Abstract
Background
Although regulatory changes towards correcting the underrepresentation of women in randomized controlled trials (RCTs) occurred (National Institutes of Health 1994), concerns exist about whether an improvement is taking place. In this systematic review and meta-analysis, we aimed to assess the inclusion rates of women in recent RCTs and to explore the potential barriers for the enrollment of women.
Methods
RCTs published in 2017 examining any type of intervention in adults were searched in PubMed and Cochrane Library. The following predefined medical fields were included: cardiovascular diseases, neoplasms, endocrine system diseases, respiratory tract diseases, bacterial and fungal infections, viral diseases, digestive system diseases, and immune system diseases. Studies were screened independently by two reviewers, and an equal number of studies was randomly selected per calendric month. The primary outcome was the enrollment rate of women, calculated as the number of randomized women patients divided by the total number of randomized patients. Rates were weighted by their inverse variance; statistical significance was tested using general linear models (GLM).
Results
Out of 398 RCTs assessed for eligibility, 300 RCTs were included. The enrollment rate of women in all the examined fields was lower than 50%, except for immune system diseases [median enrollment rate of 68% (IQR 46 to 81)]. The overall median enrollment rate of women was 41% (IQR 27 to 54). The median enrollment rate of women decreased with older age of the trials’ participants [mean age of trials’ participants ≤ 45 years: 47% (IQR 30–64), 46–55 years: 46% (IQR 33–58), 56–62 years: 38% (IQR 27–50), ≥ 63 years: 33% (IQR 20–46), p < 0.001]. Methodological quality characteristics showed no significant association with the enrollment rates of women. Out of the 300 included RCTs, eleven did not report on the number of included women. There was no significant difference between these studies and the studies included in the analysis.
Conclusions
Women are being inadequately represented, in the selected medical fields analyzed in our study, in recent RCTs. Older age is a potential barrier for the enrollment of women in clinical trials. Low inclusion rates of elderly women might create a lack of crucial knowledge in the adverse effects and the benefit/risk profile of any given treatment. Factors that might hinder the participation of women should be sought and addressed in the design of the study.
Similar content being viewed by others
Introduction
Population external validity of a randomized controlled trial (RCT) is defined as the extent to which the results of a trial can be generalized from a specific sample to a target population [1]. For many years, the population external validity of RCTs was compromised due to the inclusion of mainly male participants, while women were underrepresented [2, 3]. When applying gender-unbalanced RCTs to real-life clinical settings, concerns arise as treatment dosing and effects may not be similar between the predominantly male RCT population and women patients [2, 3]. Drug effects may vary between the sexes according to body composition and size and pharmacokinetic or pharmacodynamic parameters [3, 4]. This may lead to inappropriate dosing and inaccurate estimation of side effects in women and, ultimately, to overall less qualitative patient care and suboptimal clinical treatment outcomes for women patients.
The most significant change in the US Food and Drug Administration’s (FDA) regulations towards correcting the underrepresentation of women occurred in 2000. This particular regulation permits the FDA to place a clinical hold on investigational new drug studies for the treatment of a serious or life-threatening disease if women or men are excluded from a clinical trial due to reproductive potential [5]. This change was reinforced by an audit performed in 2001 by the US Government Accountability Office, which found that eight out of ten drugs withdrawn from the US market between 1997 and 2001 had more severe adverse events in women than in men, largely because these drugs were not sufficiently tested on women [6]. Another significant milestone is the EU Clinical Trials Regulation No 536/2014. This regulation lists specific population groups that are likely to use the investigated medicinal product, to be included in the clinical trial. This new legal addition contains provisions for including pregnant and breastfeeding women in clinical trials [7].
Pivotal trials and studies from several medical fields (cardiovascular diseases, HIV, stroke, and cancer) show that the change put forward by these regulations has yet to come [8,9,10,11,12,13,14,15,16]. There is a gap in information for many major health burden medical conditions. This has led us to perform a systematic review and meta-analysis of the literature to assess the inclusion rates of women in recently published randomized controlled trials and to further explore the potential barriers to enrollment of women.
Methods
We included randomized controlled trials in the following Medical Subject Headings (MESH) categories: cardiovascular diseases, neoplasms, endocrine system diseases, respiratory tract diseases, bacterial and fungal infections, viral diseases, digestive system diseases, and immune system diseases. These areas were chosen due to their major health burden in terms of disability and death [17]. We included any type of intervention in adults (age ≥ 18 years).
We conducted a comprehensive search to identify all RCTs published during 2017 in PubMed and Cochrane Library. Our full search phrase is presented in Additional file 1: Appendix 1. Out of 26,994 identified records, we have randomly selected and screened 1098 records. The function “RAND” in Excel was used, a unique random number was assigned to each trial, numbers were sorted from smallest to largest. An equal number of studies per calendric month was reviewed by two investigators (AT, IP). Records were excluded if they were duplicates, not RCTs, not written in English, included patients under the age of 18, and if they examined a sex-specific condition. Sex-specific condition was defined as a condition that occurs only in people of one sex, such as prostate cancer, ovarian cancer, pregnancy and delivery-related conditions, endometriosis, polycystic ovary syndrome, and bacterial vaginosis.
For each included study, we extracted data on the main disease or disorder, funding, patient characteristics (age, gender), hypothesis, intervention type, setting, countries (developed and developing economies), centers, study duration, follow-up duration, number of screened patients, number of randomized patients, methodological characteristics (allocation concealment, blinding), and study endpoints (soft, surrogate or hard outcome, outcome of primary hypothesis).
Country classifications to developed and developing economies were performed according to the United Nations’ “World Economic Situation and Prospects 2022” statistical annex [18].
Soft outcomes were defined as patient-reported outcomes and symptomatology. Surrogate outcomes were defined as a laboratory measure or physical sign that is intended to be used as a substitute for a clinical endpoint that matters to patients. Hard outcomes were defined as acute coronary syndrome and stroke, pathological diagnosis, overall survival, and mortality [19].
The risk of bias was assessed by both investigators according to the Cochrane Handbook for Systematic Reviews of Interventions [20].
Our primary outcome was the enrollment rate of women, calculated as the number of randomized women patients divided by the total number of randomized patients.
Statistical analysis
Analysis was performed using the Statistical Package for the Social Sciences 27 (SPSS Inc.).
Data are presented as percentages for categorical variables, and as median and interquartile range (IQR, 25–75 percentiles) for non-normally distributed continuous variables. Associations between median enrollment rates and trial characteristics, and median enrollment rates and medical conditions were tested in a univariate analysis. Categorical data were compared using the chi-square test. For the meta-analysis, we weighted rates by the inverse variance. Statistical significance was tested using general linear models (GLM).
Results
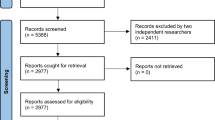
Out of 398 RCTs (~33 each month) assessed for eligibility, 300 RCTs were included in this systematic review and meta-analysis (Fig. 1).
The enrollment rate of women in all the examined diseases was lower than 50%, except for immune system diseases. The overall median enrollment rate of women was 41% (IQR 27–54; n = 289). In Table 1, we present the median enrollment rates of women in trials examining different medical conditions. The lowest median enrollment rate of women was in trials examining ischemic heart disease [22% (IQR 17–33); n = 17]. Studies examining immune system diseases had the highest proportion of women [68% (46–81); n = 12]. Many of the trials (by field) recruited less than 40% women: HIV/AIDS [33% (IQR 9–57); n = 13], bacterial and fungal infections [37% (IQR 25–49); n = 24], congestive heart failure [34% (IQR 25–53); n = 14], hypertension [38% (19–52); n = 11], liver disease [35% (IQR 26–46); n = 12], neoplasms [39% (IQR 28–47); n = 53], type 1 diabetes [37% (IQR 14–59); n = 4], and respiratory tract diseases [39% (IQR 20–60); n = 28].
Trial characteristics and their association with median enrollment rates of women are presented in Table 2. The median enrollment rate of women decreased with older age of the trial’s participants [mean age of trials’ participants ≤ 45 years: 47% (IQR 30–64); n = 69, 46 to 55 years: 46% (IQR 33–58); n = 71, 56 to 62 years: 38% (IQR 27–50); n = 76, ≥ 63 years: 33% (IQR 20–46); n = 67, p < 0.001]. Studies testing hard primary outcomes had a lower enrollment rate than trials examining soft outcomes [35% (IQR 26–47); n = 34, compared with 43% (26–59); n = 130, p ≤ 0.001]. Trials of invasive interventions included significantly fewer women than those of non-invasive interventions [31% (IQR 22–46); n = 55, compared with 42% (29–58); n = 234, p < 0.001]. A third of the trials investigating invasive interventions (20/55) were in the field of cardiovascular diseases. There was no significant difference in the median enrollment rate of women between developed and developing countries [39% (IQR 25–54); n = 170, compared with 41% (IQR 28–54); n = 86, p = 0.448].
Methodological quality characteristics (allocation concealment, blinding of participants, blinding of trial personnel) showed no significant association with the median enrollment rates of women.
Out of the 300 included RCTs, eleven did not report on the number of included women. The characteristics of these studies are presented in Additional file 3: Table S1. There was no significant difference between these studies and the studies included in the analysis.
Discussion
The overall median enrollment rate of women was 41%. Older age of study participants, invasive interventions (mostly in trials assessing cardiovascular diseases), and studies with hard primary outcomes were related to lower women’s enrollment rates. Methodological quality characteristics showed no significant association with median enrollment rates of women. The association between older age and lower enrollment rates of women has been previously described by Vitale et al. [21, 22]. This population of older women represents a large proportion of real-world drug and treatment recipients. Unfortunately, their underrepresentation creates an absence of crucial data for the estimation of the interventions’ safety, adverse events, and real-world effectiveness [21, 22].
When looking specifically at women’s enrollment rates by medical conditions, the underrepresentation of women in cardiovascular diseases, HIV, stroke, and cancer is in line with the findings published in previous systematic reviews in these fields [10,11,12,13,14,15,16]. However, we have observed low enrollment rates of women in trials for type 1 diabetes mellitus and bacterial and fungal infections. We have looked at several large cohort studies in these fields which reflect the target populations. In the Pittsburgh Epidemiology of Diabetes Complications Study, which included a large cohort of young US adults with type 1 diabetes mellitus, the proportion of women was 49% (compared to a median of 37% in the trials included here) [23]. Another large cohort included 4306 clinically diagnosed adult patients with type 1 diabetes mellitus attending the outpatient clinic at Steno Diabetes Center in Gentofte, Denmark, from 2001 to 2013. The proportion of women was 46% in this cohort and 42% in the validation cohort for this study (n = 2118) [24].
We also examined large observational cohorts in the field of bacterial and fungal infections covering most of the topics included in our systematic review: hospital-acquired surgical site infection (SSI) [25], Clostridioides difficile infection (CDI) [26], catheter-associated urinary tract infection (CA UTI) [27], and neutropenic fever (NF) [28]. The percentage of women in these cohorts was 42–54%, higher than the rate we observed in randomized trials (37%).
Explanations suggested for low recruitment rates of young women in RCTs were fluctuations in the female hormones, which could affect the outcome of the intervention, adding more variability to the data, and concerns regarding exposing women with reproductive potential, pregnant or lactating, to experimental drugs [3]. Moreover, including a woman with child-bearing potential in a study usually requires sampling of serum or urine for β-hCG and conducting a contraception check, which complicates the recruitment process, skewing recruitment towards men. For post-menopausal women, one explanation for low recruitment rates is that women suffer more from dementia [29], which might complicate the consent procedures.
There are potentially other, unmeasured characteristics that could account for this gap, especially ones that are related to cultural barriers: low literacy levels compared to men (interfering in the informed consent process), modesty, the fear of stigma that causes women to seek less help, or discrimination in health care service utilization [29]. Another explanation could be that in different regions, especially in developing countries, women are not the predominant decision-makers in matters concerning their health [30].
A limitation of our study is the exclusion of non-English papers (n = 10). In these trials, women’s enrollment rates might be even lower. Another limitation is that we did not collect data on women’s retention rates from RCTs. In some studies, women were more likely to prematurely discontinue the study drug and withdraw consent from the trial compared to men [31, 32]. It could be of value to study women’s retention rates from RCTs and to demonstrate the rates of missing information for this outcome.
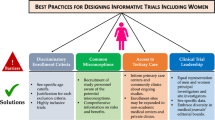
Factors that might hinder the participation of women should be sought and addressed in the design of the study. The proportion of included women can be estimated at the protocol writing stage and followed during the trial. Strategies to improve the participation of women in RCTs should be implemented: improvement of the explanation about the benefits of the trial, to dispel potential misconceptions, and obtaining feedback from both men and women, who declined to participate, to better understand potential barriers to enrollment of women. An important action towards improving gender equity in medical research is to ensure that the study leadership, including the study executive committee and site investigators, includes both men and women. Nielsen et al. showed a robust positive correlation between women’s authorship and the likelihood of a study including gender and sex analysis [33].
In conclusion, we found that women are being inadequately represented, in the selected medical fields analyzed in our study, in recent RCTs. Older age is a potential barrier to enrollment of women in clinical trials. Low inclusion rates of elderly women in clinical trials might create a lack of crucial knowledge of the adverse effects and the benefit/risk profile of any given treatment. Reporting sex-stratified outcomes for both efficacy and adverse events is of high importance. RCT investigators should increase their efforts to recruit women who are eligible for enrollment so that their proportion in the study sample will be as close to the real-life population as possible.
Availability of data and materials
No additional data is available.
Abbreviations
- CA UTI:
-
Catheter-associated urinary tract infection
- SSI:
-
Surgical site infection
- CDI:
-
Clostridium difficile infection
- CR GNB:
-
Carbapenem-resistant Gram-negative bacteria
- CONSORT:
-
Consolidated Standards of Reporting Trials
- EU:
-
European Union
- FDA:
-
Food and Drug Administration
- GLM:
-
Generalized linear models
- GAO:
-
Government Accountability Office
- HIV:
-
Human immunodeficiency virus
- IND:
-
Investigational new drug
- IQR:
-
Interquartile range
- MESH:
-
Medical Subject Headings
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews
- RCT:
-
Randomized controlled trial
- SPSS:
-
Statistical Package for the Social Sciences
References
Bracht GH, Glass GV. The external validity of experiments. Am Educ Res J. 1968;5(4):437–74.
Seydel C. The missing sex. Nat Biotechnol. 2021;39(3):260–5. https://doi.org/10.1038/s41587-021-00844-4.
Liu KA, Dipietro Mager NA. Women’s involvement in clinical trials: historical perspective and future implications. Pharmacy Practice (Granada). 2016;14(1):708.
Institute of Medicine. Exploring the biological contributions to human health: does sex matter? Washington, DC: The National Academies Press; 2001. https://doi.org/10.17226/10028
National Institutes of Health. NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research. Federal Register. March 1994.
Clinical trials on medicinal products for human use regulation (EU) No 536/ 2014. The European Parliament and of the Council. 2014.https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-1/reg_2014_536/reg_2 014_536_en.pdf.
Downing NS, Shah ND, Neiman JH, Aminawung JA, Krumholz HM, Ross JS. Participation of the elderly, women, and minorities in pivotal trials supporting 2011-2013 U.S. Food and Drug Administration approvals. Trials. 2016;17:199. https://doi.org/10.1186/s13063-016-1322-4.
Eshera N, Itana H, Zhang L, Soon G, Fadiran EO. Demographics of clinical trials participants in pivotal clinical trials for new molecular entity drugs and biologics approved by FDA from 2010 to 2012. Am J Ther. 2015;22(6):435–55. https://doi.org/10.1097/MJT.0000000000000177.
Khan SU, Khan MZ, Subramanian CR, et al. Participation of women and older participants in randomized clinical trials of lipid-lowering therapies: a systematic review. JAMA Netw Open. 2020;3(5):e205202.
Tahhan AS, Vaduganathan M, Greene SJ, et al. Enrollment of older patients, women, and racial/ethnic minority groups in contemporary acute coronary syndrome clinical trials: a systematic review. JAMA Cardiol. 2020;5(6):714–22. https://doi.org/10.1001/jamacardio.2020.0359.
Khan MS, Shahid I, Siddiqi TJ, et al. Ten-year trends in enrollment of women and minorities in pivotal trials supporting recent US Food and Drug Administration approval of novel cardiometabolic drugs. J Am Heart Assoc. 2020;9(11):e015594. https://doi.org/10.1161/JAHA.119.015594 Epub 2020 May 19.
Tsang W, Alter DA, Wijeysundera HC, Zhang T, Ko DT. The impact of cardiovascular disease prevalence on women’s enrollment in landmark randomized cardiovascular trials: a systematic review. J Gen Intern Med. 2012;27(1):93–8. https://doi.org/10.1007/s11606-011-1768-8 Epub 2011 Jun 29.
Curno MJ, Rossi S, Hodges-Mameletzis I, Johnston R, Price MA, Heidari S. A systematic review of the inclusion (or exclusion) of women in HIV research: from clinical studies of antiretrovirals and vaccines to cure strategies. J Acquir Immune Defic Syndr. 2016;71(2):181–8. https://doi.org/10.1097/QAI.0000000000000842.
Carcel C, Reeves M. Under-enrollment of women in stroke clinical trials: what are the causes and what should be done about it? Stroke. 2021;52(2):452–7.
Duma N, Vera Aguilera J, Paludo J, et al. Representation of minorities and women in oncology clinical trials: review of the past 14 years. J Oncol Pract. 2018;14(1):e1–e10. https://doi.org/10.1200/JOP.2017.025288 Epub 2017 Nov 3.
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22.
United Nations. World economic situation and prospects 2022. New York; 2022. Accessed on 20-Nov-2022
Goldberg R, Gore JM, Barton B, Gurwitz J. Individual and composite study endpoints: separating the wheat from the chaff. Am J Med. 2014;127(5):379–84.
Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Vitale C, Fini M, Spoletini I, Lainscak M, Seferovic P, Rosano GMC. Under-representation of elderly and women in clinical trials. Int J Cardiol. 2017;232:216–21. https://doi.org/10.1016/j.ijcard.2017.01.018.
Vitale C, Rosano G, Fini M. Are elderly and women under-represented in cardiovascular clinical trials? Implication for treatment. Wien Klin Wochenschr. 2016;128(Suppl 7):433–8. https://doi.org/10.1007/s00508-016-1082-x Epub 2016 Sep 21.
Miller RG, Mahajan HD, Costacou T, Sekikawa A, Anderson SJ, Orchard TJ. A contemporary estimate of total mortality and cardiovascular disease risk in young adults with type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2016;39:2296–303. https://doi.org/10.2337/dc16-1162.
Vistisen D, Andersen GS, Hansen CS, et al. Prediction of first cardiovascular disease event in type 1 diabetes mellitus: the Steno Type 1 Risk Engine. Circulation. 2016;133(11):1058–66. https://doi.org/10.1161/CIRCULATIONAHA.115.018844.
Leaper DJ, Holy CE, Spencer M, et al. Assessment of the risk and economic burden of surgical site infection following colorectal surgery using a US longitudinal database: is there a role for innovative antimicrobial wound closure technology to reduce the risk of infection? Dis Colon Rectum. 2020;63:1628–38. https://doi.org/10.1097/DCR.0000000000001799.
Esteban-Vasallo MD, de Miguel-Díez J, López-de-Andrés A, Hernández-Barrera V, Jiménez-García R. Clostridium difficile-related hospitalizations and risk factors for in-hospital mortality in Spain between 2001 and 2015. J Hosp Infect. 2019;102(2):148–56. https://doi.org/10.1016/j.jhin.2018.09.006.
Gomila A, Carratalà J, Eliakim-Raz N, et al. Clinical outcomes of hospitalised patients with catheter-associated urinary tract infection in countries with a high rate of multidrug-resistance: the COMBACTE-MAGNET RESCUING study. Antimicrob Resist Infect Control. 2019;3(8):198. https://doi.org/10.1186/s13756-019-0656-6.
Parodi RL, Lagrutta M, Tortolo M, et al. A multicenter prospective study of 515 febrile neutropenia episodes in Argentina during a 5-year period. PLoS One. 2019;14:e0224299. https://doi.org/10.1371/journal.pone.0224299.
Persampieri L. Gender and informed consent in clinical research: beyond ethical challenges. BioLaw Journal-Rivista di Biodiritto. 2019;1:65–87.
Osamor PE, Grady C. Women’s autonomy in health care decision-making in developing countries: a synthesis of the literature. Int J Women's Health. 2016;7(8):191–202. https://doi.org/10.2147/IJWH.S105483.
Lau ES, Braunwald E, Morrow DA, Giugliano RP, Antman EM, Gibson CM, et al. Sex, permanent drug discontinuation, and study retention in clinical trials: insights from the TIMI trials. Circulation. 2021;143(7):685–95. https://doi.org/10.1161/CIRCULATIONAHA.120.052339.
Navar AM, Roe MT, White JA, Cannon CP, Lokhnygina Y, Newby LK, et al. Medication discontinuation in the IMPROVE-IT Trial. Circ Cardiovasc Qual Outcomes. 2019;12(1):e005041. https://doi.org/10.1161/CIRCOUTCOMES.118.005041.
Nielsen MW, et al. One and a half million medical papers reveal a link between author gender and attention to gender and sex analysis. Nat Hum Behav. 2017;1(11):791–6.
Funding
This work was supported by the Israel National Institute for Health Policy and Health Services Research (NIHP) (grant number 2016/80). IP is supported in part by a research grant for young researchers, Rabin Medical Center, Israel. The funders had no role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; and in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
The corresponding author attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted. VD: conceptualization, data curation, formal analysis, investigation, methodology, and writing—original draft. AT: data curation, formal analysis, and investigation. NT: data curation and investigation. IP: data curation, formal analysis, investigation, funding acquisition, and methodology. IA-D: data curation and investigation. JN: data curation and investigation. DY: investigation and methodology. MP: funding acquisition, investigation, methodology, and resources. LL: conceptualization, formal analysis, funding acquisition, investigation, methodology, project administration, resources, and supervision. All authors contributed to the manuscript and provided critical feedback. The authors read and approved the final manuscript. The corresponding author has the right to grant on behalf of all authors and does grant on behalf of all authors a worldwide license to the publishers and its licensees in perpetuity, in all forms, formats, and media (whether known now or created in the future), to (i) publish, reproduce, distribute, display, and store the contribution; (ii) translate the contribution into other languages, create adaptations and reprints, include within collections, and create summaries, extracts, and/or abstracts of the contribution; (iii) create any other derivative work(s) based on the contribution; (iv) exploit all subsidiary rights in the contribution; (v) include electronic links from the contribution to third party material wherever it may be located; and (vi) license any third party to do any or all of the above. The review was not registered. This work was performed in partial fulfillment of the requirements for a Ph.D. degree of Vered Daitch, Sackler Faculty of Medicine, Tel Aviv University, Israel. PRISMA checklist is provided in Additional file 2: Appendix 2.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Appendix 1. Search phrase.
Additional file 2.
PRISMA Checklist.
Additional file 3: Table S1.
Characteristics of studies that did not report the number of included women and their comparison to studies that report the number of included women.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Daitch, V., Turjeman, A., Poran, I. et al. Underrepresentation of women in randomized controlled trials: a systematic review and meta-analysis. Trials 23, 1038 (2022). https://doi.org/10.1186/s13063-022-07004-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-022-07004-2