval
- MLST:
-
Multi-locus sequence typing
- OR:
-
Odds ratio
- qPCR:
-
quantitative polymerase chain reaction
- SFG:
-
Spotted fever group.
References
Rizzoli A, Rizzoli A, Silaghi C, Obiegala A, Rudolf I, Hubálek Z, Földvári G, et al. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: new hazards and relevance for public health. Front Public Health. 2014;2:251.
Mączka I, Tylewska-Wierzbanowska S. Life cycle of Borrelia burgdorferi spirochete in the environment. Post Mikrobiol. 2010;49:25–32.
Estrada-Peña A, Osácar JJ, Pichon B, Gray JS. Hosts and pathogens detection for immature stages of Ixodes ricinus (Acari: Ixodidae) in North-Central Spain. Exp Appl Acarol. 2005;37:257–68.
Mihalca AD, Dumitrache MO, Sándor AD, Magdaş C, Oltean M, Györke A, et al. Tick parasites of rodents in Romania: host preferences, community structure and geographical distribution. Parasit Vectors. 2012;21:266.
Silaghi C, Woll D, Hamel D, Pfister K, Mahling M, Pfeffer M. Babesia spp. and Anaplasma phagocytophilum in questing ticks, ticks parasitizing rodents and the parasitized rodents - Analyzing the host-pathogen-vector interface in a metropolian area. Parasit Vectors. 2012;5:191.
Silaghi C, Woll D, Mahling M, Pfister K, Pfeffer M. Candidatus Neoehrlichia mikurensis in rodents in an area with sympatric existence of the hard ticks Ixodes ricinus and Dermacentor reticulatus. Parasit Vectors. 2012;5:285.
Földvári G, Široký P, Szekeres S, Majoros G, Sprong H. Dermacentor reticulatus: a vector on the rise. Parasit Vectors. 2016;9:314.
Anderson JF. Epizootiology of Lyme borreliosis. Scand J Infect Dis Suppl. 1991;77:23–4.
Bowman AS, Nuttall PA, editors. Ticks: biology, disease and control. Cambridge: Cambridge University Press; 2009.
Gray J. BIOLOGY: The Spirochaete: Borrelia Strains. In: EUCLAB, European Concreted Action on Lyme Borreliosis. 2016. http://meduni09.edis.at/eucalb/cms_15/index.php?option=com_content&view=article&id=58:spirochaete-strains&catid=57:biology-cat1&Itemid=92 of Spirochaete, strains. Accessed 15 Jan 2017.
Krause PJ, Fish D, Narasimhan S, Barbour AG. Borrelia miyamotoi infection in nature and humans. Clin Microbiol Infect. 2015;21:631–9.
Gorelova N, Korenberg E, Kovalevskii Y, Shcherbakov S. Small mammals as reservoir hosts for Borrelia in Russia. Zbl Bakt. 1995;282:315–22.
Gassner F, Takken W, Lombaers-van der Plas C, Kastelein P, Hoetmer A, Holdinga M, van Overbeek L. Rodent species as natural reservoirs of Borrelia burgdorferi sensu lato in different habitats of Ixodes ricinus in the Netherlands. Ticks Tick Borne Dis. 2013;4:452–8.
Sun J, Lin J, Gong Z, Chang Y, Ye X, Gu S, et al. Detection of spotted fever group Rickettsiae in ticks from Zhejiang Province, China. Exp Appl Acarol. 2015;65:403–11.
Parola P, Paddock CD, Socolovschi C, Labruna MB, Mediannikov O, Kernif T, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev. 2013;26:657–702.
Parola P, Paddock CD, Raoult D. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev. 2005;18:719–56.
Parola P, Rovery C, Rolain JM, Brouqui P, Davoust B, Raoult D. Rickettsia slovaca and R. raoultii in tick-borne rickettsioses. Emerg Infect Dis. 2009;15:1105–8.
Ibarra V, Oteo JA, Portillo A, Santibanez S, Blanco JR, Metola L, et al. Rickettsia slovaca infection: DEBONEL/TIBOLA. Ann N Y Acad Sci. 2006;1078:206–14.
Brouqui P, Parola P, Fournier PE, Raoult D. Spotted fever rickettsioses in southern and eastern Europe. FEMS Immunol Med Microbiol. 2007;49:2–12.
Ortuno A, Pons I, Quesada M, Lario S, Anton E, Gil A, Castella J, Segura F. Evaluation of the presence of Rickettsia slovaca infection in domestic ruminants in Catalonia, Northeastern Spain. Vector Borne Zoonotic Dis. 2012;12:1019–22.
Ortuno A, Quesada M, Lopez-Claessens S, Castella J, Sanfeliu I, Anton E, Segura-Porta F. The role of wild boar (Sus scrofa) in the eco-epidemiology of R. slovaca in northeastern Spain. Vector Borne Zoonotic Dis. 2007;7:59–64.
Inokuma H, Seino N, Suzuki M, Kaji K, Takahashi H, Igota H, Inoue S. Detection of Rickettsia helvetica DNA from peripheral blood of sika deer (Cervus nippon yesoensis) in Japan. J Wildl Dis. 2008;44:164–7.
de Mera IG F, Zivkovic Z, Bolanos M, Carranza C, Perez-Arellano JL, Gutierrez C, de la Fuente J. Rickettsia massiliae in the Canary Islands. Emerg Infect Dis. 2009;15:1869–70.
de Sousa R, Lopes de Carvalho I, Santos AS, Bernardes C, Milhano N, Jesus J, Menezes D, Nuncio MS. Role of the lizard Teira dugesii as a potential host for Ixodes ricinus tick-borne pathogens. Appl Environ Microbiol. 2012;78:3767–9.
Schicht S, Schreiber T, Strube C. Rickettsia spp. and coinfections with other pathogenic microorganisms in hard ticks from northern Germany. J Med Entomol. 2012;49:766–71.
Overzier E, Pfister K, Thiel C, Herb I, Mahling M, Silaghi C. Diversity of Babesia and Rickettsia species in questing Ixodes ricinus: A longitudinal study in urban, pasture, and natural habitats. Vector Borne Zoonotic Dis. 2013;13:559–64.
Obiegala A, Oltersdorf C, Silaghi C, Kiefer D, Kiefer M, Woll D, Pfeffer M. Rickettsia spp. in small mammals and their parasitizing ectoparasites from Saxony, Germany. Vet Parasitol. 2016;5:19–24.
Schreiber C, Krücken J, Beck S, Maaz D, Pachnicke S, Krieger K, et al. Pathogens in ticks collected from dogs in Berlin/Brandenburg, Germany. Parasit Vectors. 2014;7:535.
Tappe J, Jordan D, Janecek E, Fingerle V, Strube C. Revisited: Borrelia burgdorferi sensu lato infections in hard ticks (Ixodes ricinus) in the city of Hanover (Germany). Parasit Vectors. 2014;7:441.
May K, Jordan D, Fingerle V, Strube C. Borrelia burgdorferi sensu lato and co-infections with Anaplasma phagocytophilum and Rickettsia spp. in Ixodes ricinus in Hamburg, Germany. Med Vet Entomol. 2015;29:425–9.
Mehlhorn H, Mehlhorn T, Müller M, Vogt M, Rissland J. Tick survey for prevalent pathogens in peri-urban recreation sites in Saarland and Rhineland-Palatinate (Germany). Parasitol Res. 2016;115:1167–72.
Pluta S, Harteit K, Oehme R, Mackenstedt U, Kimming P. Prevalence of Coxiella burnetti and Rickettsia spp. in ticks and rodents in southern Germany. Ticks Tick Borne Dis. 2010;1:145–7.
Schex S, Dobler G, Riehm J, Müller J, Essbauer S. Rickettsia spp. in wild small mammals in Lower Bavaria, South-Eastern Germany. Vector Borne Zoonotic Dis. 2011;11:493–50.
Speck S, Perseke L, Petney T, Skuballa J, Pfäffle M, Taraschewski H, et al. Detection of Rickettsia helvetica in ticks collected from European hedgehogs (Erinaceus europaeus, Linnaeus, 1758). Ticks Tick Borne Dis. 2013;4:222–6.
Richter D, Schlee DB, Matuschka FR. Reservoir competence of various rodents for the Lyme disease spirochete Borrelia spielmanii. Appl Environ Microbiol. 2011;77:3565–70.
Skuballa J, Petney T, Pfäffle M, Oehme R, Hartelt K, Fingerle V, et al. Occurrence of different Borrelia burgdorferi sensu lato genospecies including B. afzelii, B. bavariensis, and B. spielmanii in hedgehogs (Erinaceus spp.) in Europe. Ticks Tick Borne Dis. 2012;3:8–13.
Fietz J, Tomiuk J, Matuschka FR, Richter D. Seasonal prevalence of Lyme disease spirochetes in a heterothermic mammal, the edible dormouse (Glis glis). Appl Environ Microbiol. 2014;80:3615–21.
Silaghi C, Hamel D, Thiel C, Pfister K, Pfeffer M. Spotted fever group rickettsiae in ticks, Germany. Emerg Infect Dis. 2011;17:890–2.
Stresemann E. Exkursionsfauna von Deutschland, Wirbeltiere, vol. 3. Heidelberg: Spektrum Akademischer Verlag, Gustav Fischer; 1989.
Siuda K. Kleszcze (Acari: Ixodida) Polski: Zagadnienia ogólne. Monografie parazytologiczne. 1991.
Wölfel R, Essbauer SS, Dobler G. Diagnostics of tick-borne rickettsioses in Germany: A modern concept for a neglected disease. J Med Microbiol. 2008;298:368–74.
Schwaiger M, Peter O, Cassinotti P. Routine diagnosis of Borrelia burgdorferi (sensu lato) infections using a real‐time PCR assay. Clin Microbiol Infect. 2001;7:461–9.
Roux V, Raoult D. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int J Syst Evol Microbiol. 2000;50:1449–55.
Wang G, Liveris D, Mukherjee P, Jungnick S, Margos G, Schwartz I. Molecular typing of Borrelia burgdorferi. Curr Protoc Microbiol. 2014;34:1–31.
Asbrink E, Hovmark A. Early and late cutaneous manifestations in Ixodes-borne borreliosis (Erythema migrans borreliosis, Lyme borreliosis). Ann NY Acad Sci. 1988;539:4–15.
Steere AC. Lyme disease. N Engl J Med. 2001;345:115–25.
Pachner AR, Steere AC. The triad of neurologic manifestations of Lyme disease: meningitis, cranial neuritis, and radiculoneuritis. Neurology. 1985;111:47–53.
Strle F. Ocular manifestations of Lyme borreliosis. Acta Dermato-venereol APA. 1994;1–2:71–6.
Piesman J. Ecology of Borrelia burgdorferi sensu lato in North America. In: Gray JS, Kahl O, Lane RS, Stanek G, editors. Lyme borreliosis: biology, epidemiology and control. 1st ed. New York: CABI Publishing, New York; 2002. p. 223–50.
Gern L, Humair PF. Ecology of Borrelia burgdorferi sensu lato in Europe. In: Gray JS, Kahl O, Lane RS, Stanek G, editors. Lyme borreliosis: biology, epidemiology and control. 1st ed. New York: CABI Publishing, New York; 2002. p. 149–74.
Korenberg E, Gorelova NB, Kovalevskii YV. Ecology of Borrelia burgdorferi sensu lato in Russia. In: Gray JS, Kahl O, Lane RS, Stanek G, editors. Lyme borreliosis: biology, epidemiology and control. 1st ed. New York: CABI Publishing, New York; 2002. p. 175–200.
Halos L, Jamal T, Maillard R, Beugnet F, Le Menach A, Boulouis HJ, Vayssier-Taussat M. Evidence of Bartonella sp. in questing adult and nymphal Ixodes ricinus ticks from France and co-infection with Borrelia burgdorferi sensu lato and Babesia sp. Vet Res. 2005;36:79–87.
Ferquel E, Garnier M, Marie J, Bernède-Bauduin C, Baranton G, Pérez-Eid C, Postic D. Prevalence of Borrelia burgdorferi sensu lato and Anaplasmataceae members in Ixodes ricinus ticks in Alsace, a focus of Lyme borreliosis endemicity in France. Appl Environ Microbiol. 2006;72:3074–8.
Schouls LM, Van De Pol I, Rijpkema SG, Schot CS. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J Clin Microbiol. 1999;37:2215–22.
Lenčáková D, Hizo-Teufel C, Peťko B, Schulte-Spechtel U, Stanko M, Wilske B, Fingerle V. Prevalence of Borrelia burgdorferi sl OspA types in Ixodes ricinus ticks from selected localities in Slovakia and Poland. Int J Med Microbiol. 2006;296:108–18.
Stünzner D, Hubálek Z, Halouzka J, Wendelin I, Sixl W, Marth E. Prevalence of Borrelia burgdorferi sensu lato in the tick Ixodes ricinus in the Styrian mountains of Austria. Wien Klin Wochenschr. 2006;118:682–5.
Fingerle V, Munderloh UG, Liegl G, Wilske B. Coexistence of ehrlichiae of the phagocytophila group with Borrelia burgdorferi in Ixodes ricinus from Southern Germany. Med Microbiol Immun. 1999;188:145–9.
Hildebrandt A, Schmidt KH, Wilske B, Dorn W, Straube E, Fingerle V. Prevalence of four species of Borrelia burgdorferi sensu lato and coinfection with Anaplasma phagocytophila in Ixodes ricinus ticks in central Germany. Eur J Clin Microbiol Infect Dis. 2003;22:364–7.
Dietrich F, Schmidgen T, Maggi RG, Richter D, Matuschka FR, Vonthein R, Kempf VA. Prevalence of Bartonella henselae and Borrelia burgdorferi sensu lato DNA in Ixodes ricinus ticks in Europe. Appl Environ Microbiol. 2010;76:1395–8.
Zhioua E, Aeschlimann A, Gern L. Infection of field-collected Ixodes ricinus (Acari: Ixodidae) larvae with Borrelia burgdorferi in Switzerland. J Med Entomol. 1994;31:763–6.
Cadenas FM, Rais O, Humair PF, Douet V, Moret J, Gern L. Identification of host blood meal source and Borrelia burgdorferi sensu lato in field-collected Ixodes ricinus ticks in Chaumont (Switzerland). J Med Entomol. 2007;44:1109–17.
Kahl O, Janetzki C, Gray JS, Stein J, Bauch RJ. Tick infection rates with Borrelia Ixodes ricinus versus Haemaphysalis concinna and Dermacentor reticulatus in two locations in eastern Germany. Med Vet Entomol. 1992;6:363–6.
Scoles GA, Papero M, Beati L, Fish D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1:21–34.
Richter D, Kohn C, Matuschka FR. Absence of Borrelia spp., Candidatus Neoehrlichia mikurensis, and Anaplasma phagocytophilum in questing adult Dermacentor reticulatus ticks. Parasitol Res. 2013;112:107–11.
Reye AL, Stegniy V, Mishaeva NP, Velhin S, Hübschen JM, Ignatyev G, Muller CP. Prevalence of tick-borne pathogens in Ixodes ricinus and Dermacentor reticulatus ticks from different geographical locations in Belarus. PLoS One. 2013;8:e54476.
Gray JS, Kirstein F, Robertson JN, Stein J, Kahl O. Borrelia burgdorferi sensu lato in Ixodes ricinus ticks and rodents in a recreational park in south-western Ireland. Exp Appl Acarol. 1999;23:717–29.
Marsot M, Sigaud M, Chapuis JL, Ferquel E, Cornet M, Vourc’h G. Introduced Siberian chipmunks (Tamias sibiricus barberi) harbor more-diverse Borrelia burgdorferi sensu lato genospecies than native bank voles (Myodes glareolus). Appl Environ Microbiol. 2011;77:5716–21.
Khanakah G, Kocianová E, Vyrosteková V, Řeháček J, Kundi M, Stanek G. Seasonal variations in detecting Borrelia burgdorferi sensu lato in rodents from north eastern Austria. Wien Klin Wochenschr. 2006;118:754–8.
Kurtenbach K, De Michelis S, Etti S, Schäfer SM, Sewell HS, Brade V, Kraiczy P. Host association of Borrelia burgdorferi sensu lato - the key role of host complement. Trends Microbiol. 2002;10:74–9.
van Duijvendijk G, Sprong H, Takken W. Multi-trophic interactions driving the transmission cycle of Borrelia afzelii between Ixodes ricinus and rodents: a review. Parasit Vectors. 2015;8:1.
Tadin A, Tokarz R, Markotić A, Margaletić J, Turk N, Habuš J, Lipkin WI. Molecular survey of zoonotic agents in rodents and other small mammals in Croatia. Am J Trop Med Hyg. 2016;94:466–73.
Cerar T, Korva M, Avšič-Županc T, Ružić-Sabljić E. Detection, identification and genotyping of Borrelia spp. in rodents in Slovenia by PCR and culture. BMC Vet Res. 2015;11:188.
Tveten AK. Exploring diversity among Norwegian Borrelia strains originating from Ixodes ricinus ticks. Int J Microbiol. 2014;2014:397143.
James MC, Gilbert L, Bowman AS, Forbes KJ. The heterogeneity, distribution, and environmental associations of Borrelia burgdorferi sensu lato, the agent of Lyme borreliosis, in Scotland. Front Public Health. 2014;2:129.
Jacquot M, Abrial D, Gasqui P, Bord S, Marsot M, Masseglia S, et al. Multiple independent transmission cycles of a tick-borne pathogen within a local host community. Sci Rep. 2016;6:31273.
Jacquot M, Bisseux M, Abrial D, Marsot M, Ferquel E, Chapuis J-L, et al. High-throughput sequence typing reveals genetic differentiation and host specialization among populations of the Borrelia burgdorferi species complex that infect rodents. PLoS One. 2014;9:e88581.
Becker NS, Margos G, Blum H, Krebs S, Graf A, Lane RS, et al. Recurrent evolution of host and vector association in bacteria of the Borrelia burgdorferi sensu lato species complex. BMC Genom. 2016;17:734.
Jungnick S, Margos G, Rieger M, Dzaferovic E, Bent SJ, Overzier E, et al. Borrelia burgdorferi sensu stricto and Borrelia afzelii: Population structure and differential pathogenicity. Int J Med Microbiol. 2015;305:673–81.
Raoult D, Weiller PJ, Chagnon A, Chaudet H, Gallais H, Casanova P. Mediterranean spotted fever: clinical, laboratory and epidemiological features of 199 cases. Am J Trop Med Hyg. 1986;35:845–50.
Cotté V, Bonnet S, Cote M, Vayssier-Taussat M. Prevalence of five pathogenic agents in questing Ixodes ricinus ticks from western France. Vector Borne Zoonotic Dis. 2010;10:723–30.
Sprong H, Wielinga PR, Fonville M, Reusken C, Brandenburg AH, Borgsteede F, et al. Ixodes ricinus ticks are reservoir hosts for Rickettsia helvetica and potentially carry flea-borne Rickettsia species. Parasit Vectors. 2009;2:41.
Blaschitz M, Narodoslavsky-Gföller M, Kanzler M, Walochnik J, Stanek G. First detection of Rickettsia helvetica in Ixodes ricinus ticks in Austria. Vector Borne Zoonotic Dis. 2008;8:561–4.
Chmielewski T, Podsiadly E, Karbowiak G, Tylewska-Wierzbanowska S. Rickettsia spp. in ticks, Poland. Emerg Infect Dis. 2009;15:486–8.
Tijsse-Klasen E, Hansford KM, Jahfari S, Phipps P, Sprong H, Medlock JM. Spotted fever group rickettsiae in Dermacentor reticulatus and Haemaphysalis punctata ticks in the UK. Parasit Vectors. 2013;6:1.
Špitalská E, Štefanidesová K, Kocianová E, Boldiš V. Rickettsia slovaca and Rickettsia raoultii in Dermacentor marginatus and Dermacentor reticulatus ticks from Slovak Republic. Exp Appl Acarol. 2012;57:189–97.
Dobec M, Golubic D, Punda-Polic V, Kaeppeli F, Sievers M. Rickettsia helvetica in Dermacentor reticulatus ticks. Emerg Infect Dis. 2009;15:98–100.
Walker DH, Ismail N. Emerging and re-emerging rickettsioses: endothelial cell infection and early disease events. Nat Rev Microbiol. 2008;6:375–86.
Azad AF, Beard CB. Rickettsial pathogens and their arthropod vectors. Emerg Infect Dis. 1998;4:179–86.
Acknowledgments
We thank Dana Rüster, Dietlinde Woll, Tessa Foerster, Dr. Anneliese Balling, Dr. André F. Streck and Rayan Ababneh for their excellent practical help during fieldwork and technical assistance. The work of MP and AO was done within the framework of COST action TD1303 EURNEGVEC. This paper has been sponsored by Bayer Animal Heath in the framework of the 12th CVBD world forum symposium.
Funding
The Federal Environment Agency of Germany (FKZ 371148) funded part of this project.
Availability of data and materials
The data supporting the conclusions of this article are included within the article. The raw data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
MP and AO organized and planned the study. AO, MP and CO organized and participated in the fieldwork for the collection of wildlife samples. AO carried out the morphologic determination of ticks. AO, CO, JN and NK prepared the samples in the laboratory. AO, NK, JN and CO tested the samples for Borrelia burgdorferi (s.l.) and Rickettsia spp. AO performed the sequence analysis. AO, NK and MP drafted the manuscript and wrote the final version. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1:
Batch of Borrelia afzelii sequences according to the genes: clpA, clpX, uvrA, nifS, rplB, recG, pepX, pyrG of sequence type 165. (DOCX 17 KB)
Additional file 2:
Batch of Borrelia afzelii sequences according to the genes: clpA, clpX, uvrA, nifS, rplB, recG, pepX, pyrG of sequence type 559. (DOCX 16 KB)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Obiegala, A., Król, N., Oltersdorf, C. et al. The enzootic life-cycle of Borrelia burgdorferi (sensu lato) and tick-borne rickettsiae: an epidemiological study on wild-living small mammals and their ticks from Saxony, Germany. Parasites Vectors 10, 115 (2017). https://doi.org/10.1186/s13071-017-2053-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-017-2053-4