Abstract
Background
There is evidence to demonstrate that the coagulopathy which occurs in patients with traumatic brain injury coincides with disseminated intravascular coagulation (DIC). We hypothesized that DIC with increased fibrinolysis during the early stage of isolated traumatic brain injury (iTBI) affects the outcome of the patients and that hypoperfusion contributes to hyperfibrinolysis in the DIC.
Methods
This retrospective study included 92 patients with iTBI who were divided into DIC and non-DIC groups according to the Japanese Association Acute Medicine DIC scoring system. The DIC patients were subdivided into those with and without hyperfibrinolysis. The platelet counts and global markers of coagulation and fibrinolysis were measured. Systemic inflammatory response syndrome (SIRS), organ dysfunction (assessed by the Sequential Organ Failure Assessment score), tissue hypoperfusion (assessed by the lactate levels) and the transfusion volume were also evaluated. The outcome measure was all-cause hospital mortality.
Results
DIC patients showed consumption coagulopathy, lower antithrombin levels and higher fibrin/fibrinogen degradation products (FDP) and D-dimer levels than non-DIC patients. All of the DIC patients developed SIRS accompanied by organ dysfunction and required higher blood transfusion volumes, leading to a worse outcome than non-DIC patients. These changes were more prominent in DIC with hyperfibrinolysis. A higher FDP/D-dimer ratio suggests that DIC belongs to the fibrinolytic phenotype and involves fibrin(ogen)olysis. The mean blood pressures of the patients with and without DIC on arrival were identical. Hypoperfusion and the lactate levels were not identified as independent predictors of hyperfibrinolysis.
Conclusions
DIC, especially DIC with hyperfibrinolysis, affects the outcome of patients with iTBI. Low blood pressure-induced tissue hypoperfusion does not contribute to hyperfibrinolysis in this type of DIC.
Similar content being viewed by others
Background
There is evidence to demonstrate that the coagulopathy which occurs in patients with traumatic brain injury coincides with the definition of disseminated intravascular coagulation (DIC) by the International Society on Thrombosis and Haemostasis (ISTH): the intravascular activation of coagulation with the loss of localization and damage to the microvasculature [1, 2]. Kaufman et al. [3] showed clear evidence of consumption coagulopathy and the formation of disseminated microvascular thrombi in the brain and other organs, particularly in the lungs. Similarly, many studies using special staining techniques have revealed intravascular thrombosis in the injured brain [4]. Intravascular microthrombosis is strongly linked to neuronal death and affects the outcome as a secondary cerebral insult following initial brain damage [5,6,7]. In addition, endothelial activation and injury that occurs in association with intravascular tissue factor and thrombin formation, and systemic inflammation have been observed in patients with traumatic brain injury, especially among the patients who showed worse outcomes [8, 9].
DIC is divided into fibrinolytic (hemorrhagic) and thrombotic phenotypes [10, 11]. DIC in the early phase of trauma belongs to the fibrinolytic phenotype and is associated with a poor prognosis [12, 13]. If it does not improve, the DIC then proceeds to the thrombotic phenotype [13]. Since the first report of DIC in traumatic brain injury, many studies have pointed out that extremely elevated levels of fibrin/fibrinogen degradation products (FDP) and D-dimer are associated with a poor outcome [14,15,16,17]. These results suggest that DIC with the fibrinolytic phenotype also affects the prognosis of patients with traumatic brain injury [18].
DIC with the fibrinolytic phenotype is defined by the coexistence of both DIC and pathological systemic fibrin(ogen)olysis [10, 11]. In the early phase of trauma and traumatic shock, shock-induced hypoperfusion releases tissue-type plasminogen activator (t-PA) from the endothelial Weibel–Palade bodies, resulting in systemic primary fibrin(ogen)olysis in addition to DIC-induced secondary fibrinolysis [1, 10, 12, 13]. In the clinical setting, hemorrhagic shock is rarely associated with isolated traumatic brain injury (iTBI). Although the relationship between early coagulopathy and hypoperfusion was implicated in iTBI, the participation of hypoperfusion in increased fibrin(ogen)olysis has not been elucidated [19]. In addition, a recent study suggested that participation of endogenous plasminogen activators is involved in the increased fibrinolysis after iTBI, which causes progressive intracerebral hemorrhage [20].
Based on these insights derived from previous studies, we tested the hypotheses that DIC associated with increased fibrinolysis affects the outcome of patients with iTBI and that hypoperfusion contributes to the increased fibrinolysis in this type of DIC.
Methods
Patients
Consecutive patients with traumatic brain injury who were admitted to the emergency department (ED) from June 2000 to March 2016 were eligible for inclusion in the present study. Patients with severe iTBI who were admitted to the ICU were included in the present study. Exclusion criteria were as follows: missing data, ≤ 8 years of age, cardiac arrest and resuscitation, severe injuries to other parts of the body and cervical spinal cord injury. Our Institutional Review Board approved this study and waived informed consent.
We retrospectively conducted a systematic review of the computer-based medical records of these patients to provide baseline data and DIC-related parameters. The platelet count, prothrombin time, prothrombin time ratio, fibrinogen, antithrombin, FDP, D-dimer and lactate levels were measured at four time points within 24 hours after admission to the ED: Time Point 01, immediately after to 4 hours after arrival at the ED; Time Point 02, 4–8 hours after arrival; Time Point 03, 8–16 hours after arrival; and Time Point 04, 16–24 hours after arrival. The day 0 data showed the worst maximal values (highest or lowest) of these four measurement time points. The day 0 data were used for classifying patients with or without DIC and for determining the phenotype.
Definitions
Severe isolated traumatic brain injury (iTBI) was defined according to the Abbreviated Injury Scale (AIS) as follows: AIS of the head and neck ≥ 3 and AIS of other body parts ≤ 2. The Injury Severity Score (ISS) was used to evaluate the degree of the injury. In the present study, brain damage was defined as cerebral contusion with or without hematoma and subdural hematoma with cerebral contusion. The severity of the patient’s condition and organ dysfunction was assessed by the Acute Physiology and Chronic Health Evaluation II (APACHE II) score and the Sequential Organ Failure Assessment (SOFA) score [21], respectively. The systemic inflammatory response syndrome (SIRS) score was calculated according to the formula established in the American College of Chest Physicians/Society of Critical Care Medicine consensus conference [22]. DIC was diagnosed based on the Japanese Association for Acute Medicine (JAAM) DIC and the International Society on Thrombosis and Haemostasis (ISTH) overt DIC diagnosis criteria based on the day 0 data [1, 23]. DIC and overt DIC were diagnosed based on total scores of ≥ 4 and ≥ 5, respectively. We referred to the criteria of Asakura [11] for the definition of the DIC phenotype. Hyperfibrinolysis was defined as an FDP level ≥ 100 μg/ml, and the FDP/D-dimer ratio was used as a surrogate marker of fibrin(ogen)olysis [11]. Tissue hypoperfusion was defined as a blood lactate level ≥ 4 mmol/L based on the Surviving Sepsis Campaign Guidelines 2012 [24]. The outcome measure was the rate of all-cause hospital mortality.
Statistical analyses
The results were shown as the median and interquartile range. The IBM SPSS software program (version 24.0 for MAC OSX; IBM Japan, Tokyo, Japan) was used for all of the analyses. The Kruskal–Wallis test was used for comparison among three groups, while the Mann–Whitney U test and the chi-square test were used for comparisons between two groups. The dependent and the independent variables were evaluated by logistic regression analysis (the backward stepwise method based on likelihood) and the odds ratios and 95% confidence intervals were shown. The prediction of hospital death was evaluated using the area under the receiver operating characteristic (ROC) curve (AUC). Survival curves during hospital stay were analyzed by the Kaplan–Meier method using a log-rank test for comparison. p < 0.05 was considered to indicate statistical significance.
Results
Demographic and clinical characteristics of the patients
During the study period, a total of 335 trauma patients with brain injury presented to the ED. After the exclusion of ineligible patients and patients with incomplete data, patients with cervical spinal cord injury in whom the AIS of the head and neck was ≥ 3 were further excluded. Finally, 92 eligible patients with iTBI were identified. The patients were divided into the DIC (n = 45) and non-DIC (n = 47) groups based on the JAAM DIC scoring system (Fig. 1).
Table 1 presents the demographic data of the patients. Although the ISS and AIS were distributed evenly between the two groups, the APACHE II scores of the DIC patients were higher. All of the DIC patients developed SIRS with higher SOFA scores and required greater volume of blood transfusion, leading to a significantly worse outcome in comparison to the non-DIC patients. It is important to note that the mean blood pressures of the two groups at arrival to the ED were ≥ 90 mmHg and that only 5 patients required catecholamine, which suggests a low incidence of hemorrhagic shock in both groups of the patients. The use of tranexamic acid was evenly distributed between the two groups. No patients received an infusion or transfusion in the prehospital setting because emergency medical service personnel are not permitted perform transfusion and the infusion of Ringer’s lactate solution is only allowed for patients with a severe worsening of hypovolemic shock.
Serial changes in the platelet count and markers of coagulation and fibrinolysis
The DIC patients continuously showed significantly lower platelet counts, more prolonged prothrombin time ratios and lower levels of fibrinogen and antithrombin immediately after arrival at the ED (Time Point 01) until Time Point 04, and on day 0 in comparison to non-DIC patients (Fig. 2). The DIC patients also showed persistently higher levels of FDP and D-dimer in comparison to the non-DIC patients (Fig. 3). In addition, the FDP/D-dimer ratios of the DIC patients were significantly higher than those of the non-DIC patients (Fig. 4). These results suggest consumption coagulopathy, insufficient anticoagulation by antithrombin and increased fibrin(ogen)olysis in DIC patients. The numbers of patients at each time point are presented in Additional file 1: Table S1.
Box plots of platelet counts, prothrombin time ratios, and fibrinogen and antithrombin levels. Dark boxes, DIC patients; white boxes, non-DIC patients. Horizontal bars in the box indicate the median (middle) and interquartile ranges (upper 25%, lower 75%). Black squares in the box indicate the mean value. Top and bottom bars indicate the maximum and minimum values, respectively. T01 Time Point 01 (immediately after to 4 hours after arrival at the ED), T02 Time Point 02 (4–8 hours after arrival), T03 Time Point 03 (8–16 hours after arrival), T04 Time Point 04 (16–24 hours after arrival), Day 0 worst maximal values (highest or lowest) of these four measurement time points. *p < 0.05, **p < 0.01, †p < 0.001 vs non-DIC patients
Box plots of FDP, D-dimer and lactate levels. Dark boxes, DIC patients; white boxes, non-DIC patients. Horizontal bars in the box indicate the median (middle) and interquartile ranges (upper 25%, lower 75%). Black squares in the box indicate the mean value. Top and bottom bars indicate the maximum and minimum values, respectively. FDP fibrin/fibrinogen degradation products, T01 Time Point 01 (immediately after to 4 hours after arrival at the ED), T02 Time Point 02 (4–8 hours after arrival), T03 Time Point 03 (8–16 hours after arrival), T04 Time Point 04 (16–24 hours after arrival), Day 0 worst maximal values (highest or lowest) of these four measurement time points. *p < 0.05, **p < 0.01, †p < 0.001 vs non-DIC patients
Subgroup analyses of the DIC patients
The DIC patients were subdivided into those with (n = 17) and without (n = 28) hyperfibrinolysis based on their FDP levels (Table 2). The DIC patients’ fibrinolysis marker levels are presented in Table 3, which shows that the levels of FDP and D-dimer of the DIC patients with hyperfibrinolysis were extremely high in comparison to those without hyperfibrinolysis. The ISS of the two groups was identical; however, the patients with hyperfibrinolysis had higher DIC, APACHE II and SOFA scores and underwent transfusion with higher volumes of red blood cell concentrates in comparison to the patients without hyperfibrinolysis. The FDP/D-dimer ratios of the patients with hyperfibrinolysis were markedly higher than non-DIC patients (Fig. 4). Based on these results, DIC with hyperfibrinolysis is considered equivalent to DIC with the fibrinolytic phenotype, which is associated with fibrin(ogen)olysis, hemorrhage and organ dysfunction. DIC with hyperfibrinolysis was associated with a higher mortality rate (58.9%) in comparison to DIC without hyperfibrinolysis (10.7%).
Analyses of the outcomes and the predictors of hyperfibrinolysis
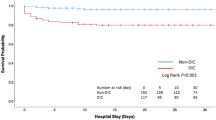
Stepwise logistic regression analyses confirmed that the DIC and APACHE II scores are independent predictors of patients’ death (Table 4). The ROC curves further showed the significant discriminative performance of the DIC and APACHE II scores for predicting death in iTBI patients (Fig. 5). These results are important because the DIC score showed good discriminative power for predicting the outcome in comparison to the APACHE II score, which is known as a superior predictor of death in various kinds of critical illness. The Kaplan–Meier curves showed that DIC, especially DIC diagnosed by the ISTH scoring system and DIC with hyperfibrinolysis, significantly affected the survival rate of patients with iTBI (Fig. 6). Table 5 shows that the DIC score and the existence of hyperfibrinolysis further influenced the death in DIC patients.
ROC curves of the DIC scores (green line) and APACHE II score (blue line) for predicting hospital death. APACHE Acute Physiology and Chronic Health Evaluation, AUC area under the receiver operating characteristic curve, DIC disseminated intravascular coagulation, SE standard error (Color figure online)
Kaplan–Meier curves showing the association between DIC (a) or DIC with hyperfibrinolysis (b) and hospital mortality. Log-rank p = 0.001 (a), p = 0.001 (b). DIC disseminated intravascular coagulation, ISTH International Society on Thrombosis and Haemostasis, JAAM Japanese Association for Acute Medicine (Color figure online)
Despite the fact that the two groups showed the same mean blood pressures on arrival at the ED, the DIC patients, especially those with hyperfibrinolysis, showed a higher prevalence of hypoperfusion and significantly higher lactate levels (Fig. 3, Tables 1 and 3). However, the lactate levels were not found to be an independent predictor of hyperfibrinolysis by the stepwise logistic regression analyses (odds ratio, 1.335; 95% confidence interval, 0.991–1.798; p = 0.058) (Table 5). In addition, neither hypoperfusion nor brain damage predicted hyperfibrinolysis in DIC patients.
Discussion
The present study demonstrated that DIC—especially DIC with hyperfibrinolysis—during the early phase of iTBI is accompanied by systemic inflammation, organ dysfunction and greater need for transfusion. Furthermore, DIC significantly affects patient outcomes, which coincides with the trauma of the other body parts. Hemorrhagic shock is rare in iTBI patients and the tissue hypoperfusion evaluated by lactate levels did not contribute to hyperfibrinolysis in DIC patients with iTBI. The higher FDP/D-dimer ratios implicate the fibrin(ogen)olysis in this type of DIC.
Previous studies have separately discussed the roles of DIC and increased markers of fibrinolysis in the outcome of patients with traumatic brain injury or iTBI. DIC-induced microvascular thrombosis is closely linked to the area of ischemic changes and neuronal death in the injured brain [4, 5, 7]. Systemic microthrombi in vital organs such as the lungs, liver, kidneys, intestines and pituitary glands give rise to organ dysfunction and result in a worse outcome [3, 5, 9, 15]. Other studies have shown that DIC increases the FDP and D-dimer levels, which are independent predictors of progressive hemorrhage and a poor outcome in patients with traumatic brain injury [15,16,17]. The results of the present study unify DIC and increased FDP and D-dimer levels as one concept of DIC with hyperfibrinolysis, which leads to SIRS, organ dysfunction, a greater need for transfusion and worse outcomes in patients with iTBI.
van der Sande et al. [14] demonstrated that DIC with a fatal outcome was associated with extremely high FDP levels and assumed the coexistence of an underlying process that differed from DIC, namely primary fibrin(ogen)olysis due to an unknown cause. In the present study, higher FDP/D-dimer ratios in DIC—especially DIC with hyperfibrinolysis—implicated the existence of fibrin(ogen)olysis and suggested that the DIC belongs to the fibrinolytic phenotype [10, 11]. Another study showed a close relationship between DIC with fibrinolysis and delayed and recurrent intracranial hematomas in patients with head injury [25]. The same group demonstrated that DIC with fibrinolytic syndrome increased the mortality rate by > 4 times that of patients without DIC [18]. These studies support our hypothesis and results, which indicates that DIC with hyperfibrinolysis (the fibrinolytic phenotype) solely affects the outcome of patients with iTBI. The present study further suggests that DIC with the fibrinolytic phenotype in iTBI equally influences the patient’s outcome similar to DIC with the fibrinolytic phenotype in patients with extracranial trauma [2, 12, 13].
The consumption coagulopathy observed in the present study indicates that the increases in the levels of FDP and D-dimer are partly due to DIC-induced secondary fibrinolysis. Fibrin(ogen)olysis, which is thought to be present based on the higher FDP/D-dimer ratios, suggests that a condition other than iTBI increases the levels of the two markers. Thus, the involvement of coexisting condition(s) in hyperfibrinolysis was investigated. The present study failed to show a difference in the mean blood pressures on arrival at the ED between the DIC and non-DIC groups or DIC with and without hyperfibrinolysis. This means that the mechanism underlying the development of hyperfibrinolysis due to the shock-induced release of t-PA from the endothelial cells is unlikely. Although apparent shock was not observed, the higher lactate levels in DIC suggest the role of tissue hypoperfusion in the mechanisms of hyperfibrinolysis. However, the logistic regression analyses clearly demonstrated that tissue hypoperfusion was not a cause of hyperfibrinolysis in patients with iTBI-associated DIC. These results suggest that the lactate levels are not increased due to low blood pressure-related hypoperfusion and that they occurred due to DIC-induced low flow-related hypoperfusion in the damaged brain tissues [19, 26, 27].
Another explanation of hyperfibrinolysis in iTBI is that traumatic brain injury induces the release of t-PA from injured brain tissues [2]. Localized fibrinolytic activity and the expression of t-PA in the brain have long been reported to occur [28,29,30,31]. A recent study indicated that endogenous t-PA and urokinase-type plasminogen activator (u-PA) increase the lysis of plasma clots and contribute to intracerebral hemorrhage after traumatic brain injury [20]. Based on these studies, we investigated the relationship between brain damage and hyperfibrinolysis. However, the logistic regression analysis did not show brain damage to be the main cause of hyperfibrinolysis. A more detailed study should be performed in order to determine how the areas of brain damage are associated with the increases in FDP and D-dimer levels.
Lastly, the present study clearly demonstrated that DIC is associated with SIRS, organ dysfunction and the need for greater transfusion volumes, especially in patients with hyperfibrinolysis, leading to a worse outcome in patients with iTBI. The ROC curve suggests that the DIC score could be used for predicting the outcome of patients with iTBI. It is important to note that the possibility of survival in DIC with hyperfibrinolysis was < 40%. These results indicate the need to treat DIC as well as hyperfibrinolysis during the early stage of iTBI. The administration of tranexamic acid within 3 hours of iTBI may be a promising treatment; however, the late application of this drug might increase intracerebral hemorrhage by potentiating u-PA-mediated plasminogen activation. [32, 33].
The present study was associated with some limitations. This was a single-center retrospective study that involved a relatively small number of patients and was limited by incomplete data. We also acknowledge that the relatively small sample size in the present study negatively impacts the robustness of the logistic regression analyses. The measured markers were collected systemically and not locally from the brain. The molecular markers of fibrinolysis were not measured, nor were the relationships between increased fibrinolysis and intracerebral hemorrhage. It is necessary to elucidate how the release of t-PA from the injured brain tissues is involved in hyperfibrinolysis. Finally, over the long years of the study period the patients have changed treatments, which may act as confounders to the data.
Conclusions
Patients with DIC during the early stage of iTBI showed lower platelet counts, consumption coagulopathy and insufficient coagulation control by antithrombin. Highly elevated FDP and D-dimer levels and increased FDP/D-dimer ratios suggest that DIC belongs to the fibrinolytic phenotype, which involves fibrin(ogen)olysis. DIC was associated with SIRS, organ dysfunction and need for greater transfusion volumes, especially in patients with hyperfibrinolysis, leading to worse outcomes in patients with iTBI. The lactate levels and the prevalence of hypoperfusion were increased in DIC patients. However, hemorrhagic shock was not observed and neither lactate levels nor hypoperfusion contributed to hyperfibrinolysis in DIC patients. The results failed to prove that damaged brain tissue contributes to hyperfibrinolysis; this mechanism should be investigated in a more detailed study in the future. To improve the outcome of the patients with iTBI, the control of both DIC and increased fibrinolysis will be necessary.
Abbreviations
- AIS:
-
Abbreviated Injury Scale
- APACHE:
-
Acute Physiology and Chronic Health Evaluation
- AUC:
-
Area under the receiver operating characteristic curve
- DIC:
-
Disseminated intravascular coagulation
- ED:
-
Emergency department
- FDP:
-
Fibrin/fibrinogen degradation products
- ISS:
-
Injury Severity Score
- ISTH:
-
International Society on Thrombosis and Haemotasis
- iTBI:
-
Isolated traumatic brain injury
- JAAM:
-
Japanese Association for Acute Medicine
- ROC:
-
Receiver operating characteristic
- SIRS:
-
Systemic inflammatory response syndrome
- SOFA:
-
Sequential Organ Failure Assessment
- t-PA:
-
Tissue-type plasminogen activator
- u-PA:
-
Urokinase-type plasminogen activator
References
Taylor Jr FB, Toh CH, Hoots WK, Wada H, Levi M. Toward definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–30.
Gando S. Hemostasis and thrombosis in trauma patients. Semin Thromb Hemost. 2015;41:26–34.
Kaufman HH, Hui KS, Mattson JC, Borit A, Childs TL, Hooots WK, et al. Clinicopathological correlations of disseminated intravascular coagulation in patients with head injury. Neurosurgery. 1984;15:34–42.
Stein SC, Smith DH. Coagulopathy in traumatic brain injury. Neuro Crit Care. 2004;1:479–88.
Stein SC, Young GS, Talucci RC, Greenbaum BH, Ross SE. Delayed brain injury after head trauma: significance of coagulopathy. Neurosurgery. 1992;30:160–5.
Stein SC, Chen XH, Sinson GP, Smith DH. Intravascular coagulation: a major secondary insult in nonfatal traumatic brain injury. J Neurosurg. 2002;97:1373–7.
Stein SC, Graham DI, Chen XH, Smith DH. Association between intravascular microthrombosis and cerebral ischemia in traumatic brain injury. Neurosurgery. 2004;54:687–91.
Yokota H, Naoe Y, Nakabayashi M, Unemoto K, Kushimoto S, Kurokawa A, et al. Cerebral endothelial injury in severe head injury: the significance of serum thrombomodulin and the von Willebrand factor. J Neurotrauma. 2002;19:1007–15.
Di Battisata AP, Rizoli SB, Lejnieks B, Min A, Shiu MY, Peng HT, et al. Sympathoadrenal activation is associated with acute traumatic coagulopathy and endotheliopathy in isolated brain injury. Shock. 2016;46 Suppl 1:96–103.
Marder VJ, Feinstein DI, Colman RW, Levi M. Consumptive thrombohemorrhagic disorders. In: Colman RW, Marder VJ, Clowes AW, George JN, Goldhaber SZ, editors. Hemostasis and Thrombosis. Basic Principles and Clinical Practice. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006. p. 1571–600.
Asakura H. Classifying types of disseminated intravascular coagulation: clinical and animal models. J Intensive Care. 2014;2:20.
Sawamura A, Hayakawa M, Gando S, Kubota N, Sugano M, Wada T, et al. Disseminated intravascular coagulation with a fibrinolytic phenotype at an early phase of trauma predicts mortality. Thromb Res. 2009;124:608–13.
Gando S. Disseminated intravascular coagulation. In: Gonzalez E, Moor HB, Moore E, editors. Trauma induced coagulopathy. Switzerland: Springer International Publishing AG; 2016. p. 195–217.
van der Sande JJ, Veltkamp JJ, Boekhout-Mussert RJ, Bouwhuis-Hoogerwerf ML. Head injury and coagulation disorders. J Neurosurg. 1978;49:357–65.
Olson JD, Kaufmann HH, Moake J, O’Gorman TW, Hoots K, Wagner K, et al. The incidence and significance of hemostatic abnormalities in patients with head injuries. Neurosurgery. 1989;24:825–32.
Tian HL, Chen H, Wu BS, Cao HL, Xu T, Hu J, Wang G, et al. D-dimer as a predictor of progressive hemorrhagic injury in patients with traumatic brain injury: analysis of 194 cases. Neurosurg Rev. 2010;33:359–66.
Nakae R, Takayama Y, Kuwamoto K, Naoe Y, Sato H, Yokota H. Time course of coagulation and fibrinolytic parameters in patients with traumatic brain injury. J Neurotrauma. 2016;33:688–95.
Miner ME, Kaufmann HH, Graham SH, Haar FH, Gildenberg PL. Disseminated intravascular coagulation fibrinolytic syndrome following head injury in children: frequency and prognostic implication. J Pediatrics. 1982;100:687–91.
Lustenberger T, Talving P, Kobayashi L, Barmparas G, Inaba K, Lam L, et al. Early coagulopathy after isolated severe traumatic brain injury: relationship with hypoperfusion challenged. J Trauma. 2010;69:1410–4.
Hijazi N, Fanne RA, Abramovitch R, Yarovoi S, Higazi M, Abdeen S, et al. Endogenous plasminogen activators mediate progressive intracerebral hemorrhage after traumatic brain injury in mice. Blood. 2015;125:2558–67.
Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–8.
Members of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference committee. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definition for sepsis and organ failure and guidelines for the use innovative therapies in sepsis. Crit Care Med. 1992;20:864–74.
Gando S, Saitoh D, Ogura H, Mayumi T, Koseki K, Ikeda T, Japanese Association for Acute Medicine Disseminated Intravascular Coagulation (JAAM DIC) study group, et al. Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: results of a multicenter, prospective survey. Crit Care Med. 2008;36:145–50.
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637.
Kaufman HH, Moake JL, Olson JD, Miner ME, duCret RP, Pruessner JL, et al. Delayed and recurrent intracranial hematomas related to disseminated intravascular clotting and fibrinolysis in head injury. Neurosurgery. 1980;7:445–9.
Lin HL, Kuo LC, Chen CW, Lee WC. Early coagulopathy resulted from brain injury rather than hypoperfusion. J Trauma. 2011;70:765.
Schwarzmaier SM, Kim SW, Trabold R, Plesnila N. Temporal profile of thrombogenesis in the cerebral microcirculation after traumatic brain injury in mice. J Neurotrauma. 2010;27:121–30.
Tovi D. Fibrinolytic activity of human brain. A histochemical study. Acta Neurol Scandinav. 1973;49:152–62.
Melchor JP, Strickland S. Tissue plasminogen activator in central nervous system physiology and pathology. Thromb Haemost. 2005;93:655–60.
Sahindranath M, Samson AL, Downes CE, Crack PJ, Lawrence AJ, Li QX, et al. Compartment- and context-specific changes in tissue-type plasminogen activator (tPA) activity following brain injury and pharmacological stimulation. Lab Invest. 2011;91:1079–91.
Lemarchant S, Docagne F, Emery E, Vivien D, Ali C, Rubio M. tPA in the injured central nervous system: different scenarios starring the same actor? Neuropharmacol. 2012;63:749–56.
Medcalf RL. The traumatic side of fibrinolysis. Blood. 2015;125:2457–8.
Dewan Y, Komolafe EO, Mejia-Mantilla JH, Perel P, Roberts I, Shakur H, on behalf of CRASH-3 Collaborators. CRASH-3-tranexamic acid for the treatment of significant traumatic brain injury: study protocol for an international randomized, double-blind, placebo-controlled trial. Trials. 2012;13:87.
Acknowledgements
None.
Funding
Departmental funding was used for this study.
Availability of data and materials
The datasets supporting the conclusions of this article will be provided based on reasonable request to the corresponding author.
Author information
Authors and Affiliations
Contributions
TW designed the study, interpreted the data, and drafted the manuscript. SG conceived the study, analyzed and interpreted the data, and drafted the manuscript. MH, AS, KM, KK and HS participated in the study design, helped to draft the manuscript, and proofread the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The Institutional Review Board of our institution (Hokkaido University Hospital Clinical Research and Medical Innovation Center) approved this study (No. 016-0148) and waived the need for informed consent.
Consent for publication
Not applicable.
Competing interests
SG received payment for lectures from Asahi Kasei Pharma. The remaining authors declare that they have no conflicts of interests in association with the present study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1: Table S1.
(XLSX 23 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wada, T., Gando, S., Maekaw, K. et al. Disseminated intravascular coagulation with increased fibrinolysis during the early phase of isolated traumatic brain injury. Crit Care 21, 219 (2017). https://doi.org/10.1186/s13054-017-1808-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-017-1808-9