Abstract
Background
The aim of the study was to describe the population pharmacokinetics (PK) of meropenem in critically ill patients receiving sustained low-efficiency dialysis (SLED).
Methods
Prospective population PK study on 19 septic patients treated with meropenem and receiving SLED for acute kidney injury. Serial blood samples for determination of meropenem concentrations were taken before, during and after SLED in up to three sessions per patient. Nonparametric population PK analysis with Monte Carlo simulations were used. Pharmacodynamic (PD) targets of 40% and 100% time above the minimal inhibitory concentration (f T > MIC) were used for probability of target attainment (PTA) and fractional target attainment (FTA) against Pseudomonas aeruginosa.
Results
A two-compartment linear population PK model was most appropriate with residual diuresis supported as significant covariate affecting meropenem clearance. In patients without residual diuresis the PTA for both targets (40% and 100% f T > MIC) and susceptible P. aeruginosa (MIC ≤ 2 mg/L) was > 95% for a dose of 0.5 g 8-hourly. In patients with a residual diuresis of 300 mL/d 1 g 12-hourly and 2 g 8-hourly would be required to achieve a PTA of > 95% and 93% for targets of 40% f T > MIC and 100% f T > MIC, respectively. A dose of 2 g 8-hourly would be able to achieve a FTA of 97% for 100% f T > MIC in patients with residual diuresis.
Conclusions
We found a relevant PK variability for meropenem in patients on SLED, which was significantly influenced by the degree of residual diuresis. As a result dosing recommendations for meropenem in patients on SLED to achieve adequate PD targets greatly vary. Therapeutic drug monitoring may help to further optimise individual dosing.
Trial registration
Clincialtrials.gov, NCT02287493.
Similar content being viewed by others
Background
In the intensive care unit (ICU), up to 42% of septic patients develop acute kidney injury (AKI) with approximately 5% of all ICU patients requiring renal replacement therapy (RRT) [1, 2]. Because of their high mortality risk optimised antibiotic dosing in these patients is considered mandatory to improve clinical outcomes [3, 4].
Meropenem is a broad-spectrum antibiotic agent commonly used in critically ill patients [5,6,7]. It has minimal protein binding (2%) and is mainly excreted unchanged in the urine (approximately 70% unchanged; 28% as inactive metabolite) [5, 7]. In patients with normal kidney function, meropenem has a half-life of approximately 1 h increasing to > 5.7 h with AKI [5]. Meropenem has a time-dependent bacterial killing characteristic, which means a plasma concentration above the minimum inhibitory concentration (MIC) for at least 40% of time of the dosing interval (40% f T > MIC) is associated with optimal activity [6,7,8,9]. Recent studies suggest a target of 100% f T > MIC to be more appropriate for critically ill patients with severe sepsis [10,11,12].
In addition to the alterations of meropenem pharmacokinetics (PK) caused by sepsis, use of RRT has additional profound effects on drug clearance (CL) [13,14,15,16,17,18,19,20,21,22]. These effects on PK differ according to the modality and intensity of RRT [21, 23,24,25,26,27].
Sustained low-efficiency dialysis (SLED) is an increasingly used modality of prolonged intermittent RRT in the ICU [28,29,30]. SLED combines both the advantage of intermittent haemodialysis (IHD) with its good solute removal and that of continuous renal replacement therapy (CRRT) with its haemodynamic stability [31,32,33]. Because of its intermittent application, the PK of drugs with predominantly renal elimination change from high CL during SLED to a significantly lower CL without SLED.
Despite its widespread use, the lack of SLED-specific dosing studies leave intensivists with considerable uncertainty about optimal antibiotic dosing [14, 25, 29, 30, 34,35,36,37]. So far, only two small studies on meropenem PK during SLED have been published and neither of them described PK over serial SLED treatments nor used a population PK approach to propose SLED-specific meropenem dosing regimens [38, 39]. The aim of this study was to describe the population PK of meropenem in critically ill septic patients receiving SLED and to develop dosing recommendations for meropenem in this population.
Methods
Setting
This was a prospective, observational population PK study. Patients were recruited between July 2013 and November 2014 in the Department of Intensive Care Medicine at the University Medical Center Hamburg-Eppendorf in Germany. Ethics approval was obtained from the local ethics committee. Written informed consent was obtained either from the patient or their appointed legal guardian. The study was registered at clincialtrials.gov (NCT02287493).
Study population
Patients aged > 18 years treated with meropenem and receiving SLED during daytime were eligible for study inclusion.
Dosing, administration and data collection
Dosing was at the discretion of the treating physician with various doses of 0.5 g, 1 g or 2 g of meropenem (Dr. Friedrich Eberth Arzneimittel GmbH, Germany) administered intravenously over 30 minutes 8-hourly.
Demographic data and clinical variables such as residual diuresis, mechanical ventilation, vasopressor support, modified sequential organ failure assessment (SOFA) score on the first day of sampling, simplified acute physiology (SAPS II) score on ICU admission, as well as laboratory variables, such as liver enzymes, C-reactive protein (CRP) and procalcitonin (PCT), were collected. Patient outcome data including ICU length of stay as well as ICU mortality were documented.
Sustained low-efficiency dialysis
SLED was performed with the Genius® batch system (Fresenius Medical Care, Bad Homburg, Germany) using a Fresenius FX 60 filter (surface area 1.4 m2, Fresenius Medical Care). Blood/dialysate and ultrafiltration flow rates as well as the duration of SLED were recorded.
Sample collection and measurements
Blood sampling from an indwelling cannula was performed on three consecutive days of SLED. Trough samples 1 h prior to infusion were taken followed by sampling 10 min and 1 h, 2 h and 4 h after the start of SLED, as well as at the end of the session. SLED was initiated no later than 3 h after meropenem infusion. Blood samples were centrifuged within 30 minutes at 3000 rpm, for 10 minutes. The serum supernatant was transferred to a tube and stored at –70 °C until analysis. According to previous stability testing, we set a maximum storage time of 3 months [40]. Samples were analysed by high-performance liquid chromatography with UV-detection (HPLC-UV) after being processed for protein precipitation. The method was validated and conducted in accordance with the guidelines of the US Food and Drug Administration’s guidance for industry on bioanalysis [41]. The coefficient of variation for the intra-day precision was 9.6%, 3.9%, and 2.2% for meropenem concentrations of 10, 20, and 80 mg/L. Inter-day precision coefficients of variation were < 15% for 10, 20, and 80 mg/L. Accuracy was > 94% with a deviation of < 15% for all tested concentrations including the lower limit of quantification of 1 mg/L.
Population pharmacokinetic modeling
One and two-compartment models were tested using the Nonparametric Adaptive Grid (NPAG) algorithm within the Pmetrics® package for R (Los Angeles, CA, USA) [42, 43]. Additive (lambda) and exponential (gamma) error models were both tested for inclusion. Demographic and clinical characteristics, which were considered biologically plausible for affecting meropenem PK, such as residual diuresis, blood/dialysate flow and bodyweight, were tested for inclusion as covariates. If a covariate improved the coefficient of determination of the linear regression (R2) and resulted in the reduction of the bias of the goodness-of-fit plots as well as in a statistically significant reduction in the log-likelihood (p < 0.05) it was included in the model. The R2 and the bias of the observed versus predicted plots as well as the log-likelihood of each run were taken into account for the goodness-of-fit evaluation. Predictive performance evaluation was based on mean prediction error (bias) and the mean bias-adjusted squared prediction error (imprecision) of the population and individual prediction models. Weighted residual plots versus time and concentration, as well as the visual predictive check (VPC) plot and the normalised prediction distribution errors (NPDE) were also used to test the suitability of the final covariate model.
Probability of target attainment
Monte Carlo simulations (n = 1000) were performed using Pmetrics® to determine the probability of target attainment (PTA) for the first 24 h of meropenem treatment for two pharmacodynamic (PD) targets. The primary PD target was set to the traditional target of 40% f T > MIC [8]. Additionally, a more aggressive and higher target was set to 100% f T > MIC [10, 11]. We simulated a 5-hour SLED treatment between the administration of 0.5 to 2 g meropenem 8-hourly and 1 to 2 g 12-hourly with an infusion time of 30 minutes. In addition we simulated both prolonged (3 hours) and continuous infusion (CI) of meropenem. In the simulation, SLED started 17 h after the first dose of meropenem, translating to a SLED session between the 2nd and the 3rd dose for 8-hourly dosing and at the end of the dosage interval for a 12-hourly dosing interval. The PTA against various MIC accounted for the plasma protein binding of 2% [5,6,7]. A dosage regimen was considered successful with a PTA > 95%.
Fractional target attainment
The fractional target attainment (FTA) identifies the achievement of target antibiotic exposures by comparing the pharmacodynamic exposure (PTA) against an MIC distribution of a chosen bacteria. To determine the FTA against P. aeruginosa with a susceptibility breakpoint of 2 mg/L, MIC data from the EUCAST database were used [8]. Additionally, MIC ≤ 8 mg/L and MIC ≤ 16 mg/L were also tested to account for P. aeruginosa strains at the resistance breakpoint. The FTA was calculated by using the targets of 40% f T >MIC and 100% f T >MIC. A dosing regimen was considered successful if the FTA was > 95%.
Results
Demographic and clinical data
A total of 308 serum samples were obtained from 19 patients. Seventy-four percent were male patients with a median [range] age, weight and SOFA score of 66 years [37–78], 81 kg [70–183] and 11 [5,6,7,8,9,10,11,12,13,14,15,16], respectively. The median residual diuresis and SLED treatment time were 0 mL/day [0–360] and 315 min [80–470], respectively. Detailed demographic and clinical data are shown in Table 1.
Pharmacokinetic model building
A two-compartment linear model using an additive error adequately described the serum concentrations of meropenem. Residual diuresis was found to be associated with CL when SLED was not being used and improved the goodness-of-fit and the log-likelihood (p < 0.01) of the model. No other covariates could be identified as significant, e.g. blood/dialysate and ultrafiltration flow rate. The final model is described as follows:
Where TVCL is the typical value of total CL, CLSLED is the population parameter estimate of meropenem CL with SLED. CLNS is the population parameter estimate of meropenem CL without SLED. The value for term SLED is 1 when SLED is on, whereas it is 0 when SLED is off. CLD is the meropenem CL referring to non-SLED CL mechanisms. CLN is the native meropenem CL associated with the residual diuresis (RD) of the patient.
The mean (SD) population PK parameter estimates from the final covariate model are displayed in Table 2.
The diagnostic plots confirmed the goodness-of-fit of the chosen model and are shown in Figs. 1 and 2. Monte Carlo simulations were performed with the final covariate model.
Probability of target attainment
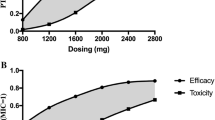
The probability of target attainment (PTA) for achieving 40% and 100% f T >MIC for the first 24 h for various meropenem doses in patients receiving a 5-hour SLED session are described in Fig. 3. These plots generally show that the PTA decreases with a higher residual diuresis. Regarding 40% f T > MIC with various residual diuresis up to 300 mL/d 0.5 g 8-hourly achieved a PTA of > 95% for MIC ≤ 2 mg/L (Fig. 3, left column).
However, aiming for 100% f T > MIC with a residual diuresis of 0 mL/d a dose of 1 g 12-hourly resulted in a sufficient PTA above 95%. With a residual diuresis of 300 mL/d a dose of 2 g 8-hourly only resulted in a PTA of 93% (Fig. 3, right column). The results for modelling prolonged (3 hours) and continuous meropenem infusions for a PK target of 100% f T > MIC are shown in Fig. 4. The PK profile of meropenem at a dose of 2 g 8-hourly in patients with a residual diuresis of 300 ml/d is shown in Fig. 5.
Fractional target attainment
The FTAs for 40% and 100% f T > MIC for the first 24 h and a 5-hour SLED session for a range of meropenem doses and degrees of residual diuresis for susceptible P. aeruginosa isolates (MIC ≤ 2 mg/L) are shown in Table 3. The FTA against P. aeruginosa strains at and below the resistance breakpoint (MIC ≤ 8 mg/L) and for strains with a MIC of ≤ 16 mg/L are shown in Tables 4 and 5, respectively. In the simulations for these two MICs we included analyses of prolonged infusions (3 h) of meropenem with the highest dose of 2 g 8-hourly.
For susceptible strains (45,715 isolates), 0.5 g 8-hourly achieved an average FTA of > 95% both for the traditional target of 40% f T > MIC and the aggressive target of 100% f T > MIC in patients without residual diuresis (Table 3). A residual diuresis of 100 or 300 mL/d did not change the results for the traditional target of 40% f T > MIC. In contrast, in simulations with a residual diuresis of 300 mL/d and with setting the target to 100% f T > MIC a meropenem dose of 2 g 8-hourly achieved an average FTA of 97%.
In the context of empiric therapy which includes P. aeruginosa strains at and below the resistance breakpoint (MIC ≤ 8 mg/L, 52771 isolates) a dose of 0.5 g 8-hourly achieved a FTA of > 95% for 40% f T > MIC in patients with residual diuresis of 0 and 100 mL/d, respectively (Table 4). For the more aggressive target of 100% f T > MIC, a dosing regimen of 2 g 8-hourly resulted in a FTA of 99% in patients with a residual diuresis ≤ 100 mL/d. In patients with a residual diuresis of 300 mL/d a meropenem dose of 2 g 8-hourly achieved a FTA of 92% (Table 4). This was further improved to 95% when simulating for prolonged infusions. In more resistant strains (MIC ≤ 16 mg/L, 56730 isolates) aiming at a PD target of 40% f T > MIC a meropenem dose of 2 g 8-hourly achieved a FTA of 95% with a residual diuresis of 300 mL/d. When aiming for a PD target of 100% f T > MIC, the highest meropenem dose of 2 g 8-hourly only resulted in a FTA > 95% in patients without residual diuresis (Table 5).
Discussion
This is the first study to describe the PK of meropenem in serial SLED treatments of critically ill septic patients in AKI using a population PK approach. The results show that achievement of optimal meropenem dosing in septic patients treated with SLED, depends on the microbiological susceptibility, the PK/PD-target (40% vs. 100% f T > MIC) and on the degree of residual diuresis.
Meropenem has been shown to have a CL that varies with the mode of RRT [24]. Therefore PK data from studies that did not include patients receiving SLED cannot be used to guide dosing during SLED. Under continuous venovenous haemofiltration (CVVH) and continuous venovenous haemodiafiltration (CVVHDF), mean meropenem CL was described to be 1.9 L/h and 3.6 L/h, respectively [44,45,46]. The meropenem CL on SLED (CLSLED = 7.9 L/h; ± 4.2 L/h) in this study was substantially higher than those reported for continuous RRT [44,45,46,47]. This can be explained by higher effluent and blood flow rates on SLED. In fact, CLSLED for meropenem was higher than the CL under intermittent RRT reported from Christensson et al. and Chimata et al. (1.2 and 4.8 L/h) who used cuprophan haemodialysis filters, which are not comparable to our filter systems [48, 49]. Compared to healthy volunteers (11–14 L/h), meropenem CL is significantly lower during SLED (7.9 L/h) [48, 49].
Not surprisingly, being on or off SLED had most impact on attaining target meropenem exposures in our model. The model fit further significantly increased by including the native clearance (CLN) linked to the residual diuresis. This is plausible since residual diuresis leads to additional meropenem elimination. This relationship has previously been shown by Ulldemolins et al. [50].
Due to the design of the PK model, CL without SLED (CLNS) is composed of CLN (1.5 L/h) and CLD (2.6 L/h) and is higher than Christenssen et al. reported within patients with end stage renal disease (CL = 1.2 L/h) [48]. This may be related to the fact, that SLED in our clinical setting was sometimes used to maintain fluid balance rather than for solute clearance. Additionally, carrying over effects of previous CRRT sessions as well as non-steady state conditions in intervals without SLED could have influenced these CL. The central volume of distribution (Vc) in our patients of 8.1 L (±7.1) was in accordance with the data of Roberts et al. who found a Vc of 7.9 L in septic patients [51].
A dosage regimen of 0.5 g 8-hourly would be adequate to achieve a PTA > 95% for the conventional target of 40% f T > MIC irrespective of the residual diuresis. However, for a target of 100% f T > MIC and a residual diuresis of up to 300 mL/d a dose of 2 g 8-hourly, which is the approved upper dosage limit, would achieve a PTA of 93%. Our results also showed that neither prolonged nor continuous meropenem infusion increased PTA compared with bolus application. This can be explained by the fact that in patients with AKI treated with SLED the meropenem clearance over a 24-hour period mainly occurs during SLED.
Regarding the FTA while aiming for the traditional target of 40% f T > MIC for susceptible P. aeruginosa (MIC ≤ 2 mg/L) a reduced dose of 0.5 g 8-hourly would ensure sufficient bactericidal activity irrespective of residual diuresis. As opposed to this, for a more aggressive target of 100% f T > MIC for susceptible P. aeruginosa (MIC ≤ 2 mg/L) an increased dose of 1 g 8-hourly would be required for patients with a residual diuresis of ≤ 100 mL/d. Further differing from this, patients with a residual diuresis of 300 mL/d would require the maximally approved dose of 2 g 8-hourly to achieve the target of 100% f T > MIC (FTA 97%). However, for P. aeruginosa strains at and below the resistance breakpoint (MIC ≤ 8 mg/L) we found that in patients with a residual diuresis of up to 300 mL/d even the maximally approved dosage of 2 g 8-hourly would result in an FTA of only 92%. The FTA was increased to 95% in this setting by simulating prolonged infusion (3 h) of 2 g 8-hourly.
Finally, for P. aeruginosa strains with a MIC ≤ 16 mg/L the maximally approved dose of 2 g 8-hourly only led to a target FTA (>95%) when aiming at a PK target of 40% fT > MIC. This was further improved by prolonged infusions in patients with a residual diuresis of 300 ml/d. When aiming for a target of 100% fT > MIC the maximal dose of 2 g 8-hourly only achieved a FTA > 95% in patients without residual diuresis. Prolonged infusions had no effect in this setting. When applying high doses of meropenem to ensure bactericidal activity as suggested by our simulations, clinicians need to balance this benefit against potential side effects such as seizures. However, the incidence of meropenem-associated seizures is described as low (<1%) and they are usually reversible on discontinuation and manageable with anticonvulsants [52].
Limitations
Endogenous renal function could not be estimated using standard approaches because serum creatinine as well as meropenem concentrations were affected by previous SLED or CRRT sessions.
Additionally, post-SLED treatment samples were not collected. Therefore, a potential rebound in serum concentrations as it has been described for vancomycin could have been missed, although this effect has not be shown for substances such as meropenem [39]. Therefore, this effect is not likely to affect the recommended dosing regimens.
Our study only simulates for 5 hours of SLED, as this was the average duration of SLED in our department, limiting extrapolation to patients receiving longer and more intensive SLED. The relatively short duration of SLED with the Genius® device in our routine clinical setting was often due to unintended interruptions for various reasons such as malfunctions of the extracorporeal circuit or early termination for patient mobilisation or transport. However, this often reflects common practice with SLED in the ICU.
Furthermore, simulating extended durations of SLED with our model would have increased the overall meropenem clearance per SLED session and thus may potentially underestimate dosing recommendations. Finally, some patients were treated with a CRRT before switching to SLED, which potentially could have led to carrying over effects from the previous CRRT.
Conclusions
In this study, we observed a relevant PK variability for meropenem in patients on SLED, which was significantly influenced by the degree of residual diuresis. As a result the given dosing recommendations for meropenem in patients on SLED to achieve adequate PD targets greatly vary. Patients with high residual diuresis, proven or suspected pathogens strains at and below the resistance breakpoint as well as aggressive PD targets require the maximum approved dosages. Further studies are required to validate our findings.
Given the high variability of meropenem PK under RRT and bacterial susceptibility, therapeutic drug monitoring may help to further optimise individual meropenem dosing in clinical practice [4, 25, 53].
Abbreviations
- AKI:
-
Acute kidney injury
- BMI:
-
Body mass index
- CI:
-
Continuous infusion
- CL:
-
Clearance
- CLSLED :
-
Meropenem clearance on SLED
- CLNS :
-
Population parameter estimate of meropenem clearance without SLED
- CLD :
-
Meropenem clearance referring to non-SLED clearance mechanisms
- CLN :
-
Native meropenem clearance associated with the residual diuresis
- CRP:
-
C-reactive protein
- CRRT:
-
Continuous renal replacement therapy
- CVVH:
-
Continuous venovenous haemofiltration
- CVVHDF:
-
Continuous venovenous haemodiafiltration
- f T > MIC :
-
Percentage of time remaining concentration above MIC
- FTA:
-
Fractional target attainment
- HPLC-UV:
-
High-performance liquid chromatography with UV-detection
- ICU:
-
Intensive care unit
- IHD:
-
Intermittent haemodialysis
- IQR:
-
Interquartile range
- KCP:
-
Constant for meropenem distribution from central to peripheral compartment
- KPC:
-
Constant for meropenem distribution from peripheral to central compartment
- MIC:
-
Minimal inhibitory concentration
- N/A:
-
Not applicable
- NPAG:
-
Nonparametric adaptive grid
- NPDE:
-
Normalised prediction distribution errors
- PCT:
-
Procalcitonin
- PD:
-
Pharmacodynamic
- PI:
-
Prolonged infusion
- PK:
-
Pharmacokinetic
- PTA:
-
Probability of target attainment
- RRT:
-
Renal replacement therapy
- R2:
-
Linear regression
- RD:
-
Residual diuresis
- SD:
-
Standard deviation
- SLED:
-
Sustained low-efficiency dialysis
- SOFA:
-
Modified sequential organ failure assessment score
- SAPS II:
-
Simplified acute physiology score
- TVCL:
-
Typical value of total clearance
- Vc :
-
Volume of distribution of the central compartment
- VPC:
-
Visual predictive check
References
Alobaidi R, Basu RK, Goldstein SL, Bagshaw SM. Sepsis-associated acute kidney injury. Semin Nephrol. 2015;35:2–11.
Hoste EA, Schurgers M. Epidemiology of acute kidney injury: how big is the problem? Crit Care Med. 2008;36:S146–51.
Kollef MH. Antibiotics for the critically ill: more than just selecting appropriate initial therapy. Crit Care. 2013;17:146.
Roberts JA, Kumar A, Lipman J. Right dose, right now: customized drug dosing in the critically ill. Crit Care Med. 2017;45:331–6.
Leroy A, Fillastre JP, Borsa-Lebas F, Etienne I, Humbert G. Pharmacokinetics of meropenem (ICI 194,660) and its metabolite (ICI 213,689) in healthy subjects and in patients with renal impairment. Antimicrob Agents Chemother. 1992;36:2794–8.
Breilh D, Texier-Maugein J, Allaouchiche B, Saux MC, Boselli E. Carbapenems. J Chemother. 2013;25:1–17.
Nicolau DP. Pharmacokinetic and pharmacodynamic properties of meropenem. Clin Infect Dis. 2008;47 Suppl 1:S32–40.
EUCAST. Meropenem Rationale for the EUCAST clinical breakpoints. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Meropenem_EUCAST_Rationale_Document_1.5_090601.pdf. Last accessed 11 Nov 2017.
Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58:1072–83.
De Waele JJ, Lipman J, Akova M, Bassetti M, Dimopoulos G, Kaukonen M, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Udy AA, Starr T, Wallis SC, Roberts JA. Risk factors for target non-attainment during empirical treatment with beta-lactam antibiotics in critically ill patients. Intensive Care Med. 2014;40:1340–51.
Li C, Du X, Kuti JL, Nicolau DP. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob Agents Chemother. 2007;51:1725–30.
McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T > MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents. 2008;31:345–51.
Beumier M, Casu GS, Hites M, Seyler L, Cotton F, Vincent JL, Jacobs F, Taccone FS. beta-lactam antibiotic concentrations during continuous renal replacement therapy. Crit Care. 2014;18:R105.
Bilgrami I, Roberts JA, Wallis SC, Thomas J, Davis J, Fowler S, Goldrick PB, Lipman J. Meropenem dosing in critically ill patients with sepsis receiving high-volume continuous venovenous hemofiltration. Antimicrob Agents Chemother. 2010;54:2974–8.
Binder L, Schworer H, Hoppe S, Streit F, Neumann S, Beckmann A, Wachter R, Oellerich M, Walson PD. Pharmacokinetics of meropenem in critically ill patients with severe infections. Ther Drug Monit. 2013;35:63–70.
Taccone FS, Laterre PF, Dugernier T, Spapen H, Delattre I, Wittebole X, De BD, Layeux B, Wallemacq P, Vincent JL, Jacobs F. Insufficient beta-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care. 2010;14:R126.
Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, Hope WW, Farkas A, Neely MN, Schentag JJ, Drusano G, Frey OR, Theuretzbacher U, Kuti JL. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14:498–509.
Goncalves-Pereira J, Povoa P. Antibiotics in critically ill patients: a systematic review of the pharmacokinetics of beta-lactams. Crit Care. 2011;15:R206.
Mattioli F, Fucile C, Del BV, Marini V, Parisini A, Molin A, Zuccoli ML, Milano G, Danesi R, Marchese A, Polillo M, Viscoli C, Pelosi P, Martelli A, Di PA. Population pharmacokinetics and probability of target attainment of meropenem in critically ill patients. Eur J Clin Pharmacol. 2016;72:839–48.
Roberts DM, Liu X, Roberts JA, Nair P, Cole L, Roberts MS, Lipman J, Bellomo R. A multicenter study on the effect of continuous hemodiafiltration intensity on antibiotic pharmacokinetics. Crit Care. 2015;19:84.
Shaw AR, Chaijamorn W, Mueller BA. We underdose antibiotics in patients on CRRT. Semin Dial. 2016;29:278–80.
Ulldemolins M, Roberts JA, Rello J, Paterson DL, Lipman J. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin Pharmacokinet. 2011;50:99–110.
Bogard KN, Peterson NT, Plumb TJ, Erwin MW, Fuller PD, Olsen KM. Antibiotic dosing during sustained low-efficiency dialysis: special considerations in adult critically ill patients. Crit Care Med. 2011;39:560–70.
Jamal JA, Udy AA, Lipman J, Roberts JA. The impact of variation in renal replacement therapy settings on piperacillin, meropenem, and vancomycin drug clearance in the critically ill: an analysis of published literature and dosing regimens*. Crit Care Med. 2014;42:1640–50.
Jamal JA, Mueller BA, Choi GY, Lipman J, Roberts JA. How can we ensure effective antibiotic dosing in critically ill patients receiving different types of renal replacement therapy? Diagn Microbiol Infect Dis. 2015;82:92–103.
Roberts DM, Roberts JA, Roberts MS, Liu X, Nair P, Cole L, Lipman J, Bellomo R. Variability of antibiotic concentrations in critically ill patients receiving continuous renal replacement therapy: a multicentre pharmacokinetic study. Crit Care Med. 2012;40:1523–8.
Roberts JA, Choi GY, Joynt GM, Paul SK, Deans R, Peake S, Cole L, Stephens D, Bellomo R, Turnidge J, Wallis SC, Roberts MS, Roberts DM, Lassig-Smith M, Starr T, Lipman J. SaMpling Antibiotics in Renal Replacement Therapy (SMARRT): an observational pharmacokinetic study in critically ill patients. BMC Infect Dis. 2016;16:103.
Jorres A. Acute kidney injury: choice of the initial modality for renal replacement therapy. Med Klin Intensivmed Notfmed. 2015;110:251–5.
Mushatt DM, Mihm LB, Dreisbach AW, Simon EE. Antibiotic dosing in slow extended daily dialysis. Clin Infect Dis. 2009;49:433–7.
Ronco C, Ricci Z, De BD, Kellum JA, Taccone FS, Joannidis M, Pickkers P, Cantaluppi V, Turani F, Saudan P, Bellomo R, Joannes-Boyau O, Antonelli M, Payen D, Prowle JR, Vincent JL. Renal replacement therapy in acute kidney injury: controversy and consensus. Crit Care. 2015;19:146.
Caires RA, Abdulkader RC, Costa E, Silva VT, Ferreira GS, Burdmann EA, Yu L, Macedo E. Sustained low-efficiency extended dialysis (SLED) with single-pass batch system in critically-ill patients with acute kidney injury (AKI). J Nephrol. 2016;29:401–9.
Villa G, Neri M, Bellomo R, Cerda J, De Gaudio AR, De RS, Garzotto F, Honore PM, Kellum J, Lorenzin A, Payen D, Ricci Z, Samoni S, Vincent JL, Wendon J, Zaccaria M, Ronco C. Nomenclature for renal replacement therapy and blood purification techniques in critically ill patients: practical applications. Crit Care. 2016;20:283.
Fieghen HE, Friedrich JO, Burns KE, Nisenbaum R, Adhikari NK, Hladunewich MA, Lapinsky SE, Richardson RM, Wald R. The hemodynamic tolerability and feasibility of sustained low efficiency dialysis in the management of critically ill patients with acute kidney injury. BMC Nephrol. 2010;11:32.
Mei JP, Ali-Moghaddam A, Mueller BA. Survey of pharmacists' antibiotic dosing recommendations for sustained low-efficiency dialysis. Int J Clin Pharm. 2016;38:127–34.
Harris LE, Reaves AB, Krauss AG, Griner J, Hudson JQ. Evaluation of antibiotic prescribing patterns in patients receiving sustained low-efficiency dialysis: opportunities for pharmacists. Int J Pharm Pract. 2013;21:55–61.
Roberts JA, Mehta RL, Lipman J. Sustained low efficiency dialysis allows rational renal replacement therapy, but does it allow rational drug dosing? Crit Care Med. 2011;39:602–3.
Roberts JA, Roberts DM. Antibiotic dosing in critically ill patients with septic shock and on continuous renal replacement therapy: can we resolve this problem with pharmacokinetic studies and dosing guidelines? Crit Care. 2014;18:156.
Deshpande P, Chen J, Gofran A, Murea M, Golestaneh L. Meropenem removal in critically ill patients undergoing sustained low-efficiency dialysis (SLED). Nephrol Dial Transplant. 2010;25:2632–6.
Kielstein JT, Czock D, Schopke T, Hafer C, Bode-Boger SM, Kuse E, Keller F, Fliser D. Pharmacokinetics and total elimination of meropenem and vancomycin in intensive care unit patients undergoing extended daily dialysis. Crit Care Med. 2006;34:51–6.
Elkhaili H, Niedergang S, Pompei D, Linger L, Leveque D, Jehl F. High-performance liquid chromatographic assay for meropenem in serum. J Chromatogr B Biomed Appl. 1996;686:19–26.
US Food and Drug Administration. Guidance for Industry Bioanalytical Method Validation. http://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf. Last accessed 11 Nov 2017.
Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit. 2012;34:467–76.
Tatarinova T, Neely M, Bartroff J, van Guilder M, Yamada W, Bayard D, Jelliffe R, Leary R, Chubatiuk A, Schumitzky A. Two general methods for population pharmacokinetic modeling: non-parametric adaptive grid and non-parametric Bayesian. J Pharmacokinet Pharmacodyn. 2013;40:189–99.
Jamal JA, Mat-Nor MB, Mohamad-Nor FS, Udy AA, Wallis SC, Lipman J, Roberts JA. Pharmacokinetics of meropenem in critically ill patients receiving continuous venovenous haemofiltration: a randomised controlled trial of continuous infusion versus intermittent bolus administration. Int J Antimicrob Agents. 2015;45:41–5
Krueger WA, Schroeder TH, Hutchison M, Hoffmann E, Dieterich HJ, Heininger A, Erley C, Wehrle A, Unertl K. Pharmacokinetics of meropenem in critically ill patients with acute renal failure treated by continuous hemodiafiltration. Antimicrob Agents Chemother. 1998;42:2421–4.
Giles LJ, Jennings AC, Thomson AH, Creed G, Beale RJ, McLuckie A. Pharmacokinetics of meropenem in intensive care unit patients receiving continuous veno-venous hemofiltration or hemodiafiltration. Crit Care Med. 2000;28:632–7.
Afshartous D, Bauer SR, Connor MJ, Aduroja OA, Amde M, Salem C, Groszek JJ, Fissell WH. Pharmacokinetics and pharmacodynamics of imipenem and meropenem in critically ill patients treated with continuous venovenous hemodialysis. Am J Kidney Dis. 2014;63:170–1.
Christensson BA, Nilsson-Ehle I, Hutchison M, Haworth SJ, Oqvist B, Norrby SR. Pharmacokinetics of meropenem in subjects with various degrees of renal impairment. Antimicrob Agents Chemother. 1992;36:1532–7.
Chimata M, Nagase M, Suzuki Y, Shimomura M, Kakuta S. Pharmacokinetics of meropenem in patients with various degrees of renal function, including patients with end-stage renal disease. Antimicrob Agents Chemother. 1993;37:229–33.
Ulldemolins M, Soy D, Llaurado-Serra M, Vaquer S, Castro P, Rodriguez AH, Pontes C, Calvo G, Torres A, Martin-Loeches I. Meropenem population pharmacokinetics in critically ill patients with septic shock and continuous renal replacement therapy: influence of residual diuresis on dose requirements. Antimicrob Agents Chemother. 2015;59:5520–8.
Roberts JA, Kirkpatrick CM, Roberts MS, Robertson TA, Dalley AJ, Lipman J. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J Antimicrob Chemother. 2009;64:142–50.
Slama TG. Clinical review: balancing the therapeutic, safety, and economic issues underlying effective antipseudomonal carbapenem use. Crit Care. 2008;12:233.
Huttner A, Harbarth S, Hope WW, Lipman J, Roberts JA. Therapeutic drug monitoring of the beta-lactam antibiotics: what is the evidence and which patients should we be using it for? J Antimicrob Chemother. 2015;70:3178–83.
Acknowledgements
The authors would like to thank all involved ICU staff and study nurses for their commitment and effort, without whom the study could not have been successfully completed.
Funding
The study was funded by Fresenius Medical Care (Bad Homburg, Germany) The company did not have any role in study design, data collection, data analysis, data interpretation, preparing the report and any decision for its publication.
J.A.R. is funded by a Practitioner Fellowship from the National Health and Medical Research Council of Australia (APP1117065). The Burns Trauma and Critical Care Research Centre would like to acknowledge other funding to our research centre from the National Health and Medical Research Council of Australia for a Project Grant (APP1044941) and Centre for Research Excellence (APP1099452).
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
SB made substantial contribution to conception, study design, data collection and interpretation of data and drafting the manuscript, and approved the final version to be published. CK made substantial contribution to conceptions, study design, sample analysis, data collection, data analysis and interpretation of data and drafting the manuscript, and approved the final version to be published. JAR made substantial contribution to conception, analysis and interpretation of data, drafting and revising the manuscript for intellectual content, and approved the final version to be published. AN made substantial contribution to conception, study design and revising the manuscript for intellectual content, and approved the final version to be published. OS made substantial contribution in revising the manuscript for intellectual content and approved the final version to be published. MB made substantial contribution to study design, sampling analysis and revising the manuscript for intellectual content, and approved the final version to be published. CL made substantial contribution to conception, study design and data collection, sample analysis and drafting and revising the manuscript for intellectual content, and approved the final version to be published. SK made substantial contribution to conception, study design and data collection and drafting and revising the manuscript for intellectual content, and approved the final version to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was obtained from the local ethics committee. Written informed consent was obtained either from the patient or their appointed legal guardian.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional information
Stephan Braune and Christina König are shared first authorship.
Stefan Kluge and Claudia Langebrake are shared last authorship.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Braune, S., König, C., Roberts, J.A. et al. Pharmacokinetics of meropenem in septic patients on sustained low-efficiency dialysis: a population pharmacokinetic study. Crit Care 22, 25 (2018). https://doi.org/10.1186/s13054-018-1940-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-018-1940-1