Abstract
Background
We aimed to assess the efficacy of a closed-loop oxygen control in critically ill patients with moderate to severe acute hypoxemic respiratory failure (AHRF) treated with high flow nasal oxygen (HFNO).
Methods
In this single-centre, single-blinded, randomized crossover study, adult patients with moderate to severe AHRF who were treated with HFNO (flow rate ≥ 40 L/min with FiO2 ≥ 0.30) were randomly assigned to start with a 4-h period of closed-loop oxygen control or 4-h period of manual oxygen titration, after which each patient was switched to the alternate therapy. The primary outcome was the percentage of time spent in the individualized optimal SpO2 range.
Results
Forty-five patients were included. Patients spent more time in the optimal SpO2 range with closed-loop oxygen control compared with manual titrations of oxygen (96.5 [93.5 to 98.9] % vs. 89 [77.4 to 95.9] %; p < 0.0001) (difference estimate, 10.4 (95% confidence interval 5.2 to 17.2). Patients spent less time in the suboptimal range during closed-loop oxygen control, both above and below the cut-offs of the optimal SpO2 range, and less time above the suboptimal range. Fewer number of manual adjustments per hour were needed with closed-loop oxygen control. The number of events of SpO2 < 88% and < 85% were not significantly different between groups.
Conclusions
Closed-loop oxygen control improves oxygen administration in patients with moderate-to-severe AHRF treated with HFNO, increasing the percentage of time in the optimal oxygenation range and decreasing the workload of healthcare personnel. These results are especially relevant in a context of limited oxygen supply and high medical demand, such as the COVID-19 pandemic.
Trial registration The HILOOP study was registered at www.clinicaltrials.gov under the identifier NCT04965844.
Similar content being viewed by others
Background
In patients with acute hypoxemic respiratory failure (AHRF), high flow nasal oxygen (HFNO) has several physiological benefits [1,2,3] and its use may reduce the need for intubation [4]. Optimal flow settings are unknown and are essentially based on expert recommendations [5]. Current guidelines recommend adjusting the fraction of inspired oxygen (FiO2) to maintain oxygenation within the predefined target range, which may vary depending on the risk of hypercapnia and the presence of a medical history of chronic respiratory failure [6].
The deleterious effects of hypoxemia are long recognized. Harmful effects of hyperoxemia are less often considered, but include vasoconstriction, inflammation, and oxidative stress. Several studies have suggested hyperoxemia is associated with poor hospital outcomes in patients under invasive ventilation [7,8,9,10], as well as patients receiving non-invasive ventilation [11]. Maintaining oxygenation within a given target range, preventing both hypoxemia and hyperoxemia, requires intensive monitoring and frequent manual adjustments of the FiO2 [12], which is time-consuming and therefore often impractical in an era of limited staff resources.
Recent studies found closed-loop oxygen delivery system with automated oxygen titration to be associated with more time spent within the SpO2 target range in patient admitted to an emergency department [13], in patients with chronic respiratory disease with exercise-induced desaturation [14], in surgical patients after extubation [15], and in patients weaning from invasive ventilation [16]. Thus far, only one study has been performed in patients with mild AHRF treated with HFNO [17]. It is worth noting that, given the greater FiO2 achieved with HFNO in comparison with conventional oxygen therapy, automated oxygen titration could be particularly useful in HFNO patients to prevent hyperoxemia.
Lastly, the COVID-19 pandemic has shown the considerable potential for HFNO to curtail the need for invasive mechanical ventilation [18,19,20] but has also simultaneously highlighted the overwhelming increase in oxygen demand and the tremendous inequities worldwide in terms of access to oxygen, some countries unable to meet oxygen demands. Limiting both times on HFNO and excessive FiO2 could contribute to reduce oxygen consumption.
The objective of this randomised clinical study was to assess the performance of closed-loop oxygen control in critically ill patients with moderate to severe AHRF treated with HFNO. We hypothesized that closed-loop oxygen control increases the time spent within a predefined SpO2 range and limits unnecessary exposure to high FiO2.
Methods
Study design
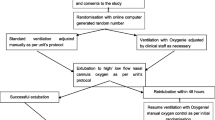
This is single-centre, single-blinded, randomised crossover trial of closed-loop oxygen control versus manual oxygen titration during HFNO. The study enrolled patients in the Hospital Universitari Vall d’Hebron, Barcelona, Spain, between April 19, 2021, to Aug 18, 2021.
Patients
Patients were eligible for participation if admitted to ICU and receiving HFNO at a flow rate ≥ 40 L/min with FiO2 ≥ 0.30, and expected to receive HFNO for at least 8 h after randomisation. Patients aged < 18 years, pregnant women, patients with an indication or at high risk for immediate intubation, and patients with an indication for non-invasive ventilation were excluded. In addition, we excluded patients that were hemodynamically unstable, patients with severe acidosis, patients with poor SpO2 signal, and patients with chronic or acute dyshaemoglobinaemia. We also excluded tracheostomised patients. Patients previously enrolled in this trial or enrolled in another interventional study could not participate.
Randomisation and masking
Patients were randomised to start with a 4-h period of closed-loop oxygen control or 4-h period of manual oxygen titration, after which each patient was switched to the alternate therapy. Randomization was 1:1, with blocks of 4, using a randomization list that was computer-generated and incorporated into the Research Electronic Data Capture (REDCap) [21] study electronic database. Patients remained unaware of the way oxygen was titrated. Due to the intervention healthcare staff could not be blinded.
Procedures
After randomisation, the physician in charge decided on the individualized optimal SpO2 range for each patient, according to current clinical situation and the medical history. These ranges were 94 to 98%, 92 to 96%, 90 to 94% or 88 to 92%.
With closed-loop oxygen control, there was an automatic adjustment of the FiO2 to maintain the patient's SpO2 in a predefined target range. The SpO2 target range was defined by setting four different cut-offs: an upper and a lower ‘optimal’ cut-off, and an upper and lower ‘suboptimal’ cut-off. These thresholds were different according to the defined target range. The SpO2 values within the target thresholds were defined as ‘optimal’. The SpO2 values outside the target but within the emergency thresholds were defined as ‘suboptimal’. And those values outside emergency thresholds were considered as out-of-range SpO2 values (Additional file 1: Table S1). When the SpO2 is in the target range, the controller fine-tunes the FiO2 setting to get the patient’s SpO2 to the middle of the target range. A detailed description of the device set-up and the way it functions, including FiO2 adjustment algorithm and alarm setting are shown in Additional file 1: Table S2. Manual adjustments were allowed in the closed-loop oxygen control group as a patient safety measure.
With manual oxygen titrations, the healthcare personnel was also aware of the optimal SpO2 target range. All FiO2 adjustments were performed by the physicians and nurses when they observed that the SpO2 was not in the predefined target range.
Clinical variables, including systemic blood pressure, heart rate, respiratory rate, SpO2, and patient comfort, and HFNO settings were recorded at the start and at the end of each period. FiO2, SpO2, flow, and temperature were continuously recorded every second using a memory box that was connected to the ventilator via the RS-232 interface port. Device alarms and settings were also recorded with a memory box.
After the first 4 h were completed, a washout period was established for 15 min, after which the patient was switched to the second period with the alternate oxygen titration strategy.
Patient care and standard activities, such as eating or physiotherapy, were uninterruptedly performed as usual, and at random in either period.
Definitions
Every recorded value of SpO2 was classified as either ‘optimal’ when within the individualized SpO2 range, ‘suboptimal’ when outside the optimal SpO2 range, but inside the suboptimal SpO2 range, as shown in Additional file 1: Table S1, or out of range boundaries when outside the suboptimal SpO2 range.
Study endpoints
The primary objective of the study was to assess the efficacy of the closed-loop oxygen control. The primary endpoint measure was the percentage of time spent in the individualized optimal SpO2 range. Secondary endpoints included the percentage of time spent in suboptimal ranges, the time spent out of range, the percentage of time with the SpO2 signal available, the mean SpO2, SpO2/FiO2, and the ROX index ([SpO2/FiO2]/respiratory rate) [22, 23], as well as the percentage of time with SpO2 below 88% and 85% and number of events of SpO2 below 88% and 85%, respectively. The percentage of time with FiO2 below 0.4 and above 0.6, the number of manual adjustments required per hour (in manual oxygen titration period), the number and frequency of alarms, and the comfort score assessed by the visual analogic scale from 0 to 10, were also secondary endpoints.
Sample size estimation
Based on the assumption that closed-loop oxygen control would increase the percentage of time spent with SpO2 in optimal range from 51 ± 30 to 81% [13], with power and significance set at 0.80 and 0.05, respectively, 45 patients were required. To account for potential dropouts, defined as a patient who required either non-invasive ventilation or intubation during the course of the study, consent withdrawal by patient or family, poor quality of the SpO2 signal for > 1 h during one of the study periods, or technical problem in recording, a sample of 50 patients was initially estimated.
Statistical analysis
Continuous data were tested for normality using Shapiro–Wilk test. According to their distribution, variables are presented as mean ± standard deviation (SD) or median (IQR, interquartile range) and compared using t-test or Wilcoxon test.
Difference estimates are shown as mean of differences in normally distributed variables or as median of differences using the Hodges–Lehman estimator in non-normally distributed variables [24]. Discontinuous data are compared using Chi-square test or Fisher’s exact test, as appropriate.
In order to assess the reproducibility and robustness of closed-loop oxygen control, a sensitivity analysis was performed for the primary endpoint by the severity of the respiratory insufficiency, using the FiO2 and the ROX index value at the time for severity classification. We also performed two sensitivity analysis according to the aetiology of AHRF, i.e., AHRF due to coronavirus disease 2019 (COVID-19) versus AHRF due to another cause, and according to the individualized optimal SpO2 ranges.
A p < 0.05 was considered significant. Statistical analysis was performed using R (version 3.5.2) [25]. We used the built-in packages, ggplot2 [26], tidyverse [27] and epitools [28].
Results
Between April 19, 2021, to Aug 18, 2021, 68 patients were screened for eligibility. Main reason for exclusion was receiving and FiO2 < 0.3 and high risk of intubation (Fig. 1). As we had a higher dropout rate than expected, we enrolled and randomised 53 patients of which 45 patients had readable data. Median age was 49 (40 to 62) years, 24 (53.3%) were male. Baseline characteristics and clinical outcomes are presented in Table 1. The majority of patients had AHRF due to COVID-19, and 41 (92.2%) patients received HFNO for de novo AHRF. The median SpO2/FiO2 at the time of inclusion was 192 (162 to 216). No differences in clinical variables were observed at the start of each study period (Table 2). Average of the total recorded time (hh:mm:ss) was 03:59:45 and 04:03:13 with 99% and 99% of time with valid SpO2 signal for closed-loop oxygen control and manual titrations of oxygen, respectively.
With closed-loop oxygen control, patients spent more time in the optimal SpO2 range compared with manual titrations of oxygen (96.5 [93.5 to 98.9] % vs. 89 [77.4 to 95.9] %; p < 0.0001) (difference estimate, 10.4 (95% confidence interval 5.2 to 17.2) (Fig. 2). With closed-loop oxygen control, patients spent less time in the suboptimal range, both above (0.6 [0.1 to 1.1] % vs. 1.0 [0.1 to 4.0] %; p = 0.0053) and below (2.3 [0.8 to 5.2] % vs. 4.2 [1.1 to 16.4] %; p = 0.0004) the cut-offs of the optimal SpO2 range (Table 3). With closed-loop oxygen control, patients spent less time beyond the suboptimal range. The number of events of SpO2 < 88% and < 85% were not significantly different between groups.
Mean FiO2 used during each period and SpO2/FiO2 ratio was not different between closed-loop oxygen control and manual oxygen titration. Time spent at different FiO2 levels, however, was different (Table 3, Additional file 1: Fig. S1). With closed-loop oxygen control, FiO2 varied much more than with manual oxygen titration. The number of manual adjustments was very different between the two approaches. Only one patient during the closed-loop oxygen control required one single manual adjustment of FiO2 due to SpO2 sensor dislodgement that was solved with SpO2 sensor repositioning. The number of alarms for a low or high SpO2 was not different between the two approaches.
Oxygen consumption during the time that the patients spent above the optimal SpO2 targets was significantly lower with closed-loop oxygen control. (Additional file 1: Table S3).
The improvement in time within optimal SpO2 range in closed-loop oxygen control versus manual oxygen titrations remained significant when patients were analysed according to initial FiO2 (Additional file 1: Table S4). Interestingly, the effect was stronger in more hypoxemic patients (Additional file 1: Fig. S2). The findings were neither affected by the cause of AHRF nor by the individualised SpO2 ranges (Additional file 1: Table S4).
Discussion
The findings of this study in patients with moderate AHRF treated with HFNC can be summarized as follows: (1) with the use of closed-loop oxygen control, patients spent more time in the optimal SpO2 range; (2) the closed-loop oxygen control decreased the time in the suboptimal range both above and below and out-of-range above the target; (3) contrary to what happened with standard of care, closed-loop oxygen control was equally effective regardless the severity of oxygenation impairment, (4) the closed loop oxygen control significantly decreased the number of manual adjustments, reducing the healthcare personnel workload, and (5) significantly decreased oxygen consumption. Given the increasing use of HFNO as highlighted worldwide during the pandemic, the growing concern of hyperoxia in critically ill patients, and the incapability of some countries to meet oxygen demands, our results bear important consequences on routine practice.
The time spent in optimal SpO2 target both during closed-loop and during the manual oxygen titration period was considerably higher than previously reported. L’Her et al. [13] reported that patients spent 81% and 51% of time in optimal SpO2 range while Harper et al. [17] had 96.2% and 71% in closed-loop and manual oxygen titration periods, respectively. Differences in the study setting and patient’s characteristics may explain these different results. One study was performed on patients admitted to the emergency department who were receiving low flow oxygen [13] while the other study focused on patients with mild AHRF treated with HFNO but who were admitted to medical or surgical wards [17]. In the present study, patients were admitted to an ICU or intermediate care unit with lower nurse-to-patient ratios, increasing the time that they spent within the optimal range in the manual oxygen titration period compared to those previously reported. Interestingly, they presented a higher respiratory impairment with higher needs for FiO2 and lower SpO2/FiO2 ratios at inclusion and during the study period, increasing the risk of being out of the optimal range. Therefore, our results demonstrate that closed-loop oxygen control improves oxygen administration also in patients with moderate to severe AHRF who required higher oxygen requirements than previously studied.
The closed-loop oxygen control decreased the time spent in suboptimal ranges both above and below limits and also the time out of range above the out-of-range limits. Overall, the closed-loop oxygen control had a higher impact in reducing hyperoxia rather than hypoxia, in line with previous results reported in patients treated with low-flow oxygen who were admitted to the emergency department [13], during the early post-extubation period [16], and in COPD patients [29]. The effect of reducing hyperoxia is extremely important for two different reasons. First, hyperoxia has been associated with worse outcomes in patients with acute respiratory distress syndrome [9] and, in the context of the shortage of oxygen that many countries have experienced during the pandemic, it may decrease the oxygen consumption in a patient and at a hospital level. In contrast, no differences were seen in time spent out of range below the limits which makes sense as all patients were admitted in the ICU or intermediate care units and they were closely monitored to prevent any clinically significant desaturation.
To our knowledge, only 2 articles have reported closed-loop oxygen control systems during HFNO therapy. The first study assessed FiO2 adjustment during a 6-min walking test (6MWT)-induced desaturation in patients with chronic respiratory diseases [14]. The second study is a recent randomized trial that included 20 patients with mild AHRF [17]. While this study demonstrated that the use of closed-loop oxygen was associated with more time in optimal SpO2 range, the median difference was rather small (− 1% for hyperoxia and − 2.4% for hypoxia). Applicability of these results to the ICU setting is limited since, contrary to our study, patients receiving FiO2 higher than 0.4 were excluded. Interestingly, our results suggest that the benefit of closed-loop oxygen control could be even more clinically relevant in more hypoxemic patients.
It should be also noted that a median of 0.21 manual adjustments per hour were needed during manual oxygen titration. This would translate into approximately 6 manual changes a day, per patient. COVID-19 pandemic has shown the critical issue of staffing and staff shortage in many hospitals and a tremendous increase in their workload. Importantly, nurse workload is a major challenge in the quality of care and it may have a direct impact on patient outcomes [30] and pre-pandemic studies [31] have shown that objective assessment of nurse workload identified excessive workload compared to the 1:1.5 nurse/patient ratio recommendation. Hence the potential benefits of automated systems that contribute to reduce nurse workload may help improve patients’ outcomes. Of note, the study was performed on ICU patients. Thus, the observed results could be even more important in areas of care with a less favourable nurse-to-patient ratios. The closed-loop oxygen control significantly reduced the number of adjustments needed and therefore, reduced the workload for medical and nursing staff, as shown previously for other automatic oxygen devices in mechanically ventilated patients [12]. These findings are especially relevant in a context of high medical demand, such as the COVID-19 pandemic.
Oxygen consumption has been an important issue during the COVID19 pandemic [32]. Some strategies aiming to reduce the oxygen consumption have been used, such as using more conservative or lower oxygen targets, the use of oxygen-saving systems or oxygen concentrators, such as the Double-Trunk Mask, which, placed over a low-flow oxygen mask, may decrease the total oxygen flow required [33]. We herein report a higher oxygen consumption during manual oxygen titration as compared to automatic closed-loop oxygen control when patients were above the optimal SpO2 range. This suggests they consumed an excess of oxygen which is wasted while keeping patients above the optimal SpO2 target. Our findings suggest that a closed-loop oxygen system may result in a more economic use of oxygen, with more oxygen spent to avoid desaturation but with less oxygen wasted, keeping the patients longer within the optimal range and decreasing the time patients spent above the optimal SpO2 targets. The fact that no significant difference in total oxygen consumption was found might be explained by our relatively short recording time and the fact that the study was underpowered to detect differences in this particular outcome. Thus, it seems reasonable to think closed-loop oxygen control system might be a way to spare oxygen, especially in high oxygen-demand periods such as COVID-19 [32].
Even though there were no differences between periods in the mean FiO2 during the 4 h of the study, during closed-loop oxygen control, patients spent more time with an FIO2 below 0.4 and above 0.6 compared with the standard of care period. This result suggests that closed-loop oxygen titration tailors the use of oxygen to patient’s individual needs during the course of the disease better than the manual titrations of oxygen and, therefore a more appropriate and adequate oxygen administration is achieved during closed-loop oxygen control in patients with moderate to severe AHRF.
The present study has important strengths. It was a randomized and cross-over study with each patient serving as its own control and replacing the dropout to finally obtain the estimated sample size. We also used individualized ranges and cut-offs according to the clinical condition of each patient and sensitivity analyses showed that closed-loop oxygen was equally effective regardless of the predefined optimal SpO2 target. Additional sensitivity analyses showed that closed-loop oxygen control was equally effective regardless of the aetiology of respiratory failure or the FiO2 at the time of enrolment.
In contrast, it has several limitations. It is a single-centre study with impossibility of blinding. Moreover, only a limited time span was analysed (4 h per period). Together with the crossover design, this does not allow for analysis of more clinically significant outcomes, such as days of HFNO therapy or ICU length of stay. However, it is the first time that a closed-loop oxygen control showed to be efficient and safe for patients with moderate to severe AHRF treated with HFNO.
Conclusions
The present study shows that a closed-loop oxygen control system improves oxygen administration in critically ill patients with moderate-to-severe AHRF treated with HFNO by mainly decreasing the time the patient spent above the limits of the clinically set oxygenation targets. The closed-loop oxygen control was also associated with a lower need for manual oxygen adjustments. These results may have important implications both at the patient level, as it decreases the risk of deleterious effects of hypoxia and hyperoxia, and at the healthcare system level as it decreases the healthcare personnel workload and it might be potentially associated with a less use of oxygen, making it a useful asset for high oxygen-demand periods such as a pandemic.
Availability of data and materials
The datasets generated during the current study are not publicly available due to patient confidentiality reasons, but are available from the corresponding author upon reasonable request. All requests will also need to be reviewed by our Ethics Committee before any data is shared.
Abbreviations
- AHRF:
-
Acute hypoxemic respiratory failure
- HFNO:
-
High flow nasal oxygen
- FIO2 :
-
Fraction of inspired oxygen
- SpO2 :
-
Pulse oxymetry
- COVID-19:
-
Coronavirus-2 disease
- ICU:
-
Intensive care unit
- ROX:
-
Respiratory rate and oxygenation index
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- 6MWT:
-
6-Minute walking test
- SAPS3:
-
Simplified Acute Physiology Score III
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation II
- SOFA:
-
Sequential Organ Failure Assessment
References
Mauri T, Turrini C, Eronia N, Grasselli G, Volta CA, Bellani G, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195(9):1207–15.
Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55(4):408–13.
Ricard JD, Roca O, Lemiale V, Corley A, Braunlich J, Jones P, et al. Use of nasal high flow oxygen during acute respiratory failure. Intensive Care Med. 2020;46(12):2238–47.
Rochwerg B, Granton D, Wang DX, Helviz Y, Einav S, Frat JP, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019;45(5):563–72.
Rochwerg B, Einav S, Chaudhuri D, Mancebo J, Mauri T, Helviz Y, et al. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med. 2020;46(12):2226–37.
O’Driscoll BR, Howard LS, Earis J, Mak V. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(Suppl 1):ii1–90.
Helmerhorst HJ, Arts DL, Schultz MJ, van der Voort PH, Abu-Hanna A, de Jonge E, et al. Metrics of arterial hyperoxia and associated outcomes in critical care. Crit Care Med. 2017;45(2):187–95.
Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, de Jonge E. Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, meta-analysis, and meta-regression of cohort studies. Crit Care Med. 2015;43(7):1508–19.
Palmer E, Post B, Klapaukh R, Marra G, MacCallum NS, Brealey D, et al. The association between supraphysiologic arterial oxygen levels and mortality in critically ill patients. A multicenter observational cohort study. Am J Respir Crit Care Med. 2019;200(11):1373–80.
Damiani E, Adrario E, Girardis M, Romano R, Pelaia P, Singer M, et al. Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Crit Care. 2014;18(6):711.
Pala Cifci S, Urcan Tapan Y, Turemis Erkul B, Savran Y, Comert B. The impact of hyperoxia on outcome of patients treated with noninvasive respiratory support. Can Respir J. 2020;2020:3953280.
Arnal JM, Garnero A, Novotni D, Corno G, Donati SY, Demory D, et al. Closed loop ventilation mode in Intensive Care Unit: a randomized controlled clinical trial comparing the numbers of manual ventilator setting changes. Minerva Anestesiol. 2018;84(1):58–67.
L’Her E, Dias P, Gouillou M, Riou A, Souquiere L, Paleiron N, et al. Automatic versus manual oxygen administration in the emergency department. Eur Respir J. 2017;50(1):1602552.
Harper JC, Kearns NA, Maijers I, Bird GE, Braithwaite I, Shortt NP, et al. Closed-loop oxygen control using a novel nasal high-flow device: a randomized crossover trial. Respir Care. 2021;66(3):416–24.
L’Her E, Jaber S, Verzilli D, Jacob C, Huiban B, Futier E, et al. Automated closed-loop versus standard manual oxygen administration after major abdominal or thoracic surgery: an international multicentre randomised controlled study. Eur Respir J. 2021;57(1):2000182.
Ouanes I, Bouhaouala F, Maatouk S, Lahmar M, Ben Abdallah S, Hammouda Z, et al. Automatic oxygen administration and weaning in patients following mechanical ventilation. J Crit Care. 2021;61:45–51.
Harper J, Kearns N, Bird G, Braithwaite I, Eathorne A, Shortt N, et al. Automatic versus manual oxygen titration using a novel nasal high-flow device in medical inpatients with an acute illness: a randomised controlled trial. BMJ Open Respir Res. 2021;8(1):e000843.
Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47(1):60–73.
Benefits and risks of noninvasive oxygenation strategy in COVID-19: a multicenter, prospective cohort study (COVID-ICU) in 137 hospitals. Crit Care. 2021;25(1):421.
Zucman N, Mullaert J, Roux D, Roca O, Ricard JD. Prediction of outcome of nasal high flow use during COVID-19-related acute hypoxemic respiratory failure. Intensive Care Med. 2020;46(10):1924–6.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Roca O, Caralt B, Messika J, Samper M, Sztrymf B, Hernández G, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019;199(11):1368–76.
Roca O, Messika J, Caralt B, Garcia-de-Acilu M, Sztrymf B, Ricard JD, et al. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: the utility of the ROX index. J Crit Care. 2016;35:200–5.
Hodges J, Lehmann E. Estimates of location based on rank tests. Ann Math Stat. 1963;34:13.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2020. http://www.r-project.org/index.html.
Wickham H. ggplot2: elegant graphics for data analysis. Springer; 2016.
Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, et al. Welcome to the tidyverse. J Open Source Softw. 2019;4(43):1686.
Sergeant E. Epitools epidemiological calculators. Ausvet; 2018.
Lellouche F, Bouchard PA, Roberge M, Simard S, L’Her E, Maltais F, et al. Automated oxygen titration and weaning with FreeO2 in patients with acute exacerbation of COPD: a pilot randomized trial. Int J Chron Obstruct Pulmon Dis. 2016;11:1983–90.
Penoyer DA. Nurse staffing and patient outcomes in critical care: a concise review. Crit Care Med. 2010;38(7):1521–8 (quiz 9).
Bruyneel A, Tack J, Droguet M, Maes J, Wittebole X, Miranda DR, et al. Measuring the nursing workload in intensive care with the Nursing Activities Score (NAS): a prospective study in 16 hospitals in Belgium. J Crit Care. 2019;54:205–11.
Schultz MJ, Roca O, Shrestha GS. Global lessons learned from COVID-19 mass casualty incidents. Br J Anaesth. 2022;128(2):e97–100.
Poncin W, Baudet L, Reychler G, Duprez F, Liistro G, Belkhir L, et al. Impact of an improvised system on preserving oxygen supplies in patients with COVID-19. Arch Bronconeumol. 2021;57:77–9.
Acknowledgements
We would like to thank the medical and nursing staff from the ICU, Hospital Universitari Vall d’Hebron, for their support during this trial.
Funding
This study is partially supported by a research grant from Hamilton Medical AG. For each patient, an anonymised file containing the recorded data from the high-flow device was sent to Hamilton Medical AG, where these data were transformed into a database of raw data and sent back to the investigators for further analysis. Hamilton Medical AG did not have a role in statistical analysis beyond this point or data interpretation.
Author information
Authors and Affiliations
Contributions
OR and JDR contributed to the study conceptualisation. OR also performed formal analysis, funding acquisition, investigation, methodology, project administration, and study supervision. OC assisted in data curation, formal analysis and performed investigation together with MS, FJR, AP, and MGdA. RF, MJS, and JDR supervised the project. OR, OC wrote the manuscript draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The trial was approved by the local Ethics Committee (PR(AG)539/2020) and was registered at www.clinicaltrials.gov (NCT04965844). Informed consent was obtained prior to inclusion from all patients or their relatives.
Consent for publication
Not applicable.
Competing interests
OR discloses a research grant from Hamilton Medical AG and speaker fees from Hamilton Medical AG, Ambu, Aerogen Ltd, and Fisher&Paykel Healthcare Ltd, and non-financial research support from Timpel and Massimo Corporation. RF discloses personal fees from MSD, Pfizer, Shionogi, Gilead, Grifols, Menarini, and GSK. MJS discloses speaker fees from Hamilton Medical AG. JDR discloses travel expenses from Fisher&Paykel Healthcare Ltd. All other authors disclose no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary Material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Roca, O., Caritg, O., Santafé, M. et al. Closed-loop oxygen control improves oxygen therapy in acute hypoxemic respiratory failure patients under high flow nasal oxygen: a randomized cross-over study (the HILOOP study). Crit Care 26, 108 (2022). https://doi.org/10.1186/s13054-022-03970-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-03970-w