Abstract
Background
Knowledge with respect to regulatory systems for cellulase production is prerequisite for exploitation of such regulatory networks to increase cellulase production, improve fermentation efficiency and reduce the relevant production cost. The target of rapamycin (TOR) signaling pathway is considered as a central signaling hub coordinating eukaryotic cell growth and metabolism with environmental inputs. However, how and to what extent the TOR signaling pathway and rapamycin are involved in cellulase production remain elusive.
Result
At the early fermentation stage, high-dose rapamycin (100 μM) caused a temporary inhibition effect on cellulase production, cell growth and sporulation of Trichoderma reesei RUT-C30 independently of the carbon sources, and specifically caused a tentative morphology defect in RUT-C30 grown on cellulose. On the contrary, the lipid content of T. reesei RUT-C30 was not affected by rapamycin. Accordingly, the transcriptional levels of genes involved in the cellulase production were downregulated notably with the addition of rapamycin. Although the mRNA levels of the putative rapamycin receptor trFKBP12 was upregulated significantly by rapamycin, gene trTOR (the downstream effector of the rapamycin–FKBP12 complex) and genes associated with the TOR signaling pathways were not changed markedly. With the deletion of gene trFKBP12, there is no impact of rapamycin on cellulase production, indicating that trFKBP12 mediates the observed temporary inhibition effect of rapamycin.
Conclusion
Our study shows for the first time that only high-concentration rapamycin induced a transient impact on T. reesei RUT-C30 at its early cultivation stage, demonstrating T. reesei RUT-C30 is highly resistant to rapamycin, probably due to that trTOR and its related signaling pathways were not that sensitive to rapamycin. This temporary influence of rapamycin was facilitated by gene trFKBP12. These findings add to our knowledge on the roles of rapamycin and the TOR signaling pathways play in T. reesei.
Similar content being viewed by others
Background
Cellulolytic fungi like Neurospora crassa and Trichoderma reesei are involved in the degradation of plant biomass and play important roles in ecosystems [1, 2]. These fungi have evolved an excellent capability to secret (hemi)cellulase to convert insoluble polysaccharides into fermentable sugars for surviving under lignocellulolytic conditions. The (hemi)cellulase should be produced in a strictly controlled way. Otherwise, too much cellular resources are directed towards protein synthesis, which is detrimental for cell survival [1]. Therefore, cellulolytic fungi have developed a complex regulatory machinery to coordinate nutrients for growth and hydrolytic enzyme production. For instance, the cellulase production of T. reesei is regulated in response to various environmental stresses such as light [3, 4], organic solvents [5], and metal ions [6]. Several signal pathways like mitogen-activated protein kinase (MAPK) pathways [7], Ca2+-responsive signaling pathway [8] and light regulation pathway [3] have been reported to be involved in the regulation of cellulase production. A better understanding of regulatory systems for cellulase production is prerequisite for exploitation of such regulatory networks to increase enzyme secretion, improve fermentation efficiency and reduce the enzyme production cost.
The target of rapamycin (TOR) signaling pathway is regarded as a central signaling hub integrating cell growth and metabolism with environmental inputs, including nutrients and growth factors in eukaryotes [9]. The TOR signaling network responds to signals such as nutritional state, cellular energy state, and growth factors to orchestrate cellular growth, proliferation, and stress responses. In response to nutrients, it activates anabolic processes like protein, lipid and nucleotide synthesis, and suppresses catabolic processes such as autophagy, thus promoting cell growth. Several studies have reported that the TOR signaling pathway plays a part in regulating cellulase production. Gene sch9 is a critical component of the TOR signaling pathway in Saccharomyces cerevisiae. Deletion of its homolog stk-10 in N. crassa severely decreased cellulase production [10]. Similarly, loss of schA (the homolog of stk-10) in Aspergillus nidulans caused defects in protein production and cellulolytic enzyme activities [11]. Also, knockdown of sch9 in T. reesei TU-6 slightly compromised the cellulase production [12]. Despite these preliminary studies, how and to what extent the TOR signaling pathway is involved in cellulase production remains elusive. Even worse, the relevant study of the TOR signaling pathway in filamentous fungi is lacking, though it has been extensively studied in yeast S. cerevisiae and Schizosaccharomyces pombe [13, 14], mammalian cells [15], and plant cells [16, 17].
The TOR kinases were first identified in S. cerevisiae as the targets of rapamycin. Rapamycin, a lipophilic macrocyclic lactone isolated from Streptomyces hygroscopicus in the early 1970s [18], displays antifungal, anticancer and immunosuppressive activities [9]. Rapamycin and its derivatives, which are called “rapalogues”, have been applied in clinic to inhibit tumor growth and prevent organ rejection [9]. When diffusing into the cell, rapamycin forms a complex with the peptidyl-prolyl cis/trans isomerase FKBP12, which subsequently binds to the TOR kinases and inhibits their functions [14, 19, 20]. Both FKBP12 and TOR kinases are conserved in eukaryotic organisms from fungi to human [21,22,23,24,25,26,27,28,29]. Rapamycin is an indispensable tool for studying the role of the TOR signaling pathway in organisms. However, the impact of rapamycin on T. reesei and cellulase production in filamentous fungi has not been reported, as far as we know.
As a first step toward understanding the role of the TOR pathway in cellulase production, the impact of rapamycin on T. reesei RUT-C30 grown on different carbon sources was investigated in terms of cellulase production, morphology, cell growth, sporulation and lipid content. T. reesei RUT-C30 was insensitive to rapamycin and only a temporary effect was observed with high concentration of rapamycin at the early cultivation stage, including decreased cellulase production, cell growth and sporulation, and altered cell morphology. The molecular mechanism behind this phenomenon was explored by comparative transcriptional profiling and gene knockout of trFKBP12.
Results
Rapamycin at high concentration temporarily inhibits cellulase production
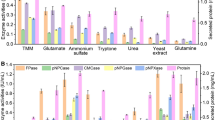
The effect of rapamycin on (hemi)cellulase production of T. reesei RUT-C30, an industrial hypercellulolytic strain [30,31,32,33], was investigated (Fig. 1). In the presence of 0.01 μM rapamycin, all the tested cellulase activities were not changed at 24 h, but was increased at 72 h and higher than those of RUT-C30 without rapamycin during the rest fermentation time. The pNPGase, pNPCase, CMCase, and FPase activities were improved by 28.7%, 24.0%, 19.2%, and 26.2% separately at 72 h. Nevertheless, when the concentration of rapamycin was increased to 1 μM, the pNPGase, pNPCase, and CMCase activities were reduced at 24 h, but increased at 120 h and beyond. The FPase activity did not reach the same level as that without rapamycin until 168 h. A higher concentration of rapamycin than 1 μM caused a more serious reduction on cellulase activities at the early fermentation process. Especially, the addition of 100 μM rapamycin led to a decline by 67.2%, 60.2%, 56.6%, and 70.3% for pNPGase, pNPCase, CMCase, and FPase activities, respectively, at 24 h. Interestingly, T. reesei RUT-C30 had the ability to recover and produce the same or even a larger amount of cellulase at 168 h, regardless of the concentrations of rapamycin. The FPase activity reached ~ 5 IU/mL at 168 h, which was comparable to those (2–8 IU/mL) reported in previous studies under similar culture conditions [34,35,36]. Obviously, rapamycin improved the cellulase production at low concentration, but at high concentration severely inhibited the cellulase production at the early stage which was restored at the late stage. The secreted protein level followed a similar trend as cellulase activity. The concentration of secreted protein was halved at 100 μM rapamycin at 24 h, as compared to the untreated sample.
The (hemi)cellulase activities and protein secretion of T. reesei RUT-C30 cultured in TMM + 2% cellulose with different concentrations of rapamycin at 24 h (a), 72 h (b), 120 h (c), and 168 h (d), respectively. FPase: the filter paper activity; pNPCase: the CBH activity; CMCase: the CMC activity; pNPGase: the β-glucosidase activity; pNPXase: the β-xylosidase activity; Secreted protein: secreted protein concentration. Data are represented as the mean of three independent experiments and error bars express the standard. Asterisks indicate significant differences (*p < 0.05, **p < 0.01, ***p < 0.001) as assessed by Student’s t test
The pNPXase activity did not have obvious change at 0.01 μM rapamycin. At 1 μM rapamycin, the pNPXase activity was decreased at 48 h and 72 h, but was restored to the level of that without rapamycin at 120 h. At 10 μM and 100 μM rapamycin, the pNPXase activity was sharply reduced by 68.0% and 71.9% at 24 h, and remained lower than that without rapamycin at 168 h, suggesting that high concentration of rapamycin decreased hemicellulase activity which was unable to be recovered.
A similar effect of 100 μM rapamycin on the (hemi)cellulase production was found in T. reesei RUT-C30 grown on glucose or lactose, except that the pNPCase activity was not changed in the presence of rapamycin when using glucose as the carbon source (Additional file 1: Figure S1). Nevertheless, the temporary inhibition effect of rapamycin on the total extracellular protein concentration was not found at the early fermentation stage of T. reesei RUT-C30 cultivated on glucose or lactose. In addition, the inhibition effect of rapamycin on the pNPXase activity can be relieved at the late stage, different from that of T. reesei RUT-C30 cultured on cellulose. Obviously, the effect of rapamycin on the (hemi)cellulase production in T. reesei RUT-C30 was not dependent on the carbon sources, though some subtle differences were observed among different carbon sources.
Rapamycin induces transit morphology defect of T. reesei RUT-C30 on cellulose, but not on lactose or glucose
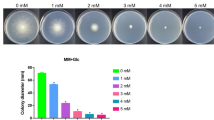
The effect of rapamycin on the morphology of T. reesei RUT-C30 cultivated in TMM + 2% cellulose, 2% lactose or 2% glucose was investigated by confocal laser scanning microscopy (CLSM) (Fig. 2 and Additional file 2: Figure S2). The morphology of T. reesei RUT-C30 was not affected by 0.01 μM rapamycin throughout the whole cellulase production on cellulose. When the concentration of rapamycin was increased to 1 μM and beyond, the abnormal hyphal morphology was observed at 24 h. The filamentation of T. reesei RUT-C30 was strongly inhibited by rapamycin, showing spherical form which was reminiscent of hypha–yeast transition. However, the morphological defects by 1 μM or 10 μM rapamycin were not found at 72 h and later. Even at 100 μM rapamycin, only a few mycelia exhibited aberrant morphology at 72 h and then disappeared after 72 h. All these findings implicated that rapamycin suppresses the hyphal formation in the early stage of T. reesei RUT-C30 grown on cellulose temporarily even at high concentration, which was restored at the late stage. However, when the carbon source was lactose or glucose, there was no notable effect of rapamycin on the mycelium morphology (Additional file 2: Figure S2).
Rapamycin hinders cell growth and sporulation of T. reesei RUT-C30 tentatively, but not the lipid content
The effect of rapamycin on the growth of T. reesei RUT-C30 on cellulose, lactose or glucose was studied (Fig. 3). Due to the interference of insoluble cellulose to the traditional growth assays like the dry biomass measurement and the optical absorbance assay, the DNA content measurement was utilized to characterize the growth of T. reesei on cellulose [7, 34, 37]. As compared to the untreated samples, the cell growth of T. reesei RUT-C30 on cellulose, lactose or glucose at 24 h was retarded notably with the treatment of rapamycin at concentrations no less than 1 μM. This cell growth impairment was rescued completely at later stage under cellulose or glucose condition, but not under lactose condition. Meanwhile, the spore amount of T. reesei RUT-C30 grown on cellulose, lactose or glucose was also reduced at 24 h, which was recovered at 120 h with no significant change even with 100 μM rapamycin (Fig. 3).
The growth (a), sporulation (b) and lipid content (c) of T. reesei RUT-C30 on TMM + 2% cellulose, lactose, or glucose with various concentrations of rapamycin. The lipid content of RUT-C30 at 24 h was stained with Nile Red. Data are represented as the mean of three independent experiments and error bars express the standard. Asterisks indicate significant differences (*p < 0.05, **p < 0.01, ***p < 0.001) as assessed by Student’s t test. Scale bar = 10 μm
It has been reported that rapamycin treatment led to an increase in the number and size of lipid droplets in the fungus S. cerevisiae [38], Podospora anserina [39, 40], and Ustilago maydis [41]. Therefore, to see whether the lipid content of T. reesei RUT-C30 was altered by rapamycin, the lipid content of T. reesei RUT-C30 grown on cellulose, lactose or glucose was stained by Nile Red and checked under CLSM (Fig. 3 and Additional file 3: Figure S3). There was no significant difference in the fluorescent intensity and lipid form between rapamycin-treated RUT-C30 and the untreated one, which was independent of the carbon source, implying that the lipid synthesis in T. reesei RUT-C30 was not affected by rapamycin. Overall, the cell growth and sporulation of T. reesei RUT-C30 were reduced by high-dose rapamycin at the early fermentation stage, but not at the late stage. On the contrary, its lipid content was not influenced by rapamycin. T. reesei RUT-C30 is highly resistant to rapamycin regardless of carbon source.
Transcription pattern of T. reesei RUT-C30 treated with high-dose rapamycin
To gain insight into how rapamycin influences T. reesei RUT-C30 at the transcriptional level, RNA-seq analysis was performed using RUT-C30 cultured in TMM medium with or without 100 μM rapamycin for 24 h. The sequences of the total reads were mapped to the reference genome of T. reesei RUT-C30 (https://www.ncbi.nlm.nih.gov/genome/323%3fgenomeassembly_id%3d49799) with coverage of 97.68–97.76%. A total of 10,048 unique transcripts were detected. Genes were considered to be differentially expressed between the two conditions when the average reads of the corresponding transcripts differed with |log2Ratio|≥ 1 and p value ≤ 0.05. By comparing rapamycin-treated RUT-C30 to the untreated one, 484 differentially expressed genes (DEGs) were obtained, of which 201 were upregulated and 283 were downregulated (Additional file 4: Table S1).
The enriched molecular function was mainly related to “catalytic activity” (Fig. 4a), which comprised 69 DEGs. Among them, 50 DEGs show hydrolase activity, of which 30 act on glycosyl bonds. DEGs in “cellulose binding”, “cellulase activity”, “beta-glucosidase activity” and “xylanase activity” categories were all downregulated, which are related to cellulose and hemicellulose degradation. In addition, the category “ATPase activity, coupled to transmembrane movement of substances” included 8 DEGs. Among them, 6 were predicted to be ABC transporters regarding multidrug resistance that were all upregulated to different degrees (Additional file 5: Table S2). The increased expressions of these ABC transporters in T. reesei might be a defense mechanism against rapamycin by exporting it out of the cells, which might be worth exploring in future study.
Gene ontology (GO) functional enrichment (a–c) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment (d) analysis of DEGs. a The most enriched molecular functions; b the most enriched cellular components; c the most enriched biological processes; d the most enriched pathways. The y-axis represents the name of the most enriched GOs or pathway that belong to different ontologies, while the x-axis represents the number of DEGs in each enriched GO or pathway
For the enriched cellular components, 37 DEGs were under “extracellular region” category, demonstrating that rapamycin greatly impacts the extracellular enzymes (Fig. 4b). Among them, two cellobiohydrolase (CBH) (CEL7A and CEL6A), seven endoglucanase (EG) (CEL7B, CEL5A, CEL12A, CEL61A, CEL45A, CLE74A, and CEL61B), three β-glucosidase (BGL) (CEL3A, CEL3C and CEL3D), and one β-xylosidase (BXL1) were downregulated. The enriched biological processes were mainly cellulose and hemicellulose catabolic processes, of which all DEGs were downregulated (Fig. 4c). These results were consistent with the remarkably decreased pNPCase, CMCase, pNPGase and pNPXase activities in RUT-C30 treated with 100 μM rapamycin for 24 h, respectively. In addition, the most enriched pathways affected by rapamycin included “Biosynthesis of secondary metabolites”, “Biosynthesis of antibiotic”, “Starch and sucrose metabolism”, “Valine, leucine and isoleucine degradation”, and “Fructose and mannose metabolism” (Fig. 4d).
DEGs involved in the cellulase production were downregulated by rapamycin
Thirty-nine DEGs were related to cellulose degradation, of which 37 were downregulated and only two were upregulated (Fig. 5 and Additional file 6: Table S3). Fifteen cellulases including two cellobiohydrolases (CEL7A and CEL6A), seven endoglucanases except CEL5B, and six β-glucosidases (CEL3A, CEL1A, CEL1B, CEL3C, CEL3H and CEL3D) were notably down-expressed, which agreed with the reduced pNPCase, CMCase, pNPGase and total filter paper FPase activities as found above. The downregulated mRNA levels of BXL1 and xylanases (XYN2, XYN3, XYN4, and XYN6) were in line with decreased pNPXase activity. Moreover, nonenzymatic cellulose attacking enzymes swollenin [42], Cip1 and Cip2 [43], which acted in synergy with cellulases and hemicellulases to enhance the hydrolytic efficiency of cellulose were also downregulated significantly. Unexpectedly, the hemicellulase α-galactosidase AGL1 and β-mannosidase (M419DRAFT_93487), which hydrolyses α-D-galactosides and mannans, were upregulated, though another α-galactosidase (M419DRAFT_71638) and two other β-mannosidases (M419DRAFT_67432 and M419DRAFT_122377) were downregulated noticeably. Two lytic polysaccharide monooxygenases (LPMOs), CEL61A (M419DRAFT_139633) and CEL61B (M419DRAFT_122518) were significantly downregulated by 21.95 (3.86)- and 22.79 (6.92)-fold, respectively (Fig. 5). In carbohydrate-active enzymes (CAZymes) database, proteins CEL61A and CEL61B were originally classified as glycoside hydrolase family 61 (GH61), but reclassified as auxiliary activity 9 (AA9) and auxiliary activity 10 (AA10), respectively [44,45,46]. They have both endoglucanase and LPMO activities [44, 47, 48].
In addition, 7 transcriptional factors involved in cellulase production were identified to be DEGs with marked downregulation (Fig. 5d). They were cellulase transcription activators Xyr1 [49], Ace3 [50] and Clr2 [51], MFS sugar transporters Crt1 and Stp1 [52, 53], xylanase promoter binding protein Xpp1 [54], and the carbon catabolite repressor Cre4 [55]. Xpp1 was firstly described as a repressor of xylanases [56] and later as a repressor of secondary metabolism [54]. The downregulation of Xpp1 did not lead to the increase of xylanase production (Fig. 1), but the expression of 11 DEGs involved in KEGG category “Biosynthesis of secondary metabolites” was found to be increased significantly (Fig. 4d and Additional file 7: Table S4), in agreement with the role of Xpp1 as a repressor of secondary metabolism.
FKBP12 is required for the temporary inhibition of rapamycin on cellulase production
Inside the cell, rapamycin first forms a gain-of-function complex with FKBP12, the well-known rapamycin cellular receptor [14, 19, 20], which then inhibits the TOR kinases. Currently, FKBP12 has been well studied in both S. cerevisiae [22, 57, 58] and human cell [59,60,61]. It is supposed that T. reesei (filamentous fungus) should be more similar to S. cerevisiae (a model fungus) than human cells. Therefore, we searched T. reesei RUT-C30 genome for the FKBP12 homolog of S. cerevisiae, leading to the identification of trFKBP12 (M419DRAFT_72966) hat has already been annotated as FKBP12 in T. reesei RUT-C30 genome database published in NCBI or Emsembl Fungi database. Protein trFKBP12 contains 119 amino acids with a predicted molecular mass of 13.3 kDa. Interestingly, the transcription level of trFKBP12 was significantly upregulated by 21.71 (3.27)-fold in T. reesei RUT-C30 treated with rapamycin (Table 1), indicating that trFKBP12 is probably the cellular receptor of rapamycin.
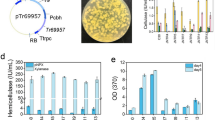
To determine whether trFKBP12 is the cellular receptor of rapamycin in T. reesei, gene trFKBP12 was knockout using T. reesei KU70 as the parent strain [62], obtaining mutant strain ΔtrFKBP12. KU70 was chosen as a host strain for its high efficiency of gene targeting, where ku70 was deleted in RUT-C30 [63]. Similar to strain RUT-C30, the inhibition effect of 100 μM rapamycin on cellulase production was observed in strain KU70 at the early fermentation stage (Fig. 6a–c), which was completely relieved at the late stage (Fig. 6d). This inhibition effect was not found in strain ΔtrFKBP12 during the whole fermentation process (Fig. 6), demonstrating that trFKBP12 was indispensable for the temporary inhibition effect of rapamycin on cellulase production in T. reesei at the early cultivation stage. It is worth noting that knockout of trFKBP12 alone led to a delay in cellulose production, similar to that caused by high-dose rapamycin. No morphology change was found in strain KU70 or ΔtrFKBP12 with the treatment of 100 μM rapamycin (Additional file 8: Figure S4).
The (hemi)cellulase activities and protein secretion of T. reesei KU70 and ΔtrFKBP12 cultured in TMM + 2% cellulose with/ without 100 μM rapamycin at 24 h (a), 72 h (b), 120 h (c) and 168 h (d), respectively. FPase: the filter paper activity; pNPCase: the CBH activity; CMCase: the CMC activity; pNPGase: the β-glucosidase activity; pNPXase: the β-xylosidase activity; Protein: secreted protein concentration. Data are represented as the mean of three independent experiments and error bars express the standard. Asterisks indicate significant differences (*p < 0.05, **p < 0.01, ***p < 0.001) as assessed by Student’s t test
DEGs involved in the TOR pathway
The TOR kinase is the target of FKBP–rapamycin complex and can interact with multiple proteins to form two complexes TORC1 and TORC2. Both TORC1 and TORC2 play important roles in cell growth and metabolism, but only TORC1 was rapamycin-sensitive [14, 64]. In silico analysis revealed there is only one TOR (M419DRAFT_24714) in T. reesei, with 49.27% and 49.77% sequence identity to TOR1 and TOR2 from S. cerevisiae, respectively, which was coined trTOR here. The expression of gene trTOR was slightly downregulated by rapamycin (Table 1). The essential components of TORC1 (Lst8 and Kog1), and TORC2 (Avo1, Avo3, and Lst8) were identified in T. reesei, with little change at transcription levels (Table 1). The other components of the TOR complexes (Tco89, Avo2, and Bit61) were not identified in T. reesei. It seems that the effect of rapamycin on the TOR complexes was not very significant in T. reesei. The effort to delete gene trTOR was failed, indicating that trTOR is an essential gene.
Moreover, based on sequence comparisons with the homologues of genes in the TOR signal pathways [14, 65, 66], we identified a series of genes involved in TOR signal pathways in T. reesei RUT-C30, including 15 genes in ribosome biogenesis, 8 genes in cell cycle/growth, 25 genes in nutrient uptake, 1 gene in stress, 5 genes in lipid metabolism, 6 genes in cell wall integrity, and 5 genes in autophagy (Additional file 9: Table S5). All these genes were not differentially expressed. These findings matched well with the phenotype profiling results that T. reesei RUT-C30 displayed high resistance to rapamycin regardless of carbon sources (Figs. 1, 2, and 3).
DEGs related to gene expression
Thirty-five out of the total 484 DEGs are related to the gene expression in T. reesei, showing that rapamycin has prominent effects on both the transcription and translation level of T. reesei RUT-C30 grown on cellulose (Additional file 10: Table S6). DEGs related to DNA metabolism were found, like DNA topoisomerase IV alpha subunit, and DNase I protein. In particular, 14 DEGs are specifically involved in regulation of transcription by RNA polymerase II that is responsible for the transcription of cellulase production [67]. On the other hand, three tRNA were downregulated, including tRNA-Arg, tRNA-Glu and tRNA-Lys, while threonyl/alanyl tRNA synthetase and eukaryotic translation initiation factor 2c were upregulated. In addition, two DEGs function as ribonuclease for RNA degradation. Therefore, 100 μM rapamycin exerted an effect on both the translation and transcription of T. reesei RUT-C30 at the early fermentation stage on cellulose.
DEGs associated with transporters
Forty-seven out of the total 484 DEGs are transporters (Additional file 5: Table S2), including 1 putative efflux pump antibiotic resistance protein, 1 xenobiotic transmembrane transporter, 3 amino acid transporters, 3 aquaporins, 8 ATPase-coupled transmembrane transporters, 10 carboxylate transporters, 11 major facilitator superfamily transporters, 3 transporters for transporting inorganic compounds (1 phosphate permease, 1 acetate transporter, and 1 ammonium permease), 4 transporters for transporting metal ions (1 metal ion transmembrane transporter, 1 siderophore transporter, 1 calcium transmembrane transporter, and 1 zinc ion transmembrane transporter), and 3 other transporters. All the four metal ion transporters were downregulated, while the three amino acid transporters were upregulated.
Discussion
The cellulase production, cell growth, sporulation ability and lipid content of T. reesei RUT-C30 grown on cellulose, lactose or glucose, were not affected by rapamycin even at the high concentration of 100 μM which was much higher than the concentrations inhibiting other organisms. For example, 0.11 μM rapamycin were enough to activate TORC1-controlled transcriptional activators Gln3p, Gat1p, Rtg1p, and Rtg3p and resulted in a fast lipid droplet replenishment in S. cerevisiae [38]. Rapamycin at 1.09 μM completely abolished the growth of P. anserina [40] and at 0.27 μM induced autophagy in hyphae of F. graminearum [21]. Obviously, T. reesei RUT-C30 is highly resistant to rapamycin, which is independent of carbon sources. The resistance to rapamycin were also observed in S. pombe [68], and land plants like Arabidopsis thaliana, the bryophyte Physcomitrella patens, the monocot Oryza sativa, and the dicots Nicotiana tabacum and Brassica napus [16], though rapamycin susceptibility is widespread among eukaryotes, inhibiting the growth of most fungi and animal cells [38, 69]. The reason why T. reesei RUT-C30 is insensitive to rapamycin is still unknown.
It has been reported that mutations of FKBP12 prevent the formation of FKBP–rapamycin complex, and mutations in the FRB (FKPB12–rapamycin-binding) domain of TOR1 block the binding of FKBP–rapamycin to TOR1, both conferring rapamycin resistance. Therefore, we performed sequence alignments of rapamycin-binding domains of trTOR and trFKBP12 with those of the corresponding homologs from organisms that are sensitive (S. cerevisiae and Homo sapiens) or insensitive (A. thaliana, S. pombe, and O. sativa) to rapamycin (Additional file 11: Figure S5). Most of the amino acids required for the formation of hydrophobic rapamycin-binding pocket [70] are well conserved in trFKBP12, including Tyr31, Phe48, Ile68, Trp71, Tyr94, Ile103 and Phe111 (numbered according to trFKBP12) (Additional file 11: Figure S5A). Similarly, the amino acid residue Trp2101, Phe2108, or Ser2035 of mTOR (mammalian target of rapamycin), mutation of which confers rapamycin resistance [71], is also present in trTOR (Additional file 11: Figure S5B). These findings demonstrate that the high resistance of T. reesei to rapamycin was not due to mutations of trFKBP12 and trTOR on comparison to counterparts from organisms that are susceptible to rapamycin.
The temporary inhibition effect of rapamycin on T. reesei RUT-C30 might due to the degradation of rapamycin either chemically or by RUT-C30 during the culture process. Currently, it is unknown how stable rapamycin is under our cultivation conditions, which is worthy of further study. As far as we know, no rapamycin degradation pathway in T. reesei has been reported. The hyphal growth of Verticillium dahliae hypersensitive to rapamycin was severely inhibited in the presence of 0.2 μM rapamycin even after 11 days’ cultivation [72]. Moreover, when firstly cultured in TMM medium for 96 h and treated with different concentrations of rapamycin for 24 h, the (hemi)cellulase activities and protein secretion of T. reesei RUT-C30 were not affected by rapamycin (Additional file 12: Figure S6). It seems that the temporary inhibition effect on RUT-C30 by rapamycin only occurs at the early fermentation stage. In addition, rapamycin can bind to ABC transporters with high affinity, acting as a substrate for transport by ABC transporters out of cells and causing rapamycin resistance [73]. Rapamycin biosynthetic gene cluster in rapamycin-producing strains S. hygroscopicus and Streptomyces rapamycinicus contains putative ABC transporter genes (OrfX and rapX) to prevent self-poisoning by exporting rapamycin from inside cells [74, 75]. In T. reesei RUT-C30, 8 ABC transporters were significantly changed at mRNA level (Additional file 5: Table S2), of which 6 transporters related to multidrug resistance were upregulated markedly, including M419DRAFT_75459, M419DRAFT_90404, M419DRAFT_109180, M419DRAFT_116127, M419DRAFT_97054 and M419DRAFT_114328 (Additional file 5: Table S2). The noticeable increase of these ABC transporters might contribute to the recovery of enzymatic activities at later fermentation process in T. reesei RUT-C30 and its rapamycin resistance. Taken together, we believe that the temporary inhibition effect of rapamycin on T. reesei RUT-C30 at the early fermentation stage should not be due to rapamycin degradation during cultivation.
T. reesei RUT-C30 might represent an excellent platform to study the resistance of cells to rapamycin which is commonly countered when rapamycin is explored as antifungal or anticancer agents. mTOR has become a focus for cancer drug development [76]. Rapamycin is a highly specific inhibitor of mTOR and potently suppresses tumor cell growth by retarding cells in G1 phase or potentially inducing apoptosis. Currently, rapamycin and its analogues are being evaluated as anticancer agents in clinical trials. However, many human cancers have displayed intrinsic resistance or acquired resistance to rapamycin [77]. Though several predicted mechanisms behind the resistance of cancer cells to rapamycin have been proposed, the detailed mechanisms remain to be explored. The high rapamycin resistance of T. reesei RUT-C30 might endow it as a model for the eukaryotic cell to unravel mechanism of rapamycin resistance.
The expression level of gene trFKBP12 was remarkably increased in the presence of high-concentration rapamycin. In Fusarium fujikuroi and Botrytis cinerea, rapamycin treatment also led to an increased transcription level of FKBP12-encoding gene fpr1 [78, 79]. Knockout of gene trFKBP12 caused a transient inhibition effect on cellulase production at the early fermentation stage (Fig. 6), as compared to the parent strain KU70. Furthermore, trFKBP12 knockout completely abolished the inhibition effect of high-dose rapamycin on cellulase production in T. reesei, as indicated by the unchanged cellulase production in strain ΔtrFKBP12 treated with high-dose rapamycin. These findings imply that the temporary inhibition of rapamycin on cellulase requires trFKBP12, and free trFKBP12 might play a role in the initial production of cellulase. Both the deletion of trFKBP12 and the addition of rapamycin can result in a reduced level of free trFKBP12. Rapamycin can bind trFKBP12 to form the rapamycin–trFKBP12 complex, leading to the reduction of free trFKBP12. In contrast to the profound upregulation of trFKBP12, the expression of trTOR was only slightly downregulated and genes involved in the TOR signaling pathways were not changed markedly, which might be associated with the high rapamycin resistance in T. reesei. FKBP12 mediated rapamycin sensitivity via TOR in most eukaryote cells [80,81,82].
Despite that T. reesei exhibited high resistance to rapamycin, its cellulase production, cell growth and sporulation were inhibited by high concentration of rapamycin at the early fermentation stage, which was independent of carbon sources and was relieved at the late stage. In addition, the presence of high-concentration rapamycin induced morphology defect in strain RUT-C30 at the early cultivation on cellulose. However, this morphology defect was not found in RUT-C30 grown on glucose or lactose, and strain KU70 or ΔtrFKBP12 propagated on cellulose, indicating that it is a very specific phenotype for RUT-C30 cultured on cellulose. Similar hyphal morphology alterations with the treatment of rapamycin were previously reported in other filamentous fungi like P. anserina [40] and U. maydis [83].
Conclusion
In this study, we have investigated the effect of rapamycin on cellulase production, filamentous morphology, cell growth, sporulation, and lipid content of T. reesei RUT-C30 grown on different carbon sources (cellulose, lactose and glucose). The high-dose rapamycin (100 μM) induced a delay in cellulase production, cell growth and sporulation regardless of the carbon sources and specifically caused morphology defect in RUT-C30 grown on cellulose, both of which were transient and restored at the late fermentation stage. In contrast, the lipid content of T. reesei RUT-C30 cultivated on cellulose, lactose or glucose was not changed by rapamycin. These findings implicate that T. reesei RUT-C30 is highly resistant to rapamycin. In line with the phenotype profiling results, transcriptomic analysis found that the mRNA levels of genes associated with the cellulase production were decreased severely by rapamycin. Furthermore, the transcriptional level of the putative rapamycin receptor trFKBP12 was increased significantly, while those of gene trTOR (the downstream effector of the rapamycin–FKBP12 complex) and genes associated with the TOR signaling pathways were not changed markedly, matching well with the super rapamycin resistance of T. reesei we observed. Upon the deletion of gene trFKBP12, there is no influence of rapamycin on cellulase production, indicating the transient inhibition of high-dose rapamycin on cellulase production is assisted by trFKBP12. Overall, we first discovered that T. reesei is highly resistant to rapamycin, probably owing to that trTOR and its relevant signaling pathways were not very sensitive to rapamycin. These results deepen our understanding of the impact of rapamycin and the role of the TOR signaling pathways in T. reesei, a cellulose-producing workhouse in industry.
Methods
Microbial strains, plasmids and cultivation conditions
Escherichia coli DH5α was explored for propagation and construction of plasmids. Agrobacterium tumefaciens AGL-1 was used as a T-DNA donor for fungal transformation. T. reesei RUT-C30 (CICC 13052, ATCC 56765) was purchased from China Center of Industrial Culture Collection. T. reesei KU70, derived from T. reesei RUT-C30 by deleting gene ku70, was provided friendly by Professor Wei Wang from East China University of Science and Technology [84]. Plasmid pXBthg was a gift from Professor Zhihua Zhou from Key Laboratory of Synthetic Biology, Shanghai [85]. E. coli DH5α and A. tumefaciens AGL-1 were cultivated in Luria–Bertani (LB) with 220 rpm at 37 °C and 28 °C, respectively. T. reesei were grown on potato dextrose agar (PDA) plates at 28 °C for conidia production and in Trichoderma minimal media (TMM) [86] with 2% (w/t) cellulose, lactose or glucose at 28 °C with 220 rpm. TMM was a well-used medium for T. reesei cultivation for cellulase production [8, 34, 35, 37, 86, 87]. Cellulose and lactose are insoluble and soluble inducers for cellulase production, respectively, while glucose represses cellulase production. All chemicals used in this research were ordered from Sigma-Aldrich, USA.
Shake-flask cultivation
Five percent (v/v) 107/mL conidia of T. reesei RUT-C30 were inoculated into 10 mL (Sabouraud dextrose broth) SDB and incubated at 28 °C with 220 rpm for 2 days. Ten percent (v/v) pre-grown mycelia were inoculated into 50 mL TMM media (pH 6) with different concentrations of rapamycin as indicated in the text using 2% cellulose, lactose, or glucose as the carbon source, and then incubated at 28 °C with 200 rpm for 7 days. The stock solution of rapamycin (50 mM) was prepared in DMSO with sonication. Samples were taken at varied time points as indicated in the context for (hemi)cellulase activity assay, confocal observation of fungal mycelia, biomass measurement, Nile Red staining and RNA-seq analysis. If necessary, samples were centrifuged at 8000 rpm for 30 min to separate the mycelia and the supernatant.
Analysis method
(Hemi)cellulase activities, confocal observation of fungal mycelia, DNA content measurement, sporulation assay and RNA-seq analysis were performed as described in our previous research [34, 37, 87]. The growth of strain T. reesei grown in TMM + 2% cellulose with and without rapamycin was assayed by DNA content measurement, while by measuring the optical density of the culture suspension at 600 nm (OD 600 nm) with a UV–vis spectrophotometer (UV-2600, Shimadzu, Japan) when cultured in TMM + 2% lactose or TMM + 2% glucose.
Lipid content analysis by Nile Red staining
For Nile Red staining, fresh mycelia collected as above were washed with PBS for two times, and incubated with Nile Red solution (25 ng/mL) at 28 °C for 10 min. Then, the samples were washed with PBS for two times, and checked under a confocal microscope (TCS SP8, Leica, Germany) with the excitation wavelength of 510–560 nm and the emission wavelength of 600–680 nm.
Deletion of gene trFKBP12 in T. reesei KU70
The upstream and downstream sequences (~ 1500 bp) of gene trFKBP-12 were amplified separately by PCR using genomic DNA of T. reesei KU70 as a template, and cloned into plasmid pXBthg at XhoI and at BamHI, respectively, using ClonExpress™ II One Step Cloning Kit (Vazyme, China), leading to plasmid pXBthg-FKBP. The resulting plasmid pXBthg-FKBP was transformed into T. reesei KU70 by the Agrobacterium tumefaciens-mediated transformation (AMT) method [88]. Sixty hygromycin-resistant transformants per 107 conidia were obtained on PDA medium with 50 μg/mL hygromycin, of which 3 transformants were selected randomly for three consecutive subcultures to achieve genetic stability. Then genome DNA of these three transformants was extracted and used as template for PCR verification. The PCR verification was performed using 2*Phanta Max Master Mix (Vazyme, China) with the following PCR reaction conditions: 95 °C for 3 min; 35 cycle of 95 °C for 15 s, 67 °C for 15 s and 72 °C for 210 s; 72 °C for 5 min. All three transformants were proved to be trFKBP12-deleted (Additional file 13: Figure S7), of which one was further confirmed by sequencing at Sangon Biotech and named as strain ΔtrFKBP12. The primers used are listed in Additional file 14: Table S7.
Availability of data and materials
The datasets supporting the conclusions of this article are included in the article and its Additional files.
Abbreviations
- TOR:
-
Target of rapamycin
- BGL:
-
β-Glucosidase
- EG:
-
Endoglucanase
- CBH:
-
Cellobiohydrolase
- GH:
-
Glycoside hydrolase
- pNPGase:
-
The β-glucosidase activity
- pNPCase:
-
The CBH activity
- CMCase:
-
The CMC activity
- FPase:
-
The filter paper activity
- pNPXase:
-
The β-xylosidase activity
- AMT:
-
Agrobacterium-Mediated fungal transformation
- SDB:
-
Sabouraud dextrose broth
- TMM:
-
Trichoderma minimal medium
- PDA:
-
Potato dextrose agar
References
Tian CG, Beeson WT, Iavarone AT, Sun JP, Marletta MA, Cate JHD, et al. Systems analysis of plant cell wall degradation by the model filamentous fungus Neurospora crassa. Proc Natl Acad Sci USA. 2009;106(52):22157–62.
Martinez D, Berka RM, Henrissat B, Saloheimo M, Arvas M, Baker SE, et al. Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn. Hypocrea jecorina). Nat Biotechnol. 2008;26(5):553–60.
Schmoll M. Regulation of plant cell wall degradation by light in Trichoderma. Fungal Biol Biotechnol. 2018;5(1):10.
Beier S, Hinterdobler W, Bazafkan H, Schillinger L, Schmoll M. CLR1 and CLR2 are light dependent regulators of xylanase and pectinase genes in Trichoderma reesei. Fungal Genet Biol. 2020;136:10.
Chen Y, Wu C, Shen Y, Ma Y, Wei D, Wang W. N, N-dimethylformamide induces cellulase production in the filamentous fungus Trichoderma reesei. Biotechnol Biofuels. 2019;12:36.
Chen Y, Shen Y, Wang W, Wei D. Mn2+ modulates the expression of cellulase genes in Trichoderma reesei Rut-C30 via calcium signaling. Biotechnol Biofuels. 2018;11(1):54.
Wang MY, Zhao QS, Yang JH, Jiang BJ, Wang FZ, Liu KM, et al. A mitogen-activated protein kinase Tmk3 participates in high osmolarity resistance, cell wall integrity maintenance and cellulase production regulation in Trichoderma reesei. PLoS ONE. 2013;8(8):12.
Chen L, Zou G, Wang J, Wang J, Liu R, Jiang Y, et al. Characterization of the Ca2+-responsive signaling pathway in regulating the expression and secretion of cellulases in Trichoderma reesei Rut-C30. Mol Microbiol. 2016;100(3):560–75.
Kim J, Guan KL. mTOR as a central hub of nutrient signalling and cell growth. Nat Cell Biol. 2019;21(1):63–71.
Lin L, Wang S, Li X, He Q, Benz JP, Tian C. STK-12 acts as a transcriptional brake to control the expression of cellulase-encoding genes in Neurospora crassa. PLoS Genet. 2019;15(11):e1008510.
Brown NA, de Gouvea PF, Krohn NG, Savoldi M, Goldman GH. Functional characterisation of the non-essential protein kinases and phosphatases regulating Aspergillus nidulans hydrolytic enzyme production. Biotechnol Biofuels. 2013;6(1):91.
Lv XX, Zhang WX, Chen GJ, Liu WF. Trichoderma reesei Sch9 and Yak1 regulate vegetative growth, conidiation, and stress response and induced cellulose production. J Microbiol. 2015;53(4):236–42.
Reidman S, Cohen A, Kupiec M, Weisman R. The cytosolic form of aspartate aminotransferase is required for full activation of TOR complex 1 in fission yeast. J Biol Chem. 2019;294(48):18244–55.
Loewith R, Hall M. Target of Rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189:1177–201.
Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–93.
Menand B, Desnos T, Nussaume L, Berger F, Bouchez D, Meyer C, et al. Expression and disruption of the Arabidopsis TOR (target of rapamycin) gene. Proc Natl Acad Sci USA. 2002;99(9):6422–7.
Dobrenel T, Caldana C, Hanson J, Robaglia C, Vincentz M, Veit B, et al. TOR signaling and nutrient sensing. Annu Rev Plant Physiol. 2016;67:261–85.
Sehgal SN, Baker H, Vezina C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot. 1975;28(10):727–32.
Heitman J, Movva NR, Hall MN. Targets for cell-cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253(5022):905–9.
Raught B, Gingras A-C, Sonenberg N. The target of rapamycin (TOR) proteins. Proc Natl Acad Sci USA. 2001;98(13):7037.
Yu F, Gu Q, Yun Y, Yin Y, Xu JR, Shim WB, et al. The TOR signaling pathway regulates vegetative development and virulence in Fusarium graminearum. N Phytol. 2014;203(1):219–32.
Koltin Y, Faucette L, Bergsma DJ, Levy MA, Cafferkey R, Koser PL, et al. Rapamycin sensitivity in Saccharomyces cerevisiae is mediated by a peptidyl-prolyl cis-trans isomerase related to human FK506-binding protein. Mol Cell Biol. 1991;11(3):1718.
Fu LW, Wang PC, Xiong Y. Target of Rapamycin signaling in plant stress responses. Plant Physiol. 2020;182(4):1613–23.
Li XJ, Cai WG, Liu YL, Li H, Fu LW, Liu ZY, et al. Differential TOR activation and cell proliferation in Arabidopsis root and shoot apexes. Proc Natl Acad Sci USA. 2017;114(10):2765–70.
Yuan X, Xu P, Yu Y, Xiong Y. Glucose-TOR signaling regulates PIN2 stability to orchestrate auxin gradient and cell expansion in Arabidopsis root. Proc Natl Acad Sci USA. 2020;117(51):32223.
Juvvadi PR, Fox D, Bobay BG, Hoy MJ, Gobeil SMC, Venters RA, et al. Harnessing calcineurin-FK506-FKBP12 crystal structures from invasive fungal pathogens to develop antifungal agents. Nat Commun. 2019;10:18.
McCready K, Spencer V, Kim M. The importance of TOR kinase in plant development. Front Plant Sci. 2020;11:7.
Liu T, Xiong J, Yi S, Zhang H, Zhou S, Gu L, et al. FKBP12 enhances sensitivity to chemotherapy-induced cancer cell apoptosis by inhibiting MDM2. Oncogene. 2017;36(12):1678–86.
Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–76.
Bischof RH, Ramoni J, Seiboth B. Cellulases and beyond: the first 70 years of the enzyme producer Trichoderma reesei. Microb Cell Fact. 2016;15(1):106.
Gupta VK, Kubicek CP, Berrin J-G, Wilson DW, Couturier M, Berlin A, et al. Fungal enzymes for bio-products from sustainable and waste biomass. Trends Biochem Sci. 2016;41(7):633–45.
Gupta VK, Steindorff AS, de Paula RG, Silva-Rocha R, Mach-Aigner AR, Mach RL, et al. The post-genomic era of Trichoderma reesei: what’s next? Trends Biotechnol. 2016;34(12):970–82.
Aro N, Pakula T, Penttila M. Transcriptional regulation of plant cell wall degradation by filamentous fungi. Fems Microbiol Rev. 2005;29(4):719–39.
Li C, Lin F, Zhou L, Qin L, Li B, Zhou Z, et al. Cellulase hyper-production by Trichoderma reesei mutant SEU-7 on lactose. Biotechnol Biofuels. 2017;10:228.
Li C, Lin F, Li Y, Wei W, Wang H, Qin L, et al. A β-glucosidase hyper-production Trichoderma reesei mutant reveals a potential role of cel3D in cellulase production. Microb Cell Fact. 2016;15(1):151.
Meng QS, Liu CG, Zhao XQ, Bai FW. Engineering Trichoderma reesei Rut-C30 with the overexpression of egl1 at the ace1 locus to relieve repression on cellulase production and to adjust the ratio of cellulolytic enzymes for more efficient hydrolysis of lignocellulosic biomass. J Biotechnol. 2018;285:56–63.
Li C, Lin F, Sun W, Yuan S, Zhou Z, Wu FG, et al. Constitutive hyperproduction of sorbicillinoids in Trichoderma reesei ZC121. Biotechnol Biofuels. 2018;11:291.
Madeira JB, Masuda CA, Maya-Monteiro CM, Matos GS, Montero-Lomeli M, Bozaquel-Morais BL. TORC1 inhibition induces lipid droplet replenishment in yeast. Mol Cell Biol. 2015;35(4):737–46.
Pinan-Lucarré B, Iraqui I, Clavé C. Podospora anserina target of rapamycin. Curr Genet. 2006;50(1):23–31.
Dementhon K, Paoletti M, Pinan-Lucarré B, Loubradou-Bourges N, Sabourin M, Saupe SJ, et al. Rapamycin mimics the incompatibility reaction in the fungus Podospora anserina. Eukaryot Cell. 2003;2(2):238–46.
Romero-Aguilar L, Guerra-Sánchez G, Tenorio EP, Tapia-Rodriguez M, Matus-Ortega G, Flores-Herrera O, et al. Rapamycin induces morphological and physiological changes without increase in lipid content in Ustilago maydis. Arch Microbiol. 2020;202(3):1211–21.
Saloheimo M, Paloheimo M, Hakola S, Pere J, Swanson B, Nyyssonen E, et al. Swollenin, a Trichoderma reesei protein with sequence similarity to the plant expansins, exhibits disruption activity on cellulosic materials. Eur J Biochem. 2002;269(17):4202–11.
Kuhad RC, Deswal D, Sharma S, Bhattacharya A, Jain KK, Kaur A, et al. Revisiting cellulase production and redefining current strategies based on major challenges. Renew Sustain Energy Rev. 2016;55:249–72.
Karkehabadi S, Hansson H, Kim S, Piens K, Mitchinson C, Sandgren M. The first structure of a glycoside hydrolase family 61 member, Cel61B from Hypocrea jecorina, at 1.6 A resolution. J Mol Biol. 2008;383(1):144–54.
Hemsworth GR, Davies GJ, Walton PH. Recent insights into copper-containing lytic polysaccharide mono-oxygenases. Curr Opin Struct Biol. 2013;23(5):660–8.
Arntzen M, Bengtsson O, Várnai A, Delogu F, Mathiesen G, Eijsink VGH. Quantitative comparison of the biomass-degrading enzyme repertoires of five filamentous fungi. Sci Rep. 2020;10(1):20267.
Karlsson J, Saloheimo M, Siika-Aho M, Tenkanen M, Penttilä M, Tjerneld F. Homologous expression and characterization of Cel61A (EG IV) of Trichoderma reesei. Eur J Biochem. 2001;268(24):6498–507.
Tanghe M, Danneels B, Camattari A, Glieder A, Vandenberghe I, Devreese B, et al. Recombinant expression of Trichoderma reesei Cel61A in Pichia pastoris: optimizing yield and N-terminal processing. Mol Biotechnol. 2015;57(11–12):1010–7.
Mello-de-Sousa TM, Rassinger A, Derntl C, Pocas-Fonseca MJ, Mach RL, Mach-Aigner AR. The relation between promoter chromatin status, Xyr1 and cellulase expression in Trichoderma reesei. Curr Genomics. 2016;17(2):145–52.
Häkkinen M, Valkonen MJ, Westerholm-Parvinen A, Aro N, Arvas M, Vitikainen M, et al. Screening of candidate regulators for cellulase and hemicellulase production in Trichoderma reesei and identification of a factor essential for cellulase production. Biotechnol Biofuels. 2014;7(1):14.
Coradetti ST, Craig JP, Xiong Y, Shock T, Tian C, Glass NL. Conserved and essential transcription factors for cellulase gene expression in ascomycete fungi. Proc Natl Acad Sci USA. 2012;109(19):7397–402.
Zhang W, Kou Y, Xu J, Cao Y, Zhao G, Shao J, et al. Two major facilitator superfamily sugar transporters from Trichoderma reesei and their roles in induction of cellulase biosynthesis. J Biol Chem. 2013;288(46):32861–72.
Novy V, Nielsen F, Seiboth B, Nidetzky B. The influence of feedstock characteristics on enzyme production in Trichoderma reesei: a review on productivity, gene regulation and secretion profiles. Biotechnol Biofuels. 2019;12(1):1–16.
Derntl C, Kluger B, Bueschl C, Schuhmacher R, Mach RL, Mach-Aigner AR. Transcription factor Xpp1 is a switch between primary and secondary fungal metabolism. Proc Natl Acad Sci USA. 2017;114(4):E560–9.
Kubicek CP, Mikus M, Schuster A, Schmoll M, Seiboth B. Metabolic engineering strategies for the improvement of cellulase production by Hypocrea jecorina. Biotechnol Biofuels. 2009;2:19.
Derntl C, Rassinger A, Srebotnik E, Mach RL, Mach-Aigner AR. Xpp1 regulates the expression of xylanases, but not of cellulases in Trichoderma reesei. Biotechnol Biofuels. 2015;8(1):112.
Sormani R, Yao L, Menand B, Ennar N, Lecampion C, Meyer C, et al. Saccharomyces cerevisiae FKBP12 binds Arabidopsis thaliana TOR and its expression in plants leads to rapamycin susceptibility. BMC Plant Biol. 2007;7:1–8.
Kasahara K. Physiological function of FKBP12, a primary target of rapamycin/FK506: a newly identified role in transcription of ribosomal protein genes in yeast. Curr Genet. 2021. https://doi.org/10.1007/s00294-020-01142-3.
Caraveo G, Soste M, Cappelleti V, Fanning S, van Rossum DB, Whitesell L, et al. FKBP12 contributes to α-synuclein toxicity by regulating the calcineurin-dependent phosphoproteome. Proc Natl Acad Sci USA. 2017;114(52):E11313.
Sanchez-Solano A, Corral N, Segura-Covarrubias G, Guzman-Hernandez ML, Arechiga-Figueroa I, Cruz-Rangel S, et al. Regulation of the Ca2+-activated chloride channel Anoctamin-1 (TMEM16A) by Ca2+-induced interaction with FKBP12 and calcineurin. Cell Calcium. 2020;89:10.
Peiffer BJ, Qi L, Ahmadi AR, Wang Y, Guo Z, Peng H, et al. Activation of BMP signaling by FKBP12 ligands synergizes with inhibition of CXCR4 to accelerate wound healing. Cell Chem Biol. 2019;26(5):652–61.
Zhang L, Zhao X, Zhang G, Zhang J, Wang X, Zhang S, et al. Light-inducible genetic engineering and control of non-homologous end-joining in industrial eukaryotic microorganisms: LML 3.0 and OFN 1.0. Sci Rep. 2016;6:20761.
Guangtao Z, Hartl L, Schuster A, Polak S, Schmoll M, Wang T, et al. Gene targeting in a nonhomologous end joining deficient Hypocrea jecorina. J Biotechnol. 2009;139(2):146–51.
Zheng XF, Florentino D, Chen J, Crabtree GR, Schreiber SL. TOR kinase domains are required for two distinct functions, only one of which is inhibited by rapamycin. Cell. 1995;82(1):121–30.
Conrad M, Schothorst J, Kankipati HN, Van Zeebroeck G, Rubio-Texeira M, Thevelein JM. Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev. 2014;38(2):254–99.
Bracharz F, Redai V, Bach K, Qoura F, Brück T. The effects of TORC signal interference on lipogenesis in the oleaginous yeast Trichosporon oleaginosus. BMC Biotechnol. 2017;17(1):27.
Zheng F, Cao Y, Yang R, Wang L, Lv X, Zhang W, et al. Trichoderma reesei XYR1 activates cellulase gene expression via interaction with the Mediator subunit TrGAL11 to recruit RNA polymerase II. PLoS Genet. 2020;16(9):e1008979.
Weisman R, Choder M, Koltin Y. Rapamycin specifically interferes with the developmental response of fission yeast to starvation. J Bacteriol. 1997;179(20):6325–34.
Paghdal KV, Schwartz RA. Sirolimus (rapamycin): From the soil of Easter Island to a bright future. J Am Acad Dermatol. 2007;57(6):1046–50.
Cruz MC, Cavallo LM, Gorlach JM, Cox G, Perfect JR, Cardenas ME, et al. Rapamycin antifungal action is mediated via conserved complexes with FKBP12 and TOR kinase homologs in Cryptococcus neoformans. Mol Cell Biol. 1999;19(6):4101–12.
Weisman R, Choder M. The fission yeast TOR homolog, tor1+, is required for the response to starvation and other stresses via a conserved serine. J Biol Chem. 2001;276(10):7027–32.
Li LX, Zhu TT, Song Y, Luo XM, Feng L, Zhuo FP, et al. Functional characterization of Target of Rapamycin signaling in Verticillium dahliae. Front Microbiol. 2019;10:18.
Arceci RJ, Stieglitz K, Bierer BE. Immunosuppressants FK506 and rapamycin function as reversal agents of the multidrug resistance phenotype. Blood. 1992;80(6):1528–36.
Méndez C, Salas JA. The role of ABC transporters in antibiotic-producing organisms: drug secretion and resistance mechanisms. Res Microbiol. 2001;152(3–4):341–50.
Yoo YJ, Hwang JY, Shin HL, Cui H, Lee J, Yoon YJ. Characterization of negative regulatory genes for the biosynthesis of rapamycin in Streptomyces rapamycinicus and its application for improved production. J Ind Microbiol Biotechnol. 2015;42(1):125–35.
Manning BD. Game of TOR—the Target of Rapamycin rules four kingdoms. New Engl J Med. 2017;377(13):1297–9.
Kurmasheva RT, Huang S, Houghton PJ. Predicted mechanisms of resistance to mTOR inhibitors. Br J Cancer. 2006;95(8):955–60.
Teichert S, Wottawa M, Schoenig B, Tudzynski B. Role of the Fusarium fujikuroi TOR kinase in nitrogen regulation and secondary metabolism. Eukaryot Cell. 2006;5(10):1807–19.
Melendez HG, Billon-Grand G, Fevre M, Mey G. Role of the Botrytis cinerea FKBP12 ortholog in pathogenic development and in sulfur regulation. Fungal Genet Biol. 2009;46(4):308–20.
Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21(4):183–203.
Dumas SN, Lamming DW. Next generation strategies for geroprotection via mTORC1 inhibition. J Gerontol A Biol Sci Med Sci. 2020;75(1):14–23.
Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369(6483):756–8.
Romero-Aguilar L, Guerra-Sánchez G, Tenorio EP, Tapia-Rodriguez M, Matus-Ortega G, Flores-Herrera O, et al. Rapamycin induces morphological and physiological changes without increase in lipid content in Ustilago maydis. Arch Microbiol. 2020;202(5):1211–21.
Wu C, Chen Y, Huang X, Sun S, Luo J, Lu Z, et al. An efficient shortened genetic transformation strategy for filamentous fungus Trichoderma reesei. J Gen Appl Microbiol. 2020;65(6):301–7.
Ma L, Zhang J, Zou G, Wang C, Zhou Z. Improvement of cellulase activity in Trichoderma reesei by heterologous expression of a beta-glucosidase gene from Penicillium decumbens. Enzyme Microb Technol. 2011;49(4):366–71.
Minty JJ, Singer ME, Scholz SA, Bae CH, Ahn JH, Foster CE, et al. Design and characterization of synthetic fungal-bacterial consortia for direct production of isobutanol from cellulosic biomass. Proc Natl Acad Sci USA. 2013;110(36):14592–7.
Li C, Pang AP, Yang H, Lv R, Zhou Z, Wu FG, et al. Tracking localization and secretion of cellulase spatiotemporally and directly in living Trichoderma reesei. Biotechnol Biofuels. 2019;12:200.
Zhong YH, Wang XL, Wang TH, Jiang Q. Agrobacterium-mediated transformation (AMT) of Trichoderma reesei as an efficient tool for random insertional mutagenesis. Appl Microbiol Biotechnol. 2007;73(6):1348–54.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (31700040), the National Key R&D Program of China (No. 2016YFA0501604), and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Contributions
APP, ZL, and FL conceived and designed the study. APP carried out the majority of the experiments. HW, FZ and XH carried out some experiments on T. reesei cultivation, enzyme activity, and confocal imaging, respectively. WW and ZZ provided strain T. reesei KU70 and plasmid pXBthg, respectively. FGW helped analyze the data. APP and FL analyzed the data and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

Additional file 1: Figure S1.
Effects of 100 μM rapamycin on (hemi)cellulase activities and protein secretion of T. reesei RUT-C30 cultured in TMM + 2% glucose (A) and TMM + 2% lactose (B) at 24 h, 72 h, 120 h, and 168 h, respectively. FPase: the filter paper activity; pNPCase: the CBH activity; CMCase: the CMC activity; pNPGase: the β-glucosidase activity; pNPXase: the β-xylosidase activity; Secreted protein: secreted protein concentration. Data are represented as the mean of three independent experiments and error bars express the standard. Asterisks indicate significant differences (*p < 0.05, **p < 0.01, ***p < 0.001) as assessed by Student’s t test.

Additional file 2: Figure S2.
Hyphal morphology of T. reesei RUT-C30 cultured in TMM + 2% glucose (A) or lactose (B) with different concentrations of rapamycin at 24, 72, 120, and 168 h, which was observed under CLSM. Scale bar = 10 μm.

Additional file 3: Figure S3.
The lipid content of T. reesei RUT-C30 on TMM + 2% cellulose, lactose, or glucose with various concentrations of rapamycin. The lipid content of RUT-C30 at 24 h stained with Nile Red was observed under CLSM. Scale bar = 10 μm.
Additional file 4: Table S1.
Transcriptional level of total DEGs in T. reesei RUT-C30 with or without 100 μM rapamycin.
Additional file 5: Table S2.
Transcriptional level of DEGs related to transporters in T. reesei RUT-C30.
Additional file 6: Table S3.
Transcriptional level of genes involved in (hemi)cellulase production in T. reesei RUT-C30.
Additional file 7: Table S4.
Transcriptional level of DEGs related to “Biosynthesis of secondary metabolites” in T. reesei RUT-C30.

Additional file 8: Figure S4.
Hyphal morphology of T. reesei △FKBP12 and KU70 cultured in TMM + 2% cellulose with/without 100 μM rapamycin at 24, 72, 120, and 168 h, which was observed under CLSM. Scale bar = 20 μm.
Additional file 9: Table S5.
Transcriptional level of genes involved in TOR signal pathways in T. reesei RUT-C30.
Additional file 10: Table S6.
Transcription level of genes associated with gene expression in T. reesei RUT-C30.

Additional file 11: Figure S5.
FKBP12 (A) and TOR (B) sequence alignments with those of Saccharomyces cerevisiae, Homo sapiens, Arabidopsis thaliana, Schizosaccharomyces pombe, and Oryza sativa. Amino acid residues that form the hydrophobic rapamycin-binding pocket of FKBP12 (A) are in red. Mutation of amino acid residues of TOR (B) in red confers rapamycin resistance.

Additional file 12: Figure S6.
The (hemi)cellulase activities and protein secretion of T. reesei RUT-C30 cultured in TMM + 2% cellulose for 96 h and treated with different concentrations of rapamycin for 24 h. FPase: the filter paper activity; pNPCase: the CBH activity; CMCase: the CMC activity; pNPGase: the β-glucosidase activity; pNPXase: the β-xylosidase activity; Secreted protein: secreted protein concentration. Data are represented as the mean of three independent experiments and error bars express the standard. Asterisks indicate significant differences (*p < 0.05, **p < 0.01, ***p < 0.001) as assessed by Student’s t test.

Additional file 13: Figure S7.
Gene trFKBP12 was deleted in T. reesei KU70 by homologous integration (A) to obtain putative ΔtrFKBP12 strains (1, 2 and 3) in which the successful deletion of trFKBP were verified by PCR (B). FKBP, trFPBP12; hyg, hygromycin B phosphotransferase; M: DL5000 marker; Primer 1: Primer 1-F and Primer 1-R; Primer 2: Primer 2-F and Primer 2-R.
Additional file 14: Table S7.
Primers for trFKBP deletion and confirmation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pang, AP., Wang, H., Zhang, F. et al. High-dose rapamycin exerts a temporary impact on T. reesei RUT-C30 through gene trFKBP12. Biotechnol Biofuels 14, 77 (2021). https://doi.org/10.1186/s13068-021-01926-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13068-021-01926-w