Abstract
Background
Vulnerable children, including those with neuro-developmental delays and disabilities, often face barriers in accessing early primary education, thus hindering progress toward Sustainable Development Goal 4.2. Evidence-based interventions are essential to enhancing inclusivity and establishing sustainable implementation strategies to address this challenge. This study, Every Newborn—Reach up Early Education Intervention for All Children (EN-REACH), builds on the previous Every Newborn- Simplified Measurement Integrating Longitudinal Neurodevelopmental and Growth (EN-SMILING) observational cohort study. This paper provides the protocol for a cluster randomized controlled trial (cRCT) to evaluate the effectiveness of a parenting group intervention program for enhancing school readiness in Bangladesh, Nepal, and Tanzania, and an embedded process evaluation to inform scalability and feasibility.
Methods
EN-REACH is a cRCT with at least 150 clusters to evaluate the impact of a parent training program led by trained parent-teacher facilitator pairs, focusing on children aged 4 ~ 6 years preparing for preschool. Approximately 500 participants from the EN-SMILING cohort at each site have been identified. A geographic information system will define ~ 50 clusters in each of the three countries, each with approximately ten parent–child dyads. Half the clusters will be randomly assigned to intervention and control groups. The primary outcome is “school readiness”, assessed using the Measuring Early Learning Quality and Outcomes tool. Secondary outcomes include Intelligence Quotient, child functioning, growth, visual, and hearing assessments. Data will be collected at baseline, and post-intervention data following implementation of the parent group intervention sessions over approximately 5 months. Quantitative data on coverage and quality care, combined with qualitative insights from children, caregivers, facilitators, and stakeholders’ perspectives, will be used to conduct a process evaluation applying the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework.
Discussion
This protocol details a trial focused on enhancing school readiness and cognitive abilities in young children, inclusive of those with disabilities, aiming to bridge gap from home to early primary education. EN-REACH aims to provide insights into the effectiveness and acceptability of a co-designed disability-inclusive school readiness program in three countries, potentially impacting national and global policies for all children, including those with disabilities.
Trial registration
The trial was retrospectively registered on clinicaltrials.gov on 29 February 2024 (NCT06334627).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Administrative information
Note: the numbers in curly brackets in this protocol refer to SPIRIT checklist item numbers. The order of the items has been modified to group similar items (see http://www.equator-network.org/reporting-guidelines/spirit-2013-statement-defining-standard-protocol-items-for-clinical-trials/).
Title {1} | Every Newborn- Reach Up Early Education Intervention for All Children (EN-REACH)- a parent group intervention for school readiness in Bangladesh, Nepal, and Tanzania: study protocol for a cluster randomized controlled trial. |
|---|---|
Trial registration {2a and 2b} | ClinicalTrails.gov: NCT06334627. Retrospectively registered on 29 February 2024. https://clinicaltrials.gov/study/NCT06334627?cond=NCT06334627&rank=1 |
Protocol version {3} | Version 3 and, dated 20.02.2023 |
Funding {4} | The EN-REACH study is funded by the UK Medical Research Council (MRC), Global Health Research Board (MR/V035274/1), and leveraged funding in Bangladesh from Stiftung Auxilium (GR-075636). |
Author details {5a} | Mohammad Abdul Awal Miah1+*, Jaya Chandna2+, Rejina Gurung3+, Nahya Salim Masoud4,5+, Proma Paul2, Shafiqul Ameen1, Omkar Basnet3, Mustafa Miraji4, Cally Tann2, Ismat Ara Mili1, A.K.M. Tanvir Hossain1, Atique Iqbal Chowdhury1, Asraful Alam1, Kate Mackinnon Milner2, Shams El Arifeen1 Ashish KC6#, Karim Manji4#, Paul Lynch7#, Joy E Lawn2#, and Jena Derakhshani Hamadani1#, and EN-REACH collaborative group 1. Maternal and Child Health Division, International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh 2. Maternal, Adolescent, Reproductive & Child Health (MARCH), London School of Hygiene & Tropical Medicine, London, United Kingdom 3. Golden Community, Kathmandu, Nepal 4. Department of Paediatrics and Child Health, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania. 5. Department of Paediatrics and Child Health, Temeke Regional Referral Hospital, Dar es Salaam, Tanzania. 6. Murdoch Children’s Research Institute, Department of Paediatrics, University of Melbourne, Melbourne, Australia 7. School of Public Health and Community Medicine, University of Gothenburg, Gothenburg, Sweden 8. School of Education, University of Glasgow, Glasgow, Scotland +Contributed equally and shared first authorship #Shared senior authorship *Corresponding author |
Name and contact information for the trial sponsor {5b} | London School of Hygiene & Tropical Medicine (LSHTM) is the trial sponsor. Naomi Panteli, Research Facilitator, Rigo@lshtm.ac.uk |
Role of sponsor {5c} | The sponsor is the LSHTM, where the principal investigator leads the study. The funders do not have a role in the design of the study, development of the intervention package and implementation, data collection and analysis, and writing the manuscript. |
Introduction
Background and rationale {6a}
Sustainable Development Goals (SDGs) include targets for the unfinished child survival agenda, since there are 5.3 million deaths of children before their 5th birthday, half in the neonatal period. Notably, the SDGs reflect families’ and national governments’ aspirations that children thrive as well as survive. SDG target 4.2 aims to ensure access to high-quality early child development (ECD) and pre-primary education (PPE) for every young child by 2030, preparing them for primary education [1]. However, children most at risk of poor educational outcomes are those with neuro-developmental delay and disability (NDD/D), who are less likely to enroll in school or preschool services. While the SDGs call for equal learning opportunities for every child, in practice, ECD/school readiness programs, and those for children with NDD/D are often disconnected, with a gap existing between early assessment and early education opportunities [2]. Rigorous and methodologically sound studies are vital to building caregivers’ skills, and inclusion of children with disabilities.
Developmental disability refers to conditions resulting from impairments that affect a child’s physical, learning, or behavioral functioning and includes sensory impairments (hearing and vision loss), epilepsy or seizures, cerebral palsy, attention deficit hyperactivity disorder, autism spectrum disorders, intellectual disability, or other learning disorders. We define NDD/D by assessing the extent of functional challenges within various domains by applying the Child Functioning Module (CFM) developed by UNICEF and the Washington Group on Disability Statistics in 2016 [3]. It is tailored for assessing functional challenges in children aged 2 to 17 and encompasses multiple domains, such as vision, hearing, mobility, self-care, communication, learning, memory, emotional wellbeing (anxiety, depression), behavior management, attention, adaptation to routine changes, and social interactions. The response options for most questions include “no difficulty,” “some difficulty,” “significant difficulty,” and “unable to perform.” Precisely, we classify moderate to severe impairment in a child as having at least one domain in which they experience either “significant difficulty” or “unable to perform” a task.
An estimated 53 million children under 5 years live with NDD/D, including vision and hearing loss, epilepsy, intellectual disability, autism spectrum disorder, and attention deficit hyperactivity disorder [4]. Most of these children (approximately 95%) live in low- and middle-income countries (LMICs). Risks of developmental delay/disability increase with stunting or extreme poverty and malnutrition [5]. The United Nations introduced the Nurturing Care Framework for Childhood Development in 2018, aiming to synchronize health, nutrition, responsive caregiving, early learning, and social protection domains [6]. Moreover, it embraces a strong emphasis on ensuring the full rights of all children, as set out in the UN Convention on the Rights of the Child [7], and promotes the active participation of children with disabilities and their family members set out in Article 24 of the UN Convention on the Rights of Persons with Disabilities [8]. The SDGs strive for equal learning opportunities for every child. However, integration of ECD programs, particularly for children with NDD/D and those in humanitarian settings, is lacking in many government departments [9]. Despite 48% of LMICs having policies addressing ECD and PPE, there is a significant gap in service provision and evidence-based, feasible programmatic approaches, especially inclusive of marginalized children [10].
Hence, we set out to address an important evidence gap, by evaluating the impact of a school readiness parenting intervention, inclusive of children with or without NDD/D in Bangladesh, Nepal, and Tanzania.
The governments of these three countries all acknowledge the need to improve children’s development and early education. For example, Bangladesh has a comprehensive policy for Early Childhood Care and Development in 2013 [11] applicable to all Bangladeshi children. In Bangladesh, rural children living in poverty have marked deficits in cognitive development beginning as early as age 7 months and increasing to substantial deficits of − 1.2 standard scores in intelligence quotients by school age [12]. To make progress to overcome this problem, the Ministry of Primary and Mass Education initiated a universal 1-year free PPE for all 5-year-old children at government primary schools in 2014. In 2020, the government approved an extension of the PPE program starting at 4 years of age to meet the objectives of the National Education Policy 2010. The Ministry of Education in Nepal has initiated a disability program assessment in selected parts of the country, and this study can provide an added value to the existing national program. In Tanzania, the current recommendation for Early Childhood Education in 2021 is 1–7-4–2-3 + , with 12 years of fee-free education with a compulsory pre-primary level is being changed from 2 years to 1 year (starting for children at age 6), thus increasing the duration of basic education from 11 to 12 years. Currently, the government is responsible for 95.2% of PPE. There are disability schools, but these are poorly attended and poorly funded. The Tanzania Ministry of Education is in the process of reviewing and changing this training further in near future.
School readiness programs contribute and positively impact on a child’s educational performance [13]. In this context, parental engagement is a crucial factor influencing a child’s school readiness [14, 15]. The success of a comprehensive school readiness program for all children, including those with NDD/D, hinges on the active involvement of families, children, schools, and the community. Parental engagement, particularly from mothers, has been linked to positive and lasting academic success [14], reducing behavioral issues and enhancing social skills [16]. School readiness is a complex concept involving multiple dimensions [17]. To understand the transition of children with disabilities into primary education in a low-income country, an intersecting model of school readiness is valuable—ready parents, ready children, and ready schools [18]. UNICEF [19] acknowledges a more comprehensive view of school readiness, considering three dimensions: school, family/community, and children, and this model will be integrated into our intervention program. An integrative supportive intervention is essential to prepare children with/without NDD/D, their parents, community, and teachers for school to reinforce the healthy transition during their early years to maximize their personal development and achieve their learning outcomes [20]. A cluster-randomized controlled study in Mozambique showed that combining preschool teacher training with parenting education significantly impacts increased language skills and socio-emotional development [21].
Our primary research question is as follows: What is the impact of a parenting programon school readiness and early literacy, numeracy, and problem-solving skillsfor4–6-year-old children, which are inclusive of children with NDD/D?
Every Newborn—Reach up Early Education Intervention for All Children (EN-REACH) trial aims to evaluate the impact of a parent group intervention by a parent-teacher pair, inclusive of disability, on outcomes, including school readiness of children aged 4–6 years of age, using a cRCT design (approximately 50 clusters of 10 parent–child dyads per country) in Bangladesh, Nepal, and Tanzania with process evaluation of the intervention for scale up and feasibility.
Multi-country trial embedded into existing cohort study
We will add EN-REACH to an existing cohort study in Bangladesh, Nepal, and Tanzania, introducing an intervention study to be used with the same cohort as two earlier observational studies:
-
Every Newborn-Birth Indicator Research Tracking in Hospitals (EN-BIRTH) study observed > 23,000 births in five hospitals (mid-2017 to mid-2018) to validate selected coverage indicators for maternal and newborn care, resulting in multiple partners from diverse LMIC sites [22].
-
Every newborn – simplified measurement integrating longitudinal neurodevelopment and growth (EN-SMILING) is a cohort following up at-risk newborns and control children who are a subset of the EN-BIRTH study (~ 2000), applying simplified measurement tools to assess ECD using an app-based platform designed by International Centre for Diarrhoeal Diseases Research, Bangladesh (icddr,b). The study was funded by Children’s Investment Fund Foundation, which is partnering with UNICEF and WHO, up to June 2020 and Stiftung Auxilium until September 2022 in Bangladesh.
Objectives {7}
Objective 1: INNOVATION
To develop a preschool package for all children aged 4 ~ 6 years old, inclusive of those with NDD/D, in three diverse LMICs. This will include a focus on the following areas:
-
Disability-inclusive material: additional material to support children with developmental disabilities and their families
-
Preschool readiness material content will be adapted locally in partnership with families, health workers, child safeguarding professionals, and educational stakeholders through participatory workshops with multi-sectoral perspectives for inclusive early childhood care and education (ECCE) in these settings.
Objective 2: IMPACT EVALUATION
To use a cRCT design to measure school readiness and child and caregiver quality of life after receiving the parenting package delivered to 50% of the cohort, estimated 500 children in each of the three countries, compared to the other 50%.
Objective 3: IMPLEMENTATION EVALUATION
Process evaluation of parent group intervention for school readiness in accordance with the RE-AIM framework covering Reach, Effectiveness, Adoption, Implementation, and Maintenance [23, 24], applying mixed methods will be undertaken to assess the process of implementation, including quantitative data (coverage and quality of care), and qualitative evaluation of the preschool package from child, caregiver, teacher, and facility and community provider perspectives [25].
Trial design {8}
The study is a cRCT determining the effect of a preschool parenting package between intervention and non-intervention arms. The allocation for the parent–child dyad will be an equal distribution with a 1:1 ratio between the two groups, assigning 25 clusters to both the intervention and control arms of the cRCT. Clusters, including preschools, community centers, and healthcare centers, will be identified based on the geospatial location of the children’s houses in the EN-SMILING cohort using a modified K-means clustering algorithm in ArcGIS. The 50 clusters will then be randomized into intervention and control arms. Before randomizing the clusters, we will determine the eligibility of participants, obtain consent from caregivers for enrolment within 1 week, and complete baseline assessments for both groups within 3 months. Following this, trained facilitators will conduct nine intervention sessions over 5 months. Endline assessments will be completed within 2 weeks after the final parent group school readiness session. We hypothesize that a culturally adapted preschool readiness parenting program will improve the school readiness of children aged 4–6 years, including those with functional disabilities, as compared to the control group.
Methods: participants, interventions, and outcomes
Study setting {9}
The study is focused on pre-school aged children from the EN-SMILING cohort, which followed up on at-risk newborns and matched control children from a subset of the EN-BIRTH study. All families and children from Bangladesh, Tanzania, and Nepal who consent to take part in the study will be included. We present the study protocol following the SPIRIT guidelines [26].
Eligibility criteria {10}
Inclusion criteria
All consenting families and children from EN-SMILING study who remained within study area.
Exclusion criteria for children
Families who have moved out of area.
Most parents shared at least one mobile or residential phone number, either their own, their husband’s, or another family member’s. Precise details are taken describing the address of the house and its relationship to other important local landmarks in particular community hubs (preschools, community centers, and healthcare centers). This information will be stored on secure servers at each institutional site. Geographical information system (GIS) for households and early childhood education centres for all EN-REACH eligible children has been collected and mapped, as listed in Table 1. Each parent group intervention will be delivered by a pair of trained facilitators consisting of one parent and one teacher. There will be a total of 25 PPE teachers and 25 parents at each site.
Who will be asked for informed consent? {26a}
During contact with parents/primary caregivers within the EN-BIRTH study, informed consent was taken, which includes potential follow-up of newborns exposed to basic interventions. Additional written informed consent from parents was explicitly obtained for the EN-SMILING study prior to the 6-month face-to-face assessments. For EN-REACH, written informed consent will be taken before the baseline assessments of children, with oral and written explanations in local languages from parents.
Additional consent provisions for collection and use of participant data and biological specimens {26b}
Not applicable. We do not intend to gather any biological samples from the study participants.
Interventions
Explanation for the choice of comparators {6b}
The control group, constituting 50% of the approximately 150 clusters, will continue to access standard early care and/or pre-primary education in line with the prevailing local health and education systems.
Intervention description {11a}
We designed a preschool parenting package with reference to MRC guidance on developing and evaluating complex interventions to include two content areas:
-
(1)
School readiness material and planning for primary school: Content will be adapted locally in partnership with families, health workers, child safeguarding professionals and educational stakeholders in each of the three countries through the use of participatory workshops and online design workshops with an invitation for multi-sectoral perspectives for inclusive early childhood development and education in these settings.
-
(2)
Disability inclusive material: additional material to support children with developmental disabilities and their families, including feeding, play, communication, and everyday activities for children with intellectual, physical, visual and hearing impairments, content on caregiver wellbeing; and participation and rights related to education and health. This content will be derived from the Ubuntu Hub child disability programs (https://www.ubuntu-hub.org), including “Getting to Know Cerebral Palsy,” which is implemented across more than 70 countries, the ABAaNA Early Intervention Programme for young infants with developmental disability and the Juntos program for children with Congenital Zika Syndrome and their families in Brazil.
In May 2022, key team members of all sites (Nepal, Bangladesh, Tanzania) plus other team members and relevant experts met at Dar-Es-Salaam in Tanzania for a week’s design workshop. This was in order to undertake formative work to inform a sustainable, contextually relevant package. During the workshop, a framework for the intervention package was co-designed. It was decided that about nine participatory sessions should be conducted approximately fortnightly with groups of approximately ten children and their main caregivers at a community hub (preschool, community center, healthcare center). Each session would have a pair of facilitators: a teacher and an “expert” parent, trained in the package. Families and facilitators will be reimbursed with a travel fare for attending.
Pilot
All intervention sessions and logistics will be piloted for around a period of 1.5 months. We will translate all materials into local languages and train four facilitators to run two parent–child dyad groups, including the children with NDD, for approximately 6 weeks to test sessions for cultural appropriateness and any necessary adaptations. We plan to collect qualitative data from the participants, facilitators, and supervisors who will participate in the piloting phase to understand the feasibility of the study before conducting the cRCT.
Recruitment of facilitators, training, and quality of intervention
Each parent group will be led by two facilitators—one parent and one teacher. These facilitators will be selected from the study area and will undergo a week-long training in the intervention material. The training will be conducted by trained master facilitators, with all master facilitators from the three country teams being trained together during a workshop in Bangladesh. All three country teams master facilitators have trained together during training workshop in Bangladesh on 25–29 September 2022.
Criteria for discontinuing or modifying allocated interventions {11b}
The participation in EN-REACH cRCT is entirely voluntary, and individuals are entitled to withdraw from the intervention at any point without providing an explanation for their decision. Health risks for parents and their children, as well as natural calamities such as heatwaves, excessive rains, and floods, may be encountered. To address these challenges, adjustments will be made to session dates. No modifications will be made to the allocation of intervention packages.
Strategies to improve adherence to interventions {11c}
The adherence to this trial protocol is considered strong, even though it does not have strict guidelines. Employing strategies such as reminder phone calls, home visits, and providing wage loss compensation for caregivers of children with disabilities, along with sensitization meetings with teachers, school management committees, and family members, may enhance their attendance and engagement in the intervention. The quality of intervention delivery will be monitored throughout the trial with supervision from master trainers at 10% of all sessions. A quality checklist will be developed to ensure consistency of delivery across sites and the intervention period to adhere to the protocol.
Relevant concomitant care permitted or prohibited during the trial {11d}
During the intervention period, the study children, particularly those who will enroll in the pre-primary class, are free to attend their regular classes or early childhood care and education programs.
Provisions for post-trial care {30}
Harms are not anticipated from this trial and benefits are expected for the individuals and the communities. The present study focuses on improving school readiness of young children with or without NDD, as well as the quality of life of caregivers. It is expected that this study will add additional support for already identified children with NDD/D. Training will include identification of criteria for referral and information on local pathways for referral, along with the provision of standardized documentation regarding screening and assessment findings for “receiving” referral health workers. Caregivers may feel distressed and uneasy (such as anxiety, upset, stress) to some extent when asked about their mental health problems. Where additional stress is identified, health workers will complete referral guidance (local government health, education, and other support services) training, supported by standard operating procedures (SOPs) for appropriate referral for further assessment and management.
Outcomes {12}
The cRCT will assess differences in the specified outcome measures between the intervention and control groups, as well as pre- and post-intervention, are listed in Table 2, as per SPIRIT guidance on the time points for measurement of primary and secondary outcomes.
Primary outcome
The study’s primary outcome is school readiness, which will be assessed using the Measurement for Early Learning and Quality of Outcomes (MELQO) framework [27]. For children with more marked developmental difficulties or disability (estimated ~ 5–10%) who may not be able to be tested using the MELQO, the “Paediatric Evaluation of Disability Inventory Computer Adaptive Test (PEDI-CAT) [28]” will be used. The PEDI-CAT is a standardized test that measures ability in three functional domains of daily activities, mobility, and social/cognitive. Normative scaled scores are obtained for children ≥ 6 months of age to provide age-related expectations of ability using an easy to administer tablet application.
Secondary outcomes
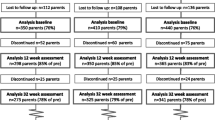
The secondary outcomes of this study encompass a comprehensive evaluation of various domains. Cognitive assessment will be conducted using the Weschler Preschool and Primary Scale of Intelligence IV (WPPSI IV) [29]. Additionally, the study will assess caregiver’s mental health by employing an adapted version of the Hopkins Symptoms Checklist (HSCL25) [30] from Tanzania. Furthermore, visual and hearing assessments will be carried out utilizing PeekVision and a standardized hearing test. The evaluation will extend to family care and quality of life using the Pediatric Quality of Life Inventory (PEDSQL) [31] and PEDICAT. Lastly, child disability will be assessed using the UNICEF and Washington Group CFM [3], providing a comprehensive understanding of the study’s secondary outcomes. Figure 1 illustrates the flowchart of the baseline measurement tests.
Careful consideration has been given to the selection of outcome measures to ensure a comprehensive measurement of the impact of the preschool intervention while considering the length of the appointment for the caregiver and the child. All tools that have not yet been used in the country context will be administered during the pilot period, and necessary adaptations will be made.
Participant timeline {13}
The schedule for enrollment, intervention, and assessment is depicted in Fig. 2. The eligibility of participants from the EN-SMILING cohort children will be confirmed, and their caregivers will be invited to provide consent within 1 week of enrolment. Baseline assessments for both groups will be completed within 3 months following enrollment. Afterward, the clusters will be randomized into intervention and control groups. Trained facilitators will conduct nine parent group intervention sessions over 5 months for the intervention group. Post-intervention assessments will be completed within 2 weeks after completing the last parent group session.
Sample size {14}
Based on the design of our intervention and existing available cohort size, we have an expected sample size of 500 parent–child dyads in each of the sites. We have predetermined approximately 50 clusters per site, with expected cluster size varying between 8 and 12 parent/child dyads. Planning for a 1:1 ratio between the 2 groups, with 25 clusters each in the intervention and control arms of the cRCT.
For the primary objective of school readiness, we will assess differences in overall direct assessment score of the child using the MELQO [27] between the two groups. Expected norms have not been generated; instead, we assumed a mean overall score of 46 in the non-intervention group. This is based on average item level statistics for 84 core items across the 4 domains (pre-numeracy, pre-literacy, executive functioning social-emotional) [32]. Therefore, we will be able to detect a 0.28 standard mean difference between the 2 groups at 80% power, 5% alpha, and an intraclass correlation (ICC) of 0.025 within our sample size of 50 clusters (25 intervention and 25 control) with 10 parent–child dyads in each cluster (N = 500 in each site).
The trial is powered to detect a minimum difference of 13% between the intervention and non-intervention group after the intervention period in pre-numeracy domain and a 12% difference in pre-literacy domain using a two-sided test in which both groups have 25 clusters of size 10, assuming 80% power, a 5% significance level, and an ICC of 0.025. This assumes that 68 and 48% of children in the non-intervention group will have average pre-numeracy and pre-literacy scores, respectively [32].
We contacted 1651 parents of children from the EN-SMILING cohort for the EN-REACH trial study. We enrolled a total of 1445 participants from 160 clusters across three countries, with each cluster typically containing 6–13 parent–child dyads. Specifically, in Bangladesh, there were 560 participants spread across 50 clusters. As a result of geographical challenges in Nepal and Tanzania, we increased the cluster count to 54 in Nepal (enrolling 482 participants) and to 56 in Tanzania (enrolling 403 participants).
Recruitment {15}
Study participants will be recruited from all available children in the EN-SMILING cohort, including parents and their children who were followed up as at-risk newborns, as well as control children from a subset of the EN-SMILING study. Two facilitators—one parent and one teacher—from each intervention cluster will also be recruited from each site.
Assignment of interventions: allocation
Sequence generation {16a}
This cRCT will include have 50 clusters per site, with 25 randomly selected intervention clusters which will receive the preschool parenting package for school readiness and 25 control clusters that will continue to have standard care that is appropriate for each of the sites provided through the health and education sectors, such as access to existing early childhood developmental resources and education programs.
The GIS coordinates for all EN-REACH eligible children will be used to create clusters for randomization. The clusters will be identified based on the geospatial location of the children using a modified K-means clustering algorithm utilizing the Python package “k-means-constrained 0.7.3.” [33]. Visual map of identified clusters to identify areas where the automated algorithm created dispersed clusters, largely due to geographic barriers and manually produce clusters in those areas using local knowledge.
Concealment mechanism {16b}
Allocation will not be concealed and will be revealed to the parents, facilitators, and researchers upon randomization to the intervention team. However, it will not be revealed to the child development assessment team or the statistical analyses team.
Implementation {16c}
Clusters will be grouped through the GIS system consisting of children (whose parents have consented). Once identified, the clusters will be randomized through a computer-generated number list of clusters by a statistician who will not be involved in the trial. The study group will be revealed to participants and those researchers who will be involved in implementing the intervention.
Assignment of interventions: blinding
Who will be blinded {17a}
Participants, facilitators, and the intervention research team will not be blinded. However, the assessment of the children’s study will be performed by a group of enumerators and a researcher from each country who are blinded to group allocation. In addition, the trial statistician will be blinded to which clusters are intervention or control.
Procedure for unblinding if needed {17b}
The trial is a cRCT not an individually randomized trial and cannot be blinded. The trial statistician will be blinded to the cluster allocation.
Data collection and management
Plans for assessment and collection of outcomes {18a}
Quantitative data will be mostly collected using a tablet-based customized application developed for the EN-SMILING study with the icddr,b team, which already tracks each child by unique ID, sets alarms for phone calls and appointments and links the previously collected data from birth. A comprehensive 7-day training session covered measurement tools, with an additional 2 days dedicated to field testing for the assessment team. The data collectors carry tablets to the assessment. Data are sent in real time to the main server in each country site. The app system checks for implausible values based on plausible ranges set in advance. Double data entry (by assessor and supervisor) is practiced for about 5% of cases and analyzed for quality improvement and also to be able to report on inter-assessor reliability. To ensure quality control, interobserver assessments will be conducted on 10 children for each assessor before the measurements began in each country site.
For objective 3, implementation evaluation, we will utilize the RE-AIM framework [23, 24], offering a systematic approach to assess implementation and inform uptake. We will collate quantitative data on the coverage and quality of the intervention provision, along with a qualitative evaluation. Perspectives will be gathered from the child, caregiver, teacher, facility, and community provider. Table 3 presents a summary of planned data collection. Qualitative approaches will be applied to assess feasibility and acceptability of the adapted package, through focus group discussions, in-depth interviews, and observation of participants. In addition, we will perform social mapping of parent networks and in-depth interviews with caregivers and staff on their perspectives and experiences of using the program. Interviews will be conducted by social scientists who have experience in qualitative research. The evaluation will also explore if the perceived value added of the intervention differs for families of children with disabilities compared to their typically developing peers.
Plans to promote participant retention and complete follow-up {18b}
The parents will receive extensive information about the assessment tools, objectives, and the importance of school readiness packages during recruitment for the EN-REACH trial. Comprehensive strategies will be adopted, involving reminder phone calls, home visits, and providing wage loss compensation for caregivers of children with disabilities to significantly enhance their attendance and engagement in the program.
Data management {19}
The three site teams already have several years of local weekly quality assurance meetings, and regular (currently monthly) all-site skype calls with LSHTM to share their local data dashboard tracking loss-to-follow-up, etc. These site teams share data quality solutions with each other. Each site has a designated software and data analyst. LSHTM-based psychologist will coordinate training materials and SOP development. Existing SOPs will be adapted to ensure data quality checks are consistent across sites.
Backup and security
Quantitative data will be transferred from tablets to a main server over secure wifi in each country site, and backed up daily to a local server which complies to an agreed specification with UPS power back up. There will also be a daily back up of cloud data and weekly to a hard drive. Qualitative data will be backed up and secured by each country site at least weekly to a hard drive and to a cloud storage system. All data will be protected by passwords and safe back up hardware. We will comply with LSHTM guidance on Data Management and Archiving https://datacompass.lshtm.ac.uk/468/ which is compatible with UK Data Archive Managing and Sharing Data Guide. Data will remain at the sites and, before being pooled and analyzed, will be fully anonymised.
For qualitative data, cross-site consistency and quality will be maximized by standard tools and a common training guide. Quality control for the qualitative data collection will be assured through focus group discussion training, translation, back translation, recording, and transcribing. The transcripts in English version of each country will be reviewed to see the adherence with framework and the predetermined themes. Where translations are undertaken, quality will be assured by one other researcher fluent in that language checking against the original recording notes. We will describe the experiences of children and caregivers relating to the intervention received, including the impact of the disability, caregiver confidence, level of inclusion in family and community life and experience of stigma/discrimination. We will examine changes in these domains over the follow-up period and explore attributions of change.
Confidentiality {27}
All information collected about caregivers, their children, and relevant respondents during the study will be kept confidential and not shared with anyone outside the study team. Anonymization of data will be carried out to maintain participant confidentiality. The data will be coded so that the personal identity and individual data from the follow-up review are traceable only with the code key which will be held by the study researchers, no one else will have access to it.
Plans for collection, laboratory evaluation, and storage of biological specimens for genetic or molecular analysis in this trial/future use {33}
Not applicable. We will not collect any biological specimens from the participants.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}
Objective 2 IMPACT EVALUATION
Quantitative analysis will be performed using Stata 15, R, and R studio. Descriptive summary statistics (means and standard deviations-SD, medians and inter-quartile ranges, numbers and percentages) by country and for each treatment arm will be used to describe the study population.
Primary analysis will be by intention to treat. For each outcome, analysis will be done at the parent/child dyad level but will be adjusted for clustering at the country and residential area/village level. We will adjust for child age and sex, neonatal intervention received, and relevant baseline scores. Additional baseline covariates, including markers of socioeconomic, will be considered in final model based on existing literature and using a stepwise selection approach. Mixed-effects regression models will be used to compare continuous (linear) and binary (logistic) outcomes between the two arms. Generalized Estimating Equations (GEE) methods may also be considered if the assumptions of mixed-effect models are not met. The mixed-effect regression models and GEE account for within-cluster correlation.
Objective 3: Implementation evaluation
Inductive thematic analysis will be undertaken using Nvivo 12 software to explore the needs identified through qualitative sources related to the intervention’s protocol, the consistency of delivery as intended, and the time required. This analysis will also encompass any adaptations made and an overall assessment of feasibility, incorporating quantitative data from quality checklists (% of correct modules delivered and accurate time taken), as well as identifying enablers and barriers to implementing the program.
Interim analyses {21b}
No interim analysis will be conducted.
Methods for additional analyses (e.g. subgroup analyses) {20b}
We will perform a per protocol analysis after the intervention is complete. We will define high fidelity as caregivers who attended all nine sessions of the parent-group intervention. We will perform subgroup analyses per country based on gender, socioeconomic status, and parental education.
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data {20c}
Protocol non-adherence may occur if a participant did not attend all the education sessions or completed primary outcome assessment outside of the study window, which will be documented and considered in the analysis. The primary intention-to-treat analysis allows for missing data under the missing at-random assumption. We will also do a sensitivity analyses, restricting to complete case population and explore handling of missing data using inverse probability weighting.
Plans to give access to the full protocol, participant-leveldata, and statistical code {31c}
We are committed to open access, with a transparent protocol, and will share relevant data and code as appropriate. Data will be owned by each country institution and pooled data by the study team. We have a long track record since 2017 of equitable partnership between these three country teams and LSHTM with data sharing agreements already in place. We have already established a committee including the principal investigator (PI) and each site PI to review any requests for data beyond the original team. Authorship and analyses will be led by country teams and agreed in advance.
Oversight and monitoring
Composition of the coordinating centre and trial steering committee {5d}
The trial management group overall consists of the joint PIs (JEL and JDH), the three site PIs, the project coordinator, the site coordinators, and the site data leads. This group meets virtually every month and has scientific responsibility for the study, discussing adherence to the protocol, quality of data collection, and participant safety. In addition, the local teams will meet frequently. The two joint PIs will be responsible for dealing with trial quality and the data.
Composition of the data monitoring committee, its role and reporting structure {21a}
We will convene an independent EN REACH Technical Advisory Committee (TAG)/ Data Monitoring Committee who will meet approximately every 6 months and be chaired by Professor Maureen Black, Distinguished Fellow at the University of Maryland. The committee will provide insights on design, overall safety, implications of results, and pathways to uptake.
Adverse event reporting and harm {22}
The study involves an intervention and assessing children to detect disabilities, but it is considered “low risk” with no anticipated adverse events. Given that our research will identify children with NDD/D SOP for referral and follow-up within existing health, education, and social services will be developed. It is expected that some children with developmental delay and/or impairment will be identified for the first time during this study. Further, given the use of screening tools that are sensitive, there is also a risk of over diagnosis. These situations may be distressing to families and without adequate follow-up care, potentially harmful. To prepare for this, health worker training will include supportive communication approaches with families, aiming to minimize parent concern. Where problems are identified, health workers will complete referral (local government health, education, and other support services), supported by SOP for further assessment and management. This training will include identification of criteria for referral and information on local pathways for referral, and provision of standardized documentation regarding screening and assessment findings for “receiving” referral health workers. Intervention services in the 3 countries, do exist, although limited, and where a need is identified, children will be referred for further assessment and management. In addition, health worker, teacher, and expert parent training will include an overview of simple strategies to support and promote child development. Intervention facilities have been identified and mapped in the respective countries and this information will be used for referrals. Adverse events will be monitored, and serious adverse events will be reported to the PI in a timely manner.
Frequency and plans for auditing trial conduct {23}
Trial conduct will be closely monitored by independent subgroups of each country and the Institutional Review Board (IRB) of each country and TAG.
Plans for communicating important protocol amendments to relevant parties (e.g., trial participants, ethical committees) {25}
Protocol amendments will be communicated to the ethics committees of each site, as well as IRB of LSHTM, the trial register, and TAG.
Dissemination plans {31a}
The research report will be produced based on the study findings and will be presented at scientific conferences. Additionally, the manuscript will be published in international, peer-reviewed journals. Dissemination workshops will be organized with the collaboration of the Ministry of Primary Education, the Ministry of Health and Family Welfare, the Ministry of Social Welfare, and stakeholders in ECCE/PPE. We aim to conduct at least two dissemination meetings: one at the district level of each site, where we will inform the district education officers, and the other at the central level of each country to invite high officials from the education ministry.
Discussion
The EN-REACH trial presented in this protocol is developing an innovative, disability-inclusive, play-based school readiness package for parents and their children, considering the contexts of Bangladesh, Nepal, and Tanzania. The trial has a robust cRCT design and embedded implementation evaluation, and aims to address the gap for a feasible package for disability inclusive education in LMICs contexts.
There is evidence to support our approach, yet few feasible packages exist for this school entry age group of children. The United Nations Convention on the Rights of the Child [7] and the Convention on the Rights of Persons with Disabilities [8] emphasize that each child possesses the right to reach their full developmental potential, noting the imperative for governments to ensure that young children with disabilities receive high-quality education [34,35,36]. Reviews indicate that psychosocial interventions for children under 3 years old can have shown short-term improvements in cognition and language [37,38,39,40], with additional benefits observed in math and language among preschoolers in India [41,42,43]. Despite the ambitious SDGs for child survival, development, and education, the gap between policy and effective implementation of inclusive ECD and pre-primary education programs is evident in LMICs [34, 44]. Our 9-session intervention is co-designed in three LMIC and planned to be delivered over approximately 9 weeks. The content is adapted from UNICEF and others to focus on foundational skills for children’s health, and emotional wellbeing as well as on introducing pre-academic literacy and numeracy skills. Moreover, the program promotes positive parenting techniques, problem-solving, and safeguarding measures, with customized play activities tailored for all children, including disabled children utilizing local, low-cost play-based learning materials that can be used to support the acquisition of early literacy foundation skills [45].
The multi-country design and large cohort of children enables a rigorous evaluation through at least 150 clusters in a cRCT to measure the effectiveness of the intervention. A strength of the trial is this robust design aiming to inform policymakers on impact and hence catalyze uptake. To evaluate the realities of implementation, we will use and adapt RE-AIM [23, 24], which is widely used approach to assess barriers and enablers to uptake and sustainability. Embedded in the cRCT will be both qualitive and qualitative process evaluation.
Another strength is the codesign of the intervention across three countries and with many constituents and experts from health, education, and including local government. Inclusive partnerships have been forged with key entities such as the Directorate of Primary Education (DPE) and the Ministry of Primary and Mass Education in Bangladesh, the Ministry of Education, Science & Technology in Nepal, and the Ministry of Education, Science, and Technology in Tanzania.
While conducting the trial using the existing EN-SMILING cohort presents an opportunity, challenges arise from the scattered locations of the children’s households, making it difficult to create clusters due to the distance between the children’s home and school locations. To address this issue and minimize travel time and hassles, GIS data for all households of EN-REACH eligible children and primary schools will be utilized. In areas with geographic barriers, clusters will be manually identified using local knowledge as intervention points to overcome these challenges.
Another challenge that this geographic dispersion poses is the distance some families may have to travel. This is a notable barrier especially for those with children with disabilities. Specifically, we will offer travel cost reimbursement and compensation for parents of disabled children to encourage their participation in the sessions. Additionally, information on accessing facilities such as stipends and assistive devices from the Ministry of Social Welfare office and other organizations will be collected, and we will provide guidance as a support mechanism.
Data generated from this intervention trial will inform intervention design and implementation for inclusive ECD programs across a diverse range of LMIC settings. It will provide important information on integration of such interventions into current government programs and most importantly will reflect the needs from communities and the families of these children inclusive of those with children with disabilities.
Trial status
The protocol version is 3.0, which is approved on 20.02.2023 by the LSHTM ethics committee. Participants’ enrolment for EN-REACH trial commenced on 26.01.2023 at the site in Bangladesh because ethical approval from the IRB of icddr,b was received on 20.12.2022, with protocol version 2.0. Participants’ enrollment closed on 27.05.2023 in Nepal and on 18.08.2023 at Tanzania site. Two of the three sites are in the process of conducting child assessments and session visits, while one site has just completed them. No data have been received or analyzed yet. The trial is retrospectively registered on clinicaltrials.gov, and the identifier is NCT06334627 (https://clinicaltrials.gov/study/NCT06334627?cond=NCT06334627&rank=1). Obtaining ethical approval from the IRBs of each country is not required to conduct the study from an official registry. However, the study design, consent form, and measures were approved by local IRBs in each country. The trial has been closely monitored by both IRBs in each country and an independent TAG committee to ensure adherence to ethical guidelines.
Availability of data and materials {29}
The PI (Joy Lawn) and Co-PIs from each country will have access to the trial data. The dataset generated from the trial will be made available upon responsible request from https://datacompass.lshtm.ac.uk/.
Change history
09 September 2024
A Correction to this paper has been published: https://doi.org/10.1186/s13063-024-08445-7
Abbreviations
- CFM:
-
Child functioning module
- cRCT :
-
Cluster randomized controlled trial
- ECCE:
-
Early childhood care and education
- ECD :
-
Early child development
- EN-BIRTH :
-
Every newborn- birth indicator research tracking in hospitals
- EN-REACH :
-
Every newborn- reach up early education intervention for all children
- EN-SMILING :
-
Every newborn – simplified measurement integrating longitudinal neurodevelopment and growth
- GEE :
-
Generalized Estimating Equations
- GIS:
-
Geographical information system
- HSCL :
-
The Hopkins symptoms checklist
- ICDDR,B:
-
International centre for diarrhoeal diseases research, Bangladesh
- ICC :
-
Intraclass correlation
- IRB:
-
Institutional review board
- LMIC :
-
Low- and middle-income countries
- LSHTM :
-
London school of hygiene and tropical medicine
- MRC :
-
Medical research council
- MELQO :
-
Measurement for early learning and quality of outcomes
- NDD/D :
-
Neuro-developmental developmental delays and disabilities
- PEDI-CAT :
-
The Paediatric Evaluation of Disability Inventory—Computer Adaptive Test
- PEDQL :
-
Pediatric Quality of Life Inventory
- PI:
-
Principal investigator
- PPE:
-
Pre-primary education
- RE-AIM:
-
Reach, effectiveness, adoption, implementation, and maintenance
- SDG :
-
Sustainable development goals
- SOP:
-
Standard operating procedures
- TAG:
-
Technical Advisory Committee
- WPPSI :
-
Weschler preschool and primary scale of intelligence
References
United Nations. Transforming our world: the 2030 Agenda for Sustainable Development United Nations. New York: Department of economic and social affairs. Division for sustainable development goals; 2015.
UNİCEF. A brief overview: inclusive education for children with disabilities in the CEECIS Region. Geneva: UNICEF; 2010.
UNICEF, WG. The Washington Group / UNICEF Child Functioning Module (CFM) – Ages 5-17 years. 2016. https://www.washingtongroup-disability.com/question-sets/wg-unicef-child-functioning-module-cfm/.
Olusanya BO, Davis AC, Wertlieb D, Boo N-Y, Nair M, Halpern R, et al. Developmental disabilities among children younger than 5 years in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Glob Health. 2018;6(10):e1100–21.
Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B, et al. Developmental potential in the first 5 years for children in developing countries. The lancet. 2007;369(9555):60–70.
World Health Organization, UNICEF, World Bank. Nurturing care for early childhood development: a framework for helping children survive and thrive to transform health and human potential. 2018.
UNICEF. Convention on the Rights of the Child. New York: United Nations. 1989. Available from: https://www.unicef.org/child-rights-convention/convention-text.
United Nations. Convention on the rights of persons with disabilities New York: UN. 2006. Retrieved from www.un.org/disabilities.
Milner KM, Salazar RB, Bhopal S, Brentani A, Britto PR, Dua T, et al. Contextual design choices and partnerships for scaling early child development programmes. Arch Dis Child. 2019;104(Suppl 1):S3–12.
Boggs D, Milner KM, Chandna J, Black M, Cavallera V, Dua T, et al. Rating early child development outcome measurement tools for routine health programme use. Arch Dis Child. 2019;104(Suppl 1):S22–33.
Bangladesh. Ministry of women and children affairs. National Children Policy 2011, Dhaka: ministry of women and children affairs; 2011. http://ecd-bangladesh.net/document/documents/National-Children-Policy-2011-English-04.12.2012.pdf. Accessed 12 Nov 2023.
Hamadani JD, Tofail F, Huda SN, Alam DS, Ridout DA, Attanasio O, et al. Cognitive deficit and poverty in the first 5 years of childhood in Bangladesh. Pediatrics. 2014;134(4):e1001–8.
Abbott-Shim M, Lambert R, McCarty F. A comparison of school readiness outcomes for children randomly assigned to a head start program and the program’s wait list. J Educ Stud Placed Risk. 2003;8(2):191–214.
Bustamante AS, White LJ, Greenfield DB. Approaches to learning and school readiness in head start: applications to preschool science. Learn Individ Differ. 2017;56:112–8.
Smythe T, Almasri NA, Moreno Angarita M, Berman BD, Kraus de Camargo O, Hadders-Algra M, et al. The role of parenting interventions in optimizing school readiness for children with disabilities in low and middle income settings. Front Pediatr. 2022;10:1072.
Powell DR, Son S-H, File N, San Juan RR. Parent–school relationships and children’s academic and social outcomes in public school pre-kindergarten. J Sch Psychol. 2010;48(4):269–92.
Scott-Little C, Kagan SL, Frelow VS. Conceptualization of readiness and the content of early learning standards: the intersection of policy and research? Early Childhood Res Quart. 2006;21(2):153–73.
Lynch P, Soni A. Widening the focus of school readiness for children with disabilities in Malawi: a critical review of the literature. Int J Incl Educ. 2021:1–15. https://doi.org/10.1080/13603116.2021.1965801.
UNICEF. School readiness: a conceptual framework. New York: UNICEF; 2012.
Mathwasa J, Sibanda L. Inclusion in Early Childhood Development Settings: A Reality or an Oasis. Education in Childhood. 2021. https://doi.org/10.5772/intechopen.99105.
Özler B, Fernald LC, Kariger P, McConnell C, Neuman M, Fraga E. Combining pre-school teacher training with parenting education: a cluster-randomized controlled trial. J Dev Econ. 2018;133:448–67.
Day LT, Ruysen H, Gordeev VS, Gore-Langton GR, Boggs D, Cousens S, et al. “Every Newborn-BIRTH” protocol: observational study validating indicators for coverage and quality of maternal and newborn health care in Bangladesh, Nepal and Tanzania. J Global Health. 2019;9(1):010902.
Glasgow RE, Harden SM, Gaglio B, Rabin B, Smith ML, Porter GC, et al. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health. 2019;7: 64.
Glasgow RE, Estabrooks PA, Ory MG, Characterizing evolving frameworks: issues from Esmail, et al. review. Implement Sci. 2020;2020(15):1–3.
Yousafzai AK, Aboud FE, Nores M, Kaur R. Reporting guidelines for implementation research on nurturing care interventions designed to promote early childhood development. Ann N Y Acad Sci. 2018;1419(1):26–37.
Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7.
UNESCO, UNICEF, Brookings Institution, World Bank. Overview Measuring Early Learning Quality and Outcomes. 2017. Report No.: ISBN 9789231002205. https://www.brookings.edu/wp-content/uploads/2017/06/melqo-measuring-early-learning-quality-outcomes.pdf . Accessed 20 Jan 2024.
Haley SM, Coster WJ, Dumas HM, Fragala-Pinkham MA, Kramer J, Ni P, et al. Accuracy and precision of the Pediatric Evaluation of Disability Inventory computer-adaptive tests (PEDI-CAT). Dev Med Child Neurol. 2011;53(12):1100–6.
Wechsler D. Wechsler preschool and primary scale of intelligence—fourth edition. TX: The Psychological Corporation San Antonio; 2012.
Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behav Sci. 1974;19(1):1–15.
Varni JW, Sherman SA, Burwinkle TM, Dickinson PE, Dixon P. The PedsQL family impact module: preliminary reliability and validity. Health Qual Life Outcomes. 2004;2: 55.
Pushparatnam A, Luna Bazaldua DA, Holla A, Azevedo JP, Clarke M, Devercelli A. Measuring early childhood development among 4–6 year olds: the identification of psychometrically robust items across diverse contexts. Front Public Health. 2021;9:17.
Levy-Kramer J. k-means-constrained. 0.7.3. Available from: https://pypi.org/project/k-means-constrained/. Accessed 2 Nov 2022.
Black MM, Walker SP, Fernald LC, Andersen CT, DiGirolamo AM, Lu C, et al. Early childhood development coming of age: science through the life course. Lancet. 2017;389(10064):77–90.
Lu C, Black MM, Richter LM. Risk of poor development in young children in low-income and middle-income countries: an estimation and analysis at the global, regional, and country level. Lancet Glob Health. 2016;4(12):e916–22.
McGregor G, Cheung Y, Cueto S, Glewwe P, Richter L, Strupp B. International child development steering group: Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369:60–70.
Aboud FE, Yousafzai AK. Global health and development in early childhood. Annu Rev Psychol. 2015;66:433–57.
Attanasio OP, Fernández C, Fitzsimons EO, Grantham-McGregor SM, Meghir C, Rubio-Codina M. Using the infrastructure of a conditional cash transfer program to deliver a scalable integrated early child development program in Colombia: cluster randomized controlled trial. BMJ. 2014;349:g5785.
Jeong J, Franchett EE, Ramos de Oliveira CV, Rehmani K, Yousafzai AK. Parenting interventions to promote early child development in the first three years of life: a global systematic review and meta-analysis. PLoS Med. 2021;18(5): e1003602.
Hamadani JD, Mehrin SF, Tofail F, Hasan MI, Huda SN, Baker-Henningham H, et al. Integrating an early childhood development programme into Bangladeshi primary health-care services: an open-label, cluster-randomised controlled trial. Lancet Glob Health. 2019;7(3):e366–75.
Meghir C, Attanasio O, Jervis P, Day M, Makkar P, Behrman J, et al. Early stimulation and enhanced preschool: a randomized trial. Pediatrics. 2023;151:151 Supple 2.
Dillon MR, Kannan H, Dean JT, Spelke ES, Duflo E. Cognitive science in the field: a preschool intervention durably enhances intuitive but not formal mathematics. Science. 2017;357(6346):47–55.
Ganimian AJ, Muralidharan K, Walters CR. Augmenting state capacity for child development: Experimental evidence from India. J Pol Econ. 2024;132(5):1565–602. https://doi.org/10.1086/728109.
UNICEF. UNICEF programme guidance for early childhood development. 2017.
UNICEF, Lego Foundation. Learning through play strengthening learning through play in early childhood education programmes. 2018. https://www.unicef.org/sites/default/files/2018-12/UNICEF-Lego-Foundation-Learning-through-Play.pdf.
Acknowledgements
Most importantly we express our gratitude to the parents and children who participated in the EN-BIRTH study and subsequent EN-SMILING cohort. We acknowledge Md. Mahfuzur Rahman Jewel, Shamsun Naher, and Md. Shariful Islam from the Directorate of Primary Education under the Ministry of Primary and Mass Education, Government of Bangladesh, Devina Pradhananga from the Ministry of Education, Science, and Technology in Nepal and Abdul Buheti from the District Education Officer in the Temeke District, Dar-es-Salaam, Tanzania for their permission, support, and oversight. We would like to extend our sincere gratitude to the TAG of the EN-REACH study, with special appreciation to Maureen Black, Professor and Distinguished Fellow at the University of Maryland, the chair. The committee also includes esteemed members: Boniface Kakhobwe, ECD Specialist at UNICEF Headquarters; Nafisa Baboo, Director of Inclusive Education at LIGHT FOR THE WORLD in Cape Town, South Africa; and Tracey Smythe, Professor in Disability and Global Health at LSHTM, as well as Hannah Boycott, the MRC funder representative. Additionally, we express our thanks to all IRBs listed in Additional file 1 for their thorough evaluation and ethical approval of the EN-REACH and EN-SMILING protocols.
Group authorship (for manuscripts involving a collaboration group):
EN-REACH Collaborative Group (all to be included on PubMed):
Bangladesh: Mohammad Abdul Awal Miah, Shafiqul Ameen, Ismat Ara Mili, AKM. Tanvir Hossain, Asraful Alam, MD. Ziaul Haque Shaikh, Md. Nazmul Hasan, Mst Salma Khatun, Ms Adori Khatun, Monira Aktar, Atique Iqbal Chowdhury, Ahmed Ehsanur Rahman, Shams El Arifeen, and Jena Derakhshani Hamadani
Nepal: Rejina Gurung, Omkar Basnet, Chudamani Poudel, Basanta Prasad Koirala, Shova Kumari Adhikari, Arjun Dhakal, Dhanasudhan Chaulagain, Prof. Bharat Khatri, Dr Ram Chandra Bastola and Ashish KC
Tanzania: Nahya Salim Masoud, Mustafa Miraji, Donat Shamba, Josephine Shabani, Mohamed Bakari, Hajra Kizibo, Mohamed Akida, Aisha Mfinanga, Hellena Mariki, Ramadhani Gunda, Seif Bakari, and Karim Manji
UK: Jaya Chandna, Proma Paul, Kate Mackinnon Milner, Rachel Lassman, Maria Zurmond, Cally Tann, Paul Lynch, and Joy E Lawn
Funding
The EN-REACH is funded by the UK Medical Research Council (MRC) Global Health Research Board (MR/V035274/1), JEL as the PI and leveraged funding in Bangladesh through Stiftung Auxilium (Grant # GR-075636), JDH as PI. The funders have no role in the intervention design or implementation.The original EN-BIRTH study and set-up of the EN-SMILING cohort was funded by the Children's Investment Fund Foundation (CIFF) as a grant to LSHTM, with JEL as the PI.
Author information
Authors and Affiliations
Consortia
Contributions
All authors reviewed the protocol paper considering their areas of expertise and the local context of the EN-REACH multi-county trial. JEL and JDH led the intervention and trial design and will provide overall guidance, overseeing, implementation, process evaluation, and the data management. JC is responsible for ensuring training, day-to-day coordination, and spot checks on backup and other quality control activities. PL, JC, CT, and MAAM will be responsible for intervention co-design, and organizing intervention and measurement training. MAAM, RJ, and MM will be in charge of contextualizing the intervention package with the lead PI in each site (NS, KM, AKC, JDH). IM, OB, and NSM will be responsible for adapting measurement tools and training for the enumerators. AIC, AA, and PP will be in charge of coordinating the creation of the GIS system and randomization of clusters. TH and PP will be responsible for EN-REACH software application development and coordination with all country data management teams. JDH, SEA, SA, AER, MAAM, NSM, KM, MM, AKC, RJ, and OB will be responsible for local data collection and implementation of the protocol, including quality control at each site.
Corresponding author
Ethics declarations
Ethics approval and consent to participate {24}
For EN-REACH, ethics approval is obtained from LSHTM and each country’s relevant IRBs. The following procedures are proposed to ensure that research conduct complies with the protocols set out and agreed upon in applications to the LSHTM Research Ethics Committee and the above IRBs for EN-REACH study trials, as listed in Additional file 1.
Consent for publication {32}
Not applicable, as no identifying images or personal/clinical details of participants are shown here, nor will they be included in the trial results reports.
Competing interests {28}
The authors declare that they have no known competing financial conflicts or personal relationships that could have influenced the research in this protocol paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ashish KC, Karim Manji, Paul Lynch, Joy E Lawn, and Jena Derakhshani Hamadani shared senior authorship.
The original online version of this article was revised: Following the publication of the original article, we were notified that administrative box under section Author details {5a} was not adjusted as per proof feedback. Incorrect sequence: 6. School of Public Health and Community Medicine, University of Gothenburg, Sweden 7. School of Education, University of Glasgow, Glasgow, Scotland. Correct sequence: 6. Murdoch Children’s Research Institute, Department of Paediatrics, University of Melbourne, Melbourne, Australia 7. School of Public Health and Community Medicine, University of Gothenburg, Gothenburg, Sweden 8. School of Education, University of Glasgow, Glasgow, Scotland.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Miah, M.A.A., Chandna, J., Gurung, R. et al. Every Newborn-Reach Up Early Education Intervention for All Children (EN-REACH)- a parent group intervention for school readiness in Bangladesh, Nepal, and Tanzania: study protocol for a cluster randomized controlled trial. Trials 25, 556 (2024). https://doi.org/10.1186/s13063-024-08381-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-024-08381-6
Keywords
- School readiness
- Parenting group intervention
- Early child development (ECD)
- Pre-primary education
- Neuro-developmental delay
- Disabilities
- Cluster randomized controlled trial (cRCT)
- Measurement for early learning and quality of outcomes (MELQO)
- Weschler preschool and primary scale of intelligence (WPPSI)
- Newborn