Abstract
Background
Cyanobacterial carbohydrates, such as sucrose, have been considered as potential renewable feedstock to support the production of fuels and chemicals. However, the separation and purification processes of these carbohydrates will increase the production cost of chemicals. Co-culture fermentation has been proposed as an efficient and economical way to utilize these cyanobacterial carbohydrates. However, studies on the application of co-culture systems to achieve green biosynthesis of platform chemicals are still rare.
Results
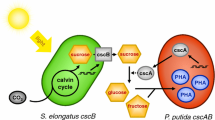
In this study, we successfully achieved one-step conversion of sucrose derived from cyanobacteria to fine chemicals by constructing a microbial consortium consisting of the fast-growing cyanobacterium Synechococcus elongatus UTEX 2973 and Escherichia coli to sequentially produce sucrose and then the platform chemical 3-hydroxypropionic acid (3-HP) from CO2 under photoautotrophic growth conditions. First, efforts were made to overexpress the sucrose permease-coding gene cscB under the strong promoter Pcpc560 in S. elongatus UTEX 2973 for efficient sucrose secretion. Second, the sucrose catabolic pathway and malonyl-CoA-dependent 3-HP biosynthetic pathway were introduced into E. coli BL21 (DE3) for heterologous biosynthesis of 3-HP from sucrose. By optimizing the cultivation temperature from 37 to 30 °C, a stable artificial consortium system was constructed with the capability of producing 3-HP at up to 68.29 mg/L directly from CO2. In addition, cell growth of S. elongatus UTEX 2973 in the consortium was enhanced, probably due to the quick quenching of reactive oxygen species (ROS) in the system by E. coli, which in turn improved the photosynthesis of cyanobacteria.
Conclusion
The study demonstrated the feasibility of the one-step conversion of sucrose to fine chemicals using an artificial consortium system. The study also confirmed that heterotrophic bacteria could promote the cell growth of cyanobacteria by relieving oxidative stress in this microbial consortium, which further suggests the potential value of this system for future industrial applications.
Similar content being viewed by others
Background
Cyanobacteria are capable of producing organic matter from inorganic carbon (CO2) by using solar energy. Due to the challenges associated with global climate change and sustainable energy supply, cyanobacteria have recently attracted significant attention as environmentally friendly and sustainable “microbial cell factories” for the production of biofuels and valuable chemicals directly from CO2 [1]. In addition, cyanobacteria have also been considered as a means of producing carbohydrate feedstocks to support industrial fermentative processes [2]. Moreover, it has been reported that several cyanobacterial species are capable of synthesizing and secreting sucrose as an osmolyte under appropriate environmental stimuli, such as osmotic pressure [3], and this production can be sustained over long time periods and at higher levels than that from plant-based feedstocks such as sugarcane and beet [4, 5]. As sucrose is an easily fermentable feedstock for many microorganisms [6, 7], significant efforts have been made to improve the production of extracellular sucrose in cyanobacteria [8]. For example, Du et al. achieved sucrose productivity at 1.43 mg/L/h in wild-type Synechocystis sp. PCC 6803 under 600 mM NaCl stress in a bioreactor and doubled the productivity to 3.13 mg/L/h by co-overexpressing key genes related to sucrose synthesis, namely, sps (slr0045), spp (slr0953) and ugp (slr0207), and deleting the glucosylglycerol phosphate synthase gene ggpS (sll1566) [9]. In another study, Ducat et al. integrated a cscB gene encoding sucrose permease from Escherichia coli W [10, 11] into the Synechococcus elongatus PCC 7942 genome and silenced the carbon competition pathway by knocking out the invertase invA and ADP-glucose pyrophosphorylase glgC to achieve sucrose secretion at a rate of 36.1 mg/L/h [12]. Recently, the fast-growing cyanobacterium Synechococcus elongatus UTEX 2973 (hereafter S. elongatus UTEX 2973) with a growth rate similar to that of yeast was identified [13], and an extracellular sucrose productivity of 35.5 mg/L/h was demonstrated in an engineered S. elongatus UTEX 2973 carrying the sucrose transporter cscB in a bioreactor experiment [14]. Sucrose productivity was further increased to 79.2 mg/L/h through upregulation of sps, which encodes a sucrose-phosphate synthase enzyme, and sucrose synthesis genes in S. elongatus UTEX 2973 [15]. In addition to the high rate of sucrose secretion and growth, this strain exhibits high tolerance to high-temperature (41 °C) and high-light (500 μmol photons∙m−2∙s−1) conditions, suggesting significant advantages for outdoor cultivation in the future [13]. However, as purification of sucrose from culture supernatant is costly and the system is easily contaminated when sucrose is produced at a large scale [16], alternative ways of utilizing sucrose produced by cyanobacteria need to be developed for potential biotechnological applications.
In nature, microorganisms typically live and interact with other microbes by establishing a stable interchange of substances in complex communities [17, 18]. Inspired by the commonly found symbiotic relationships of various microbes in nature, studies have been conducted to simulate symbiotic systems by designing artificial routes for the interchange of substances [19, 20]. Very recently, Ducat et al. constructed a co-culture system with the cyanobacterium S. elongatus PCC 7942 and the heterotrophic bacterium Halomonas boliviensis, in which the growth of H. boliviensis was supported by sucrose produced by S. elongatus PCC 7942 [21], and Li et al. constructed a co-culture system consisting of the sucrose secretory cyanobacterium S. elongatus PCC 7942 and three different yeasts to mimic lichen and research the interaction between the autotrophic and heterotrophic strains [22]. Although these studies have established new alternatives for the utilization of sucrose derived from cyanobacteria, the use of co-culture systems to achieve one-step conversion of sucrose to fine chemicals is still rare [23]. In addition, compared with axenic cultures of cyanobacteria, autotrophic–heterotrophic symbiotic systems have been found to resist contamination effectively and exhibit good robustness in fluctuating environments [24, 25].
3-Hydroxypropionic acid (3-HP, C3H6O3), as an important platform chemical, is widely used for the production of many chemicals, such as acrylic acid, malonic acid and biodegradable plastic poly-3-hydroxypropionic acid, and can also be used as a food additive or preservative [26]. As chemical synthesis of 3-HP causes severe environmental pollution [27], biosynthesis of 3-HP has attracted significant attention recently. Several 3-HP biosynthetic pathways have been reported, and at least four substrates have been used to produce 3-HP, including β-alanine [28], lactate [29], malonyl-CoA [30] and glycerol [31]. Among these pathways, the malonyl-CoA-dependent pathway, which employs acetyl-CoA carboxylase to convert the precursor acetyl-CoA to malonyl-CoA and malonyl-CoA reductase to convert malonyl-CoA to 3-HP [32, 33], was reported to have some distinct advantages over other pathways, such as a broad feedstock spectrum, thermodynamic feasibility, and redox neutrality [34]. To date, the malonyl-CoA-dependent pathway has been engineered in E. coli [35], Saccharomyces cerevisiae [36], Synechocystis sp. PCC 6803 [37] and S. elongatus PCC 7942 [38] for both heterotrophic and photoautotrophic production of 3-HP [35]. However, until now, no study about the biosynthesis of 3-HP by a co-culture system has been reported.
In this study, we reported the construction of an artificial consortium system consisting of the fast-growing cyanobacterium S. elongatus UTEX 2973 and an engineered E. coli BL21(DE3) to produce 3-HP under photoautotrophic conditions. In the consortium system, E. coli BL21(DE3) was genetically modified to synthesize 3-HP using sucrose produced by the engineered S. elongatus UTEX 2973 (Fig. 1). With the application of this co-culture system, the final yield of 3-HP was approximately 68.29 mg/L, which is comparable to that obtained in E. coli when only malonyl-CoA reductase was overexpressed [30]. In addition to the relationship where S. elongatus provides sucrose as a carbon source for growth and 3-HP production in E. coli, the study also found that increased expression of reactive oxygen species (ROS)-quenching genes in E. coli may promote cyanobacterial growth by relieving oxidative stress in the environment.
Results
Growth of S. elongatus cscB+ 2973 and sucrose secretion
Synechococcus elongatus UTEX 2973 was engineered to secrete sucrose by expressing the sucrose permease-encoding gene cscB (ECW_m2594) under the strong promoter Pcpc560. Sucrose secretion from S. elongatus cscB+ 2973 is mainly dependent upon the pH and NaCl concentration of the medium, and an alkaline environment was previously reported to be beneficial for sucrose secretion from cyanobacterial cells [39]. We used an alkaline environment (pH ≈ 8.3) with 150 mM NaCl (37 °C) to ensure the production and secretion of sucrose from S. elongatus cscB+ 2973 [14, 22]. The sucrose yield and the growth of S. elongatus cscB+ 2973 in different culture media are compared in Fig. 2. The results showed that no sucrose was produced from S. elongatus cscB+ 2973 cells without NaCl in the culture medium. However, sustainable production and secretion of sucrose could be observed for 6 days when 150 mM NaCl was added, and titers of 612.0 mg/L and 576.5 mg/L sucrose were achieved when S. elongatus cscB+ 2973 was grown in BG-11 and CoBG-11, respectively. To maintain the growth of E. coli in co-culture medium, the effect of different salt concentrations on cell growth was also examined (Additional file 1: Fig. S1), and the results showed that E. coli was able to grow normally under the tested range of salt concentrations. In this study, a sucrose titer of 576.5–612.0 mg/L (4.00–4.25 mg/L/h) was achieved by S. elongatus cscB+ 2973 cells over 6 days, which is comparable to the levels observed in similar studies conducted previously (Table 1). For example, although no CO2 aeration occurred during S. elongatus cultivation, sucrose secretion in this study was still higher than the 2.2 mg/L/h value reported in a previous study [22].
Effects of NaCl on the growth and sucrose yield of S. elongatus cscB+. Growth of strain S. elongatus cscB+ in BG-11 medium (a) and CoBG-11 medium (c). Sucrose yield of strain S. elongatus cscB+ in BG-11 medium (b) and CoBG-11 medium (d). The error bars represent the calculated standard deviation of the measurements of three biological replicates
Growth of an engineered E. coli mutant in co-culture medium
To ensure that E. coli BL21 utilizes sucrose as the sole carbon source, we cloned and expressed the essential genes for sucrose metabolism, namely, cscB (ECW_m2594), cscK (ECW_m2595) and cscA (ECW_m2596), into E. coli BL21 to generate an engineered strain, E. coli cscN. In addition, to synthesize 3-HP, the malonyl-CoA reductase-coding gene mcr (Caur_2614) was introduced into E. coli cscN, resulting in the engineered strain E. coli ABKm. A growth comparison of these two strains is shown in Additional file 2: Fig. S2. As shown, under the same conditions, the final cell density of cscN without the mcr gene was slightly increased. In a previous study, an artificial consortium was constructed by inoculating a heterotrophic bacterium into a S. elongatus PCC 7942 culture with OD750 = 0.5 [25]. In our study, the sucrose yield of S. elongatus cscB+ 2973 was ~ 200 mg/L when the cells reached OD750 = 0.5. Therefore, we selected four concentrations, namely, 50, 100, 150, and 200 mg/L, to examine whether E. coli ABKm could be stably maintained in the system using these levels of sucrose as the sole carbon source in M9 and CoBG-11 media (Fig. 3a, b). The growth of E. coli ABKm could be detected under 100, 150, and 200 mg/L sucrose. A previous study showed that the E. coli ΔcscR strain required a minimal sucrose concentration of 1.2 g/L for growth [25], which is much higher than our result for strain ABKm, suggesting that after expression of cscA, cscB and cscK, the efficiency of sucrose utilization might have improved in strain ABKm [25, 40]. To demonstrate that this effect was not caused by a strain-specific difference, the sucrose utilization pathway was also engineered into E. coli MG1655 and BW25113, and a similar result was observed (data not shown). Additionally, we also determined the 3-HP yield in strain ABKm with the different concentrations of sucrose mentioned above (i.e., 50–200 mg/L) in CoBG-11, and the results showed that strain ABKm was able to produce 3-HP under all the concentrations except 50 mg/L sucrose (Fig. 3c).
Growth and 3-HP production of pure E. coli ABKm culture under different sucrose concentrations. a Cultivated in M9 medium; b cultivated in CoBG-11 medium. The error bars represent the calculated standard deviation of the measurements of three biological replicates. c 3-HP production in the E. coli ABKm strain under different initial sucrose concentrations in co-culture medium (“*” indicates that no detectable amount was observed). The error bars represent the calculated standard deviation of the measurements of three biological replicates
Establishing a stable artificial consortium to produce 3-HP
Since the optimal growth temperature for both E. coli and S. elongatus UTEX 2973 is 37 °C, we initially set this as the incubation temperature for the co-culture system. However, the analysis showed that E. coli strain ABKm grew poorly after 1–2 days in this system compared with the growth observed in a previous study [25] (Additional file 3: Fig. S3). According to the data (Fig. 2d), at 37 °C, S. elongatus cscB+ 2973 cells produce a sufficient amount of sucrose, which prompted us to hypothesize that the rapid cell growth of E. coli and utilization of sucrose destroy the balance of the two species in this system. To confirm this hypothesis, we determined the rates of sucrose secretion and sucrose utilization in S. elongatus UTEX 2973 and E. coli, respectively. The results showed that the sucrose utilization rate of E. coli strain ABKm increased gradually with increasing initial sucrose concentration, reaching ~ 4.20 mg/L/h at an initial sucrose concentration of 200 mg/L with growth at 37 °C for 48 h (Fig. 4b). Although the sucrose secretion rate of S. elongatus cscB+ could reach ~ 4.11 mg/L/h, we speculated that with the accumulation of E. coli biomass, the sucrose consumption rate of ABKm could be faster than the sucrose secretion rate of S. elongatus cscB+ at 37 °C. Therefore, the “production–consumption” balance was disrupted, leading to collapse of the consortium. These results led us to adjust the cultivation temperature from 37 °C to 30 °C, aiming to slow down the consumption of E. coli and achieve balanced growth of S. elongatus UTEX 2973 and E. coli in the system. The growth of strain ABKm at 30 °C was then observed (Fig. 4a), and the sucrose utilization rate of this strain was determined to be ~ 2.00 mg/L/h at 30 °C at 48 h (Fig. 4b). Interestingly, there was no significant difference between 37 °C and 30 °C in terms of cell growth and sucrose production of the S. elongatus cscB+ 2973 strain (Fig. 4c, d). As a result, the artificial consortium with S. elongatus cscB+ 2973 and E. coli strain ABKm was successfully constructed and could be maintained stably for at least 7 days at 30 °C (Fig. 4e).
Construction of an artificial consortium system with engineered S. elongatus UTEX 2973 and E. coli BL21. a Growth of pure ABKm culture in CoBG-11 medium with different concentrations of sucrose at 30 °C. b Sucrose utilization rate of pure ABKm culture. c Growth of pure S. elongatus UTEX 2973 culture at 30 °C and 37 °C. d Sucrose yield of engineered S. elongatus cscB+. e Growth of two strains in the artificial consortium system at 30 °C. The error bars represent the calculated standard deviation of the measurements of three biological replicates
To evaluate the production capacity of the artificial consortium, the 3-HP yield of E. coli strain ABKm was analyzed. As shown in Fig. 5a, 3-HP production reached ~ 68.29 mg/L in 7 days. In parallel, we also determined 3-HP production in E. coli strain ABKm under pure culture conditions with continuous supplementation of sucrose according to the calculated sucrose secretion rate of S. elongatus cscB+ 2973 (Fig. 5b), and the results showed that the 3-HP yield under pure conditions was at the same level. In addition, we also observed that S. elongatus cscB+ 2973 cultivated in the consortium grew better than the cells cultivated in pure culture conditions (Fig. 6a), consistent with previous findings [25]. In addition, the results showed that almost no free sucrose could be detected in the co-culture medium, suggesting that sucrose produced by the cyanobacterium was completely consumed by the E. coli ABKm strain to support cell growth and accumulate the desired product (Additional file 4: Fig. S4) [25].
3-HP yield under different conditions. a 3-HP yield in an artificial consortium system. b 3-HP production in strain ABKm under continuous supplementation with sucrose according to the calculated sucrose secretion rate of S. elongatus cscB+. The error bars represent the calculated standard deviation of the measurements of three biological replicates
Growth of cyanobacteria and H2O2 content in different culture conditions. a Difference in the growth of S. elongatus cscB+ in the artificial consortium and under axenic conditions. The blue rhombus represents the cell number in the axenic consortium, the orange rectangle represents the cell number in the artificial consortium, and the gray triangle represents the ratio between the two conditions. b H2O2 content in blank CoBG-11 (control), axenic culture and co-culture systems under the same conditions (30 °C, 100 μE m−2s−1 light, 150 mM NaCl; “*” indicates that no detectable amount was observed under the conditions). The error bars represent the calculated standard deviation of the measurements of three biological replicates
Effect of oxidative stress on cyanobacteria in an artificial consortium system
ROS are common byproducts of aerobic metabolic processes, such as photoreactions and respiration, in oxygenic photosynthetic organisms [41], and ROS accumulation could cause oxidative damage to cyanobacterial cells. In addition, previous studies have found that organic buffers in culture media may also contribute to the generation of H2O2 [42]. For example, 1 ~ 10 mM 4-(2-hydroxyethyl)1-piperazineethanesulfonic acid (HEPES) in culture medium could produce enough H2O2 to kill Prochlorococcus [43]. Since there was also organic buffer (TES) used to maintain pH in our study, to clarify whether this organic buffer generates H2O2, we determined the titer of H2O2 in blank CoBG-11 under the same culture conditions. The results showed that no H2O2 was detected in blank culture medium, suggesting that the H2O2 in culture medium was mostly synthesized from living cells. Next, we examined the impact of E. coli co-cultivation on the H2O2 level, and the results showed that the H2O2 content was significantly reduced when the heterotrophic partner of E. coli was included in the system (Fig. 6b), which is consistent with a previous study [22].
To further understand this phenomenon at the molecular level, the expression levels of several H2O2-quenching genes in the E. coli ABKm strain under pure and co-culture conditions were comparatively analyzed by qRT-PCR (Additional file 5: Table S1). It is well known that E. coli contains three types of catalases: hydroperoxidase I (HPI) (katG), hydroperoxidase II (HPII) (katF), and hydroperoxidase III (HPIII) (katE) [44,45,46]. In addition, the synthesis of HPII often increases markedly when cells enter the stationary phase [47, 48]. The transcriptional expression of these three genes was determined. As shown in Fig. 7, the relative expression levels of katG, katF and katE in the E. coli ABKm strain were dramatically upregulated under co-culture conditions compared with those in CoBG-11 under continuous supplementation with sucrose according to the calculated sucrose secretion rate of S. elongatus cscB+ 2973, suggesting that E. coli might be able to remove ROS when co-cultivated with cyanobacterial partners and thus possibly alleviate the overall oxidative stress in the consortium system.
Discussion
Photosynthetic cyanobacteria have been considered an important alternative for providing sustainable feedstock, and cyanobacterial carbohydrates have been considered a potential renewable feedstock to support the production of fuels and chemicals. However, since axenic cultures of cyanobacteria are vulnerable to contamination [49] and it is expensive to separate and purify these products from culture medium [50], their application is limited. To address these issues, the use of synthetic consortia of cyanobacteria paired with specific heterotrophic partners has been proposed. The fast-growing S. elongatus UTEX 2973 strain reported recently exhibits high tolerance to high-temperature and high-light conditions [13]. In this study, we reported the construction of an artificial co-culture system utilizing S. elongatus UTEX 2973 and E. coli BL21 to produce the heterologous chemical 3-HP directly from CO2. In addition, analysis of the mechanism underlying the co-culture systems also provided a fundamental basis for further optimization of artificial consortium systems via metabolic engineering.
Sucrose is a commonly used carbon source for industrial microbial fermentation [51, 52]. In this study, we transformed the sucrose transporter cscB into the fast-growing cyanobacterium S. elongatus UTEX 2973 and obtained the sucrose-secreting strain S. elongatus cscB+ 2973 with a productivity of 612.0 mg/L BG-11 in 6 days. To construct an artificial consortium using sucrose as the sole carbon source, the sucrose utilization ability of E. coli was also improved. It was previously shown that sucrose utilization could be endowed in the Pseudomonas putida cscAB strain by introducing the cscA and cscB genes from E. coli W [23]. To achieve the growth of E. coli BL21 in the designed co-culture system, genes related to sucrose utilization, namely, cscA, cscB and cscK, were introduced [53]. The results showed that this engineering strategy enabled E. coli strain ABKm to grow on sucrose as the sole carbon source (Fig. 3). A previous study reported that E. coli ΔcscR strain required a minimal sucrose concentration of 1.2 g/L for growth, which is much higher than the concentration required by ABKm here, suggesting that this engineering strategy significantly improved the efficiency of sucrose utilization [40].
It was previously reported that ROS accumulation in the culture medium severely inhibited axenic growth of cyanobacteria; however, this effect was efficiently alleviated through co‑culture with Rhodotorula glutinis [22]. We also observed improved growth of S. elongatus UTEX 2973 in the co-culture system (Fig. 6a). The H2O2 content in the consortium system was found to be lower than that in the axenic culture, suggesting that E. coli might contribute to the alleviation of ROS stress and thus promote the growth of cyanobacteria in the artificial consortium, which was further confirmed by the upregulation of ROS quenching-related genes in E. coli strain ABKm (Fig. 7). In addition, to confirm our hypothesis, we also tested the influence of H2O2 on the cell growth of axenic cyanobacterial cultures, and the results showed that the addition of 10 μM H2O2 significantly decreased cell growth (Additional file 6: Fig. S5). The upregulation of ROS quenching capability could be important, as ROS may deleteriously affect cellular metabolic processes, such as nutrient bioavailability, photosynthesis and carbon flux, in plants [54]. With the decreased H2O2 content and increased cell growth of S. elongatus UTEX 2973, we also evaluated the expression levels of several genes involved in photosynthesis, including the carbon dioxide-concentrating mechanism protein-coding gene ccmM [55], ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO)-coding gene rbcL [56], PSII-related subunit-coding genes cp43 and cp47, PSI essential subunits of reaction center-coding genes psaA and psaB [57], and chlorophyll a synthesis-related genes chlaA and pcrA [58] (Additional file 5: Table S1); as expected, all these genes were upregulated (Additional file 7: Fig. S6). Several studies have also reported that heterotrophic partners can provide the necessary inorganic carbon by decomposing organic matter and growth factors such as vitamin B12 to cyanobacteria in the natural and artificial consortium [18, 59, 60], which we will also analyze in future work. Further analysis of the interactions in the consortium could provide the necessary theoretical basis for the potential application of artificial co-culture systems in many areas, such as controlling blooms [61], degradation of pollutants [62,63,64], and soil remediation [65,66,67]. For example, Fedeson et al. created an artificial consortium composed of two bacterial species (S. elongatus PCC 7942 and P. putida) that enables the degradation of the industrially produced environmental pollutant 2,4-DNT while simultaneously producing polyhydroxyalkanoates (PHA) bioplastic [68].
Our study also provided some insights into the potential relationships between the two species. The first layer of interaction is that the cyanobacterium provides the carbon source for the E. coli strain, and the engineered E. coli strain consumes this carbon source to grow and produce fine chemicals. Interestingly, we also found that the cell growth of the cyanobacterium was somewhat improved in the co-culture system. By taking a close look at the changes in the factors involved in determining cell growth, we found that E. coli may help quench ROS, which in turn promotes the growth of cyanobacteria, which is considered the second layer of interaction between these two species. Collectively, we concluded that the relationship between these two species in the co-culture system is mutualistic.
Although several previous studies have demonstrated promising characteristics of co-culture systems [21, 25], our work showed their new application: first, the final product was different. Unlike polyhydroxybutyrate (PHB), 3-HP is a heterologous compound, and our study provides proof of the concept that the production of heterologous fine chemicals can be achieved in co-culture systems by rational design. Second, S. elongatus UTEX 2973 exhibits high tolerance to high-light conditions compared with other model cyanobacterial species, such as S. elongatus PCC 7942, which means that the robustness of our system may be improved. Furthermore, compared with traditional “two-stage” fermentation, this “one-step” strategy also has several unique advantages. Besides the issue of bacterial contamination as the excess sugar in the system is consumed by heterotrophic partners [21], the “two-stage” culture strategy typically requires two steps to obtain the chemical production: obtaining the supernatant of the cyanobacterial, and then inoculating the E. coli strain to achieve cell growth and chemical production, which can greatly increase the production cost.
Although the artificial consortium system was successfully established and the desired product was obtained in this study, there remain many aspects that need to be improved in the future, such as the yield of 3-HP. To increase the yield of the target product, on the one hand, we could enhance the supply of the carbon source. In addition to silencing the competing consumption pathway [12], Qiao et al. reported that sucrose yield in S. elongatus PCC 7942 can be enhanced from 6.5 to 8.0 mg/L/h by overexpressing sucrose-phosphate synthase (sps) and glucose-1-phosphate adenylyltransferase (glgC) at the same time [69]. The research also suggested that glycogen could serve as a supporting rather than a competitive carbon pool for sucrose synthesis. In addition, Weiss et al. used alginate to encapsulate S. elongatus and enhanced the sucrose yield rates ~ twofold within 66 h [21]. On the other hand, heterotrophic partners also need to be improved. Chelladurai et al. developed a variety of recombinant E. coli strains by expressing the heterologous gene mcr and overexpressing the endogenous acetyl-CoA carboxylase and biotinidase-encoding genes accADBCb and nicotinamide nucleotide transhydrogenase-encoding gene pntAB, which converts NADH to NADPH in E. coli. In addition, several deletion mutations in phosphotransacetylase (pta) acetate kinase (ackA) and lactate dehydrogenase (ldhA) or the α-ketoglutarate dehydrogenase complex (sucAB) were carried out with the recombinant strains. The final 3-HP titer was enhanced approximately threefold from 0.71 to 2.14 mM [30]. Moreover, Cheng et al. reported the overexpression of heterogeneous acetyl-CoA carboxylase (from Corynebacterium glutamicum) and codon-optimized mcr in E. coli BL21; three types of modified E. coli strains with different host–vector systems were constructed and investigated, and the results showed that the combination of E. coli BL21 and pET28a was the most efficient host–vector system for 3-HP production. The concentration of 3-HP was enhanced from 0.68 g/L to 1.80 g/L in shake flask cultivation [70]. These studies provide valuable guidance for further metabolic engineering in E. coli.
Conclusion
With defined composition and controllable functions, synthetic consortia hold great promise for diverse value-added production, bioenergy and environmental applications. In this study, we demonstrated the feasibility of constructing an artificial consortium to achieve the one-step conversion of sucrose to the platform chemical 3-HP directly from CO2. With the application of this co-culture system, the final production of 3-HP was approximately 68.29 mg/L, which is comparable to that in E. coli when only malonyl-CoA reductase was overexpressed. Meanwhile, this study also confirmed that in this microbial consortium, heterotrophic bacteria could promote the cell growth of cyanobacteria by relieving oxidative stress, which further demonstrates the potential value of this system for the green biosynthesis of chemicals in the future.
Materials and methods
Strains, plasmid construction and culture conditions
Synechococcus elongatus UTEX 2973 and E. coli BL21(DE3) were engineered and applied to the construction of the artificial consortium system. The essential genes for sucrose metabolism, namely, the permease-coding gene cscB, invertase-coding gene cscA and fructokinase-coding gene cscK, were derived from E. coli W [53], while the malonyl-CoA reductase gene mcr was from Chloroflexus aurantiacus [71]. The super-strong promoter Pcpc560 was used to direct gene expression in S. elongatus UTEX 2973 [72]. All plasmids were prepared in E. coli DH5α. The sucrose hydrolysis system was integrated into pET-30a in E. coli with kanamycin resistance, and mcr was integrated into pACYC184 under the constitutive promoter PJ23100 in E. coli with spectinomycin resistance.
Synechococcus elongatus UTEX 2973 and the resulting engineered strains were cultivated in BG-11 medium (pH 7.5) under a light intensity of approximately 100 μmol photons m−2s−1 in an illuminating shaking incubator (HNYC-202T, Honour, Tianjin, China) at 130 rpm and 37 °C or on BG-11 agar plates in an incubator (SPX-250B-G, Boxun, Shanghai, China) [73]. To maintain the stable phenotype of sucrose secretion, appropriate antibiotics were added when necessary. E. coli strains were grown on LB medium or agar plates with appropriate antibiotics added to maintain plasmids at 37 °C in a shaking incubator (HNY-100B, Honour, Tianjin, China) at 200 rpm or in an incubator, respectively. All strains used in this study are listed in Table 2.
Conjugation of S. elongatus UTEX 2973
Constructs were delivered into S. elongatus UTEX 2973 through conjugation [74]. E. coli HB101 harboring pRL443 and pRL623 (named “helper”) and E. coli DH5α harboring the plasmid with the target gene were cultivated overnight and then transferred separately into fresh liquid LB medium with the appropriate antibiotics at a 1:50 ratio. When both strains grew to exponential phase (OD600 = 0.3–0.5), 10 mL of the cells of each strain was collected by centrifugation and washed with fresh LB medium three times to remove all the antibiotics. Then, 0.1 mL of fresh LB was used to resuspend each strain, and the cells were mixed together and incubated at 37 °C for 30 min. During this time, 10 mL of S. elongatus UTEX 2973 cells at exponential phase (OD750 ≈ 1) was collected by centrifugation and resuspended in 0.2 mL of fresh BG-11 medium. S. elongatus UTEX 2973 cells were mixed with the E. coli mixture mentioned above and incubated at 37 °C under light for 30 min. Then, the mixture was spread on BG-11 agar plates, which were then covered by sterile cellulose filters (0.45 μm pore size). The plates were incubated under light at an intensity of approximately 100 μmol photons m−2s−1 for 24 h, and then, the cellulose filters were transferred onto new BG-11 agar plates with appropriate antibiotics [75].
Construction of the artificial consortium system
Co-culture medium (named CoBG-11) was designed based on BG-11 medium and optimized for E. coli growth by supplementing with 150 mM NaCl, 4 mM NH4Cl and 3 g/L 2-[[1,3-dihydroxy-2-(hydroxymethyl) propan-2-yl] amino] ethanesulfonic acid (TES). The pH value was adjusted with NaOH to 8.3. NaCl and NH4Cl were used to maintain the cell survival of E. coli, and NaCl was used as a stress inducer for sucrose accumulation in S. elongatus UTEX 2973.
Before the two strains were cultivated together, S. elongatus UTEX 2973 was propagated in BG-11 at 37 °C with appropriate antibiotics to the exponential phase (OD750 ≈ 1.0), collected by centrifugation, inoculated into 25 mL of CoBG-11 medium and grown at 30 °C for 48 h to an OD750 of 0.5. E. coli was incubated in CoBG-11 with 1 g/L sucrose for 48 h and then collected by centrifugation, resuspended in deionized water and inoculated into the 25-mL S. elongatus culture described above at an initial OD600 of 0.01.
Quantification of cyanobacteria and E. coli
For pure cultures of S. elongatus UTEX 2973 and E. coli, cell density was measured at OD750 and OD600, respectively, using a UV-1750 spectrophotometer (Shimadzu, Kyoto, Japan). For co-culture, serial dilutions were made, and solid LB agar plates were used to determine E. coli viability and cell number by counting colony-forming units (CFU) after 24 h of incubation at 37 °C. The cell number of S. elongatus UTEX 2973 was determined by a hemocytometer under a microscope (BX43, Olympus, Shinjuku, Tokyo, Japan).
Determination of extracellular sucrose content
Supernatants of pure S. elongatus UTEX 2973 cultures were collected and analyzed for sucrose content via a colorimetric glucose–sucrose assay (Megazyme, Ireland) that employs high-purity glucose oxidase, peroxidase and β-fructosidase (invertase). At pH 4.6, sucrose is hydrolysed by invertase to d-glucose and d-fructose, and then, the free d-glucose content is determined by conversion to a red-colored quinoneimine dye compound through the action of glucose oxidase and peroxidase at pH 7.4 and employing p-hydroxybenzoic acid and 4-aminoantipyrine. Measurements were conducted at 510 nm.
Quantification of 3-HP
The 3-HP concentration was quantified according to a previously described method [37]. A 3-HP standard of analytical purity was purchased from Tokyo Chemical Industry (Tokyo, Japan). The supernatant containing 3-HP was collected from the co-culture medium by centrifuging at 12,000 rpm for 2 min at room temperature (Eppendorf 5430R, Hamburg, Germany) and used for 3-HP analysis. Sample derivatization was carried out according to the two-stage technique described previously [76]. GC–MS analysis was conducted on a GC–MS system-GC 7890 coupled to an MSD 5975 (Agilent Technologies, Inc., Santa Clara, CA) equipped with an HP-5MS capillary column (30 mm × 250 mm id).
Quantitative real-time RT-PCR analysis
Approximately 4 × 106 pure or co-cultured S. elongatus UTEX 2973 cells were collected by centrifugation at 12,000 rpm and 4 °C for 1 min. The supernatant was removed, and the cells were used for RNA extraction and RT-qPCR analysis using methods described previously [77]. The relative abundance of different mRNA molecules could be estimated using 2−ΔΔCT [78].
Analysis of H2O2 concentration
The H2O2 content in the supernatant was analyzed using the H2O2 Quantitative Assay Kit (Sangon Biotech, Shanghai, China). In the reaction, Fe2+ is oxidized to Fe3+ by H2O2 when the pH is less than 7.0, and then, the generated Fe3+ combines with dye molecules to form a claret-colored Fe3+–dye complex with a maximum absorption wavelength of 560 nm or 595 nm, and the absorption value is directly proportional to the concentration of H2O2 in cells.
Availability of supporting data
All data generated or analyzed during this study are included in this published article and its additional files.
Abbreviations
- GC–MS:
-
Gas chromatography–mass spectrometry
- WT:
-
Wild type
- 3-HP:
-
3-Hydroxypropionic acid
- F6P:
-
Fructose 6-phosphate
- PHA:
-
Polyhydroxyalkanoates
- PHB:
-
Polyhydroxybutyrate
- ROS:
-
Reactive oxygen species
References
Quintana N, Van der Kooy F, Van de Rhee MD, Voshol GP, Verpoorte R. Renewable energy from Cyanobacteria: energy production optimization by metabolic pathway engineering. Appl Microbiol Biotechnol. 2011;91:471–90.
Hays SG, Ducat DC. Engineering cyanobacteria as photosynthetic feedstock factories. Photosynthesis Res. 2015;123:285–95.
Hagemann M. Molecular biology of cyanobacterial salt acclimation. FEMS Microbiol Rev. 2011;35:87–123.
Niederholtmeyer H, Wolfstädter BT, Savage DF, Silver PA, Way JC. Engineering cyanobacteria to synthesize and export hydrophilic products. Appl Environ Microbiol. 2010;76:3462–6.
Han Y, Watson M. Production of microbial levan from sucrose, sugarcane juice and beet molasses. J Ind Microbiol. 1992;9:257–60.
Reid SJ, Abratt VR. Sucrose utilisation in bacteria: genetic organisation and regulation. Appl Microbiol Biotechnol. 2005;67:312–21.
Feng J, Gu Y, Quan Y, Gao W, Dang Y, Cao M, Lu X, Wang Y, Song C, Wang S. Construction of energy-conserving sucrose utilization pathways for improving poly-γ-glutamic acid production in Bacillus amyloliquefaciens. Microb Cell Fact. 2017;16:98.
Kirsch F, Klähn S, Hagemann M. Salt-regulated accumulation of the compatible solutes sucrose and glucosylglycerol in cyanobacteria and its biotechnological potential. Front Microbiol. 2019;10:2139.
Du W, Liang F, Duan Y, Tan X, Lu X. Exploring the photosynthetic production capacity of sucrose by cyanobacteria. Metab Eng. 2013;19:17–25.
Sahintoth M, Frillingos S, Lengeler JW, Kaback HR. Active transport by the cscB permease in Escherichia coli K-12. Biochem Biophys Res Commun. 1995;208:1116–23.
Vadyvaloo V, Smirnova IN, Kasho VN, Kaback HR. Conservation of residues involved in sugar/H+ symport by the sucrose permease of Escherichia coli relative to lactose permease. J Mol Biol. 2006;358:1051–9.
Ducat DC, Avelar-Rivas JA, Way JC, Silver PA. Rerouting carbon flux to enhance photosynthetic productivity. Appl Environ Microbiol. 2012;78:2660–8.
Yu J, Liberton M, Cliften PF, Head RD, Jacobs JM, Smith RD, Koppenaal DW, Brand JJ, Pakrasi HB. Synechococcus elongatus UTEX 2973, a fast growing cyanobacterial chassis for biosynthesis using light and CO2. Sci Rep. 2015;5:8132.
Song K, Tan X, Liang Y, Lu X. The potential of Synechococcus elongatus UTEX 2973 for sugar feedstock production. Appl Microbiol Biotechnol. 2016;100:7865–75.
Lin P-C, Zhang F, Pakrasi HB. Enhanced production of sucrose in the fast-growing cyanobacterium Synechococcus elongatus UTEX 2973. Sci Rep. 2020;10:1–8.
Chisti Y. Constraints to commercialization of algal fuels. J Biotechnol. 2013;167:201–14.
Beliaev AS, Romine MF, Serres M, Bernstein HC, Linggi BE, Markillie LM, Isern NG, Chrisler WB, Kucek LA, Hill EA. Inference of interactions in cyanobacterial–heterotrophic co-cultures via transcriptome sequencing. The ISME journal. 2014;8:2243.
Christie-Oleza JA, Sousoni D, Lloyd M, Armengaud J, Scanlan DJ. Nutrient recycling facilitates long-term stability of marine microbial phototroph–heterotroph interactions. Nature microbiology. 2017;2:17100.
Fredrickson JK. Ecological communities by design. Science. 2015;348:1425–7.
Brenner K, You L, Arnold FH. Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol. 2008;26:483–9.
Weiss TL, Young EJ, Ducat DC. A synthetic, light-driven consortium of cyanobacteria and heterotrophic bacteria enables stable polyhydroxybutyrate production. Metab Eng. 2017;44:236–45.
Li T, Li C-T, Butler K, Hays SG, Guarnieri MT, Oyler GA, Betenbaugh MJ. Mimicking lichens: incorporation of yeast strains together with sucrose-secreting cyanobacteria improves survival, growth, ROS removal, and lipid production in a stable mutualistic co-culture production platform. Biotechnol Biofuels. 2017;10:55.
Löwe H, Hobmeier K, Moos M, Kremling A, Pflüger-Grau K. Photoautotrophic production of polyhydroxyalkanoates in a synthetic mixed culture of Synechococcus elongatus cscB and Pseudomonas putida cscAB. Biotechnol Biofuels. 2017;10:190.
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology. 1979;111:1–61.
Hays SG, Yan LL, Silver PA, Ducat DC. Synthetic photosynthetic consortia define interactions leading to robustness and photoproduction. J Biol Eng. 2017;11:4.
Gokarn RR, Selifonova OV, Jessen HJ, Gort SJ, Selmer T, Buckel W: 3-Hydroxypropionic acid and other organic compounds. Google Patents; 2007.
Jiang X, Meng X, Xian M. Biosynthetic pathways for 3-hydroxypropionic acid production. Appl Microbiol Biotechnol. 2009;82:995–1003.
Borodina I, Kildegaard KR, Jensen NB, Blicher TH, Maury J, Sherstyk S, Schneider K, Lamosa P, Herrgård MJ, Rosenstand I. Establishing a synthetic pathway for high-level production of 3-hydroxypropionic acid in Saccharomyces cerevisiae via β-alanine. Metab Eng. 2015;27:57–64.
Henry CS, Broadbelt LJ, Hatzimanikatis V. Discovery and analysis of novel metabolic pathways for the biosynthesis of industrial chemicals: 3-hydroxypropanoate. Biotechnol Bioeng. 2010;106:462–73.
Rathnasingh C, Raj SM, Lee Y, Catherine C, Ashok S, Park S. Production of 3-hydroxypropionic acid via malonyl-CoA pathway using recombinant Escherichia coli strains. J Biotechnol. 2012;157:633–40.
Raj SM, Rathnasingh C, Jo J-E, Park S. Production of 3-hydroxypropionic acid from glycerol by a novel recombinant Escherichia coli BL21 strain. Process Biochem. 2008;43:1440–6.
Zhang W, Song X. Synthetic biology of cyanobacteria. New York: Springer; 2018.
Hügler M, Menendez C, Schägger H, Fuchs G. Malonyl-coenzyme A reductase from Chloroflexus aurantiacus, a key enzyme of the 3-hydroxypropionate cycle for autotrophic CO2 fixation. J Bacteriol. 2002;184:2404–10.
Liu C, Ding Y, Xian M, Liu M, Liu H, Ma Q, Zhao G. Malonyl-CoA pathway: a promising route for 3-hydroxypropionate biosynthesis. Crit Rev Biotechnol. 2017;37:933–41.
Liu C, Wang Q, Xian M, Ding Y, Zhao G. Dissection of malonyl-coenzyme A reductase of Chloroflexus aurantiacus results in enzyme activity improvement. PLoS ONE. 2013;8:e75554.
Chen Y, Bao J, Kim I-K, Siewers V, Nielsen J. Coupled incremental precursor and co-factor supply improves 3-hydroxypropionic acid production in Saccharomyces cerevisiae. Metab Eng. 2014;22:104–9.
Wang Y, Sun T, Gao X, Shi M, Wu L, Chen L, Zhang W. Biosynthesis of platform chemical 3-hydroxypropionic acid (3-HP) directly from CO2 in cyanobacterium Synechocystis sp PCC 6803. Metab Eng. 2016;34:60–70.
Lan EI, Chuang DS, Shen CR, Lee AM, Ro SY, Liao JC. Metabolic engineering of cyanobacteria for photosynthetic 3-hydroxypropionic acid production from CO2 using Synechococcus elongatus PCC 7942. Metab Eng. 2015;31:163–70.
Chi X, Zhang S, Mao S, Luan G, Luo Q, Lü X. Cyanobacteria based photosynthetic production of sucrose: development and prospect. Chin J Biotechnol. 2019;35:1411–23.
Sabri S, Nielsen LK, Vickers CE. Molecular control of sucrose utilization in Escherichia coli W, an efficient sucrose-utilizing strain. Appl Environ Microbiol. 2013;79:478–87.
Polerecky L, Bachar A, Schoon R, Grinstein M, Jørgensen BB, De Beer D, Jonkers HM. Contribution of Chloroflexus respiration to oxygen cycling in a hypersaline microbial mat from Lake Chiprana, Spain. Environ Microbiol. 2007;9:2007–24.
Ferreira CM, Pinto IS, Soares EV, Soares HM. (Un) suitability of the use of pH buffers in biological, biochemical and environmental studies and their interaction with metal ions–a review. RSC Adv. 2015;5:30989–1003.
Jeffrey Morris J, Zinser ER. Continuous hydrogen peroxide production by organic buffers in phytoplankton culture media. J Phycol. 2013;49:1223–8.
Loewen PC. Isolation of catalase-deficient Escherichia coli mutants and genetic mapping of katE, a locus that affects catalase activity. J Bacteriol. 1984;157:622–6.
Loewen P, Triggs B. Genetic mapping of katF, a locus that with katE affects the synthesis of a second catalase species in Escherichia coli. J Bacteriol. 1984;160:668–75.
Loewen PC, Triggs BL, George CS, Hrabarchuk BE. Genetic mapping of katG, a locus that affects synthesis of the bifunctional catalase-peroxidase hydroperoxidase I in Escherichia coli. J Bacteriol. 1985;162:661–7.
Hassan HM, Fridovich I. Regulation of the synthesis of catalase and peroxidase in Escherichia coli. J Biol Chem. 1978;253:6445.
Loewen PC, Switala J, Triggs-Raine BL. Catalases HPI and HPII in Escherichia coli are induced independently. Arch Biochem Biophys. 1985;243:144–9.
Gavrilescu M, Chisti Y. Biotechnology-a sustainable alternative for chemical industry. Biotechnol Adv. 2005;23:471–99.
Ziolkowska JR. Prospective technologies, feedstocks and market innovations for ethanol and biodiesel production in the US. Biotechnol Rep. 2014;4:94–8.
Wittmann C, Kiefer P, Zelder O. Metabolic fluxes in Corynebacterium glutamicum during lysine production with sucrose as carbon source. Appl Environ Microbiol. 2004;70:7277–87.
Lee IY, Seo WT, Kim GJ, Kim MK, Park C, Park YH. Production of curdlan using sucrose or sugar cane molasses by two-step fed-batch cultivation of Agrobacterium species. J Ind Microbiol Biotechnol. 1997;18:255–9.
Li F, Ma J, Wu M, Ji Y, Chen W, Ren X, Jiang M. Succinic acid production from sucrose and sugarcane molasses by metabolically engineered Escherichia coli. Chin J Biotechnol. 2015;31:534–41.
Hasanuzzaman M, Bhuyan M, Anee TI, Parvin K, Nahar K, Mahmud JA, Fujita M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants. 2019;8:384.
Kerfeld CA, Melnicki MR. Assembly, function and evolution of cyanobacterial carboxysomes. Curr Opin Plant Biol. 2016;31:66–75.
Liang F, Lindberg P, Lindblad P. Engineering photoautotrophic carbon fixation for enhanced growth and productivity. Sustain Energy Fuels. 2018;2:2583–600.
Armond P, Staehelin L, Arntzen C. Spatial relationship of photosystem I, photosystem II, and the light-harvesting complex in chloroplast membranes. J Cell Biol. 1977;73:400–18.
Papageorgiou GC, Tsimilli-Michael M, Stamatakis K. The fast and slow kinetics of chlorophyll a fluorescence induction in plants, algae and cyanobacteria: a viewpoint. Photosynth Res. 2007;94:275–90.
Bernstein HC, McClure RS, Thiel V, Sadler NC, Kim Y-M, Chrisler WB, Hill EA, Bryant DA, Romine MF, Jansson JK. Indirect interspecies regulation: transcriptional and physiological responses of a cyanobacterium to heterotrophic partnership. MSystems. 2017;2:e00181.
Ren M, Zhang G, Ye Z, Qiao Z, Xie M, Lin Y, Li T, Zhao J. Metagenomic analysis reveals potential interactions in an artificial coculture. Amb Express. 2017;7:193.
Zhu L, Zancarini A, Louati I, De Cesare S, Duval C, Tambosco K, Bernard C, Debroas D, Song L, Leloup J. Bacterial communities associated with four cyanobacterial genera display structural and functional differences: evidence from an experimental approach. Front Microbiol. 2016;7:1662.
Liu J, Wu Y, Wu C, Muylaert K, Vyverman W, Yu H-Q, Muñoz R, Rittmann B. Advanced nutrient removal from surface water by a consortium of attached microalgae and bacteria: a review. Bioresour Technol. 2017;241:1127–37.
Yang Z, Pei H, Hou Q, Jiang L, Zhang L, Nie C. Algal biofilm-assisted microbial fuel cell to enhance domestic wastewater treatment: nutrient, organics removal and bioenergy production. Chem Eng J. 2018;332:277–85.
Alcántara C, Domínguez JM, García D, Blanco S, Pérez R, García-Encina PA, Muñoz R. Evaluation of wastewater treatment in a novel anoxic–aerobic algal–bacterial photobioreactor with biomass recycling through carbon and nitrogen mass balances. Bioresour Technol. 2015;191:173–86.
Abou-Shady A. Reclaiming salt-affected soils using electro-remediation technology: PCPSS evaluation. Electrochim Acta. 2016;190:511–20.
Dahlawi S, Naeem A, Rengel Z, Naidu R. Biochar application for the remediation of salt-affected soils: challenges and opportunities. Sci Total Environ. 2018;625:320–35.
Nain L, Rana A, Joshi M, Jadhav SD, Kumar D, Shivay Y, Paul S, Prasanna R. Evaluation of synergistic effects of bacterial and cyanobacterial strains as biofertilizers for wheat. Plant Soil. 2010;331:217–30.
Fedeson DT, Saake P, Calero P, Nikel PI, Ducat DC: Biotransformation of 2, 4-dinitrotoluene in a phototrophic co-culture of engineered Synechococcus elongatus and Pseudomonas putida. bioRxiv 2018:404988.
Qiao C, Duan Y, Zhang M, Hagemann M, Luo Q, Lu X. Effects of reduced and enhanced glycogen pools on salt-induced sucrose production in a sucrose-secreting strain of Synechococcus elongatus PCC 7942. Appl Environ Microbiol. 2018;84:e02023.
Cheng Z, Jiang J, Wu H, Li Z, Ye Q. Enhanced production of 3-hydroxypropionic acid from glucose via malonyl-CoA pathway by engineered Escherichia coli. Bioresour Technol. 2016;200:897–904.
Mattozzi M, Ziesack M, Voges MJ, Silver PA, Way JC. Expression of the sub-pathways of the Chloroflexus aurantiacus 3-hydroxypropionate carbon fixation bicycle in E coli: toward horizontal transfer of autotrophic growth. Metab Eng. 2013;16:130–9.
Wang B, Eckert C, Maness P-C, Yu J. A genetic toolbox for modulating the expression of heterologous genes in the cyanobacterium Synechocystis sp PCC 6803. ACS synthetic biology. 2017;7:276–86.
Stanier R, Kunisawa R, Mandel M, Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971;35:171.
Elhai J, Wolk CP: Conjugal transfer of DNA to cyanobacteria. In Methods in enzymology. Volume 167: Elsevier; 1988. p. 747-754.
Li S, Sun T, Xu C, Chen L, Zhang W. Development and optimization of genetic toolboxes for a fast-growing cyanobacterium Synechococcus elongatus UTEX 2973. Metab Eng. 2018;48:163–74.
Roessner U, Luedemann A, Brust D, Fiehn O, Linke T, Willmitzer L, Fernie AR. Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant systems. Plant Cell. 2001;13:11–29.
Gao W, Zhang W, Meldrum DR. RT-qPCR based quantitative analysis of gene expression in single bacterial cells. J Microbiol Methods. 2011;85:221–7.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8.
Acknowledgements
None.
Funding
This research was supported by Grants from the National Key Research and Development Program of China (Grant nos. 2019YFA0904600, 2018YFA0903000 and 2018YFA0903600), the National Natural Science Foundation of China (nos. 91751102, 31901016, 31770035, 31770100, 31972931, 21621004, 31370115 and 31470217), and Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (no. TSBICIP-KJGG-007).
Author information
Authors and Affiliations
Contributions
XYS and WZ designed the research; LZ performed the major experiments and wrote the draft manuscript; JJD and XYS helped with some of the experiments; MLS helped with the GC–MS analysis; and LZ, LC, XYS and WZ analyzed the data and drafted and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have agreed to the publication of this manuscript.
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Fig. S1.
Growth of E. coli ABKm under different concentrations of NaCl in CoBG-11 medium at 37 °C.
Additional file 2: Fig. S2.
Growth of E. coli cscN and ABKm. A), B), C) Cultivated in M9 medium; D), E), F) cultivated in CoBG-11 medium.
Additional file 3: Fig. S3.
Growth in the artificial consortium system at 37 °C. S. elongatus cscB+ (green square) and E. coli ABKm (gray diamond).
Additional file 4: Fig. S4.
Consumption of sucrose in the co-culture system.
Additional file 5: Table S1.
Related genes and primers used in this study.
Additional file 6: Fig. S5.
Growth of the cyanobacterium S. elongatus cscB+ with H2O2 added.
Additional file 7: Fig. S6.
Expression level analysis of genes involved in photosynthesis. Gene expression analysis of ccmM, rbcL, cp43, cp47, psaB, psaA. chlaA and pcrA in S. elongatus cscB+. The error bars represent the calculated standard deviation of the measurements of three biological replicates.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, L., Chen, L., Diao, J. et al. Construction and analysis of an artificial consortium based on the fast-growing cyanobacterium Synechococcus elongatus UTEX 2973 to produce the platform chemical 3-hydroxypropionic acid from CO2. Biotechnol Biofuels 13, 82 (2020). https://doi.org/10.1186/s13068-020-01720-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13068-020-01720-0