Abstract
Background
Pathogenic BRCA1 founder mutations (c.4035delA, c.5266dupC) contribute to 3.77% of all consecutive primary breast cancers and 9.9% of all consecutive primary ovarian cancers. Identifying germline pathogenic gene variants in patients with primary breast and ovarian cancer could significantly impact the medical management of patients. The aim of the study was to evaluate the rate of pathogenic mutations in the 26 breast and ovarian cancer susceptibility genes in patients who meet the criteria for BRCA1/2 testing and to compare the accuracy of different selection criteria for second-line testing in a founder population.
Methods
Fifteen female probands and 1 male proband that met National Comprehensive Cancer Network (NCCN) criteria for BRCA1/2 testing were included in the study and underwent 26-gene panel testing. Fourteen probands had breast cancer, one proband had ovarian cancer, and one proband had both breast and ovarian cancer. In a 26-gene panel, the following breast and/or ovarian cancer susceptibility genes were included: ATM, BARD1, BLM, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, EPCAM, FAM175A, MEN1, MLH1, MRE11A, MSH2, MSH6, MUTYH, NBN, PALB2, PMS2, PTEN, RAD50, RAD51C, RAD51D, STK11, TP53, and XRCC2. All patients previously tested negative for BRCA1 founder mutations.
Results
In 44% (7 out of 16) of tested probands, pathogenic mutations were identified. Six probands carried pathogenic mutations in BRCA1, and one proband carried pathogenic mutations in BRCA2. In patients, a variant of uncertain significance was found in BRCA2, RAD50, MRE11A and CDH1. The Manchester scoring system showed a high accuracy (87.5%), high sensitivity (85.7%) and high specificity (88.9%) for the prediction of pathogenic non-founder BRCA1/2 mutations.
Conclusion
A relatively high incidence of pathogenic non-founder BRCA1/2 mutations was observed in a founder population. The Manchester scoring system predicted the probability of non-founder pathogenic mutations with high accuracy.
Similar content being viewed by others
Background
Hereditary breast cancers account for approximately 10% of all breast cancers, and approximately 23% of all ovarian cancers are considered hereditary [1, 2]. According to Plakhins et al., BRCA1 pathogenic founder mutations (c.4035delA, c.5266dupC) contribute to 3.77% of all consecutive primary breast cancers and 9.9% of all consecutive primary ovarian cancers [3]. BRCA1 and BRCA2 pathogenic founder mutation analysis is a relatively straightforward and cost-effective screening strategy to identify mutation carriers [4]. In Latvia, all consecutive breast and ovarian cancer cases are eligible for BRCA1 pathogenic founder mutations (c.181 T > G, c.4035delA, c.5266dupC) screening [5], and the costs of the test are covered by the public health care system. However, according to recent studies, non-founder BRCA1 and BRCA2 pathogenic mutations account for up to 21.6% of all BRCA1 and BRCA2 pathogenic mutations in the Aschkenazi Jewish population [6, 7]. There is little information about pathogenic BRCA1/2 non-founder mutations in Latvia. In a study published by Berzina et al., pathogenic non-founder mutations in BRCA1 and BRCA2 were identified in 4 out of 30 high-risk breast/ovarian cancer families from the Latvian population [8]. In another study published by Tihomirova et al., non-founder pathogenic mutations in BRCA1 and BRCA2 were detected in 9 out of 160 patients with breast and ovarian cancer [5]. These findings suggest that the proportion of pathogenic BRCA1/2 non-founder mutations is small and that family cancer history alone is of limited value to find subgroups of individuals, where expensive complete BRCA1/2 testing is indicated.
The remaining hereditary breast and ovarian cancer cases are associated with mutations in other breast and ovarian cancer susceptibility genes, such as BRCA1/2, TP53, PTEN, CDH1, STK11, MLH1, MSH2, MSH6, PMS2, PALB2, CHEK2, ATM, RAD51C, RAD51D, BRIP1 and other [9]. Patients and their relatives harbouring mutations in hereditary cancer predisposing genes could benefit prevention and screening strategies or novel therapeutic approaches [10, 11]. Advances in next-generation sequencing allowed the implementation of low-cost multi-gene panel testing in clinical practice to detect pathogenic mutations in hereditary cancer predisposing genes [12].
Therefore, knowledge of the frequency and phenotypical features of pathogenic mutations beyond BRCA1 pathogenic founder mutations in breast and ovarian cancer susceptibility genes is essential for determining the role of second-line testing with multi-gene panels in counselling unsolved high-risk breast and ovarian cancer patients.
The aim of the study was to evaluate the rate of pathogenic mutations in the 26 breast and ovarian cancer susceptibility genes in patients who meet the criteria for BRCA1/2 testing and to compare the accuracy of different selection criteria for second-line testing in a founder population.
Methods
Patient group
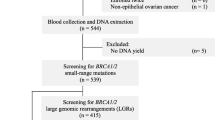
Sixteen sequential patients with primary breast and/or ovarian cancer who met all inclusion criteria were included in the study between October 2016 and August 2017. The inclusion criteria were as follows: 1) fulfil at least one of the National Comprehensive Cancer network (NCCN) BRCA1/2 testing criteria (Table 1) (www.nccn.org); 2) previously tested negative for BRCA1 pathogenic founder mutations (c.181 T > G, c.4035delA, c.5266dupC); 3) able to cover the cost of the 26 multi-gene tests.
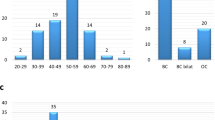
The following clinical information was obtained: age at testing, personal cancer history, age at cancer diagnosis, breast and/or ovarian cancer pathology, BRCA1/2 testing history, a family cancer history that covers a 3-generation pedigree according to probands information. The median patient age was 45.6 years (33–63 years). Fifteen out of 16 (93.75%) patients were females, and 1 out of 16 (6.25%) patients was male. Thirteen patients had unilateral breast cancer, 1 patient had bilateral breast cancer, 1 patient had ovarian cancer, and in 1 patient had both breast and ovarian cancer. Four out of 16 (25%) breast cancers were luminal-like HER2 negative, 2 out of 16 (12.5%) breast cancers were luminal B HER2 positive, 8 out of 16 (50%) breast cancers were triple-negative, and 1 out of 16 (6.25%) breast cancers was HER2 positive. The patient characteristics are summarized in Table 2.
DNA testing
Informed consent for genetic testing was obtained for all patients. All patients underwent DNA testing with a 26-gene panel (myBRCA HiRisk Hereditary Breast and Ovarian Cancer screening Test, VeritasGenetics, USA) that is a targeted next-generation sequencing assay for the detection of mutations in 26 breast and ovarian cancer susceptibility genes. The genes included high-penetrance breast-ovarian genes (BRCA1, BRCA2, PTEN, TP53, CDH1, STK11, PALB2), moderate-penetrance breast and/or ovarian genes (CHEK2, BRIP1, ATM), and additional genes (BARD1, BLM, EPCAM, RAD50, RAD51C, RAD51D, MEN1, MRE11A, MUTYH MSH2, MLH1, NBN, MSH6, PMS2, FAM175A, XRCC2). In all patients, the test was performed using saliva. The specificity and sensitivity of the assay are 99.9% for point mutations and small insertions/deletions in the 24 sequenced genes and 99.9% for structural variations in BRCA1 and BRCA2.
Statistical analysis
The specificity, sensitivity and accuracy of the NCCN criteria, Manchester scoring system and Swedish Breast cancer group criteria for the prediction of pathogenic non-founder mutations were evaluated. The Manchester score of 15 points threshold was used to assess the likelihood of BRCA1/2 pathogenic mutation [13]. The specificity, sensitivity and accuracy of different selection criteria for BRCA1/2 testing in our cohort were calculated using MedCalc Statistical Software version 17.9.
Results
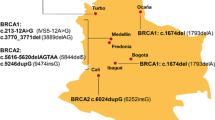
In seven out of sixteen (44%) patients included, pathogenic non-founder BRCA1/2 mutations were identified. Six patients carried pathogenic variants of BRCA1 and one of BRCA2. In four patients, variants of uncertain significance of BRCA2, RAD50, MRE11A and CDH1 were found. Detailed results are shown in Table 3. The NCCN criteria showed a high sensitivity (100%) with low specificity (50%) for the prediction of non-founder pathogenic BRCA1/2 mutations. The Swedish Breast cancer group criteria showed a low sensitivity (57.1%) with three false negative results. The Manchester scoring system showed a high accuracy (87.5%) for the prediction of pathogenic non-founder BRCA1/2 mutations with high sensitivity (85.7%) and specificity (88.9%). The sensitivity, specificity and accuracy of different criteria/scoring systems for the detection of probability of BRCA1/2 pathogenic mutations in our cohort are compared in Table 4.
Discussion
Our study is the first report on the use of a 26 gene panel in to examine breast and ovarian cancer susceptibility genes in patients in Latvia. We demonstrated a high frequency of pathogenic non-founder germline mutations in BRCA1 and BRCA2 genes. In seven out of sixteen (44%) primary breast and ovarian cancer patients matching the criteria for BRCA1/2 testing pathogenic non-founder BRCA1/2 mutations were identified. All 7 pathogenic mutations, including 2 large deletions, are novel in populations of Latvia [5, 8]. These results may suggest that the present practice of testing only the 3 most frequent BRCA1 pathogenic founder mutations is insufficient and fails to detect a considerable number of pathogenic mutations in BRCA1/2. However, our study comprises a relatively small cohort of selected patients. In a study published by Frank et al., 21.6% of patients with Ashkenazi ancestry pathogenic non-founder BRCA1 and BRCA2 mutations were identified [6]. In contrast, in the Finnish population of high-risk individuals tested negative for 28 BRCA1/2 pathogenic founder mutations, additional pathogenic mutations in BRCA1 and BRCA2 accounted for just 1.2% [12]. Much larger numbers are necessary to assess the real proportion of pathogenic non-founder mutations in the population of Latvia.
Despite the drawbacks of such a small study group, the initial results raised some observations.
Interestingly, probands that carried a pathogenic non-founder mutation had some common features. All six breast cancer patients in our study with proven pathogenic non-founder BRCA1/2 mutations had a triple-negative phenotype. It is well established that approximately 80% of all BRCA1/2– related tumours have a triple-negative phenotype [14,15,16,17,18]. The prevalence of pathogenic germline BRCA1/2 mutations in the selected triple-negative breast cancer patients ranged from 9.2 to 34.4% [19,20,21,22]. Additional analyses of cDNA microarray data from van’t Veer showed that BRCA1-related tumours have a sporadic basal-like breast cancer gene expression profile [23]. Additionally, according to Richardson et al., loss of BRCA1 function could play a role in the development of basal-like breast cancers [24]. Couch et al. identified BRCA1/2 pathogenic mutations in 11.2% of triple-negative breast cancer patients and other breast-ovarian cancer predisposing gene mutations in 3.7% of triple-negative breast cancer patients [25].
In our study we used the NCCN criteria for screening pathogenic mutations in BRCA1 and BRCA2, where triple-negative breast cancer is used as a criterion together with an age limit < 60. Only one out of six breast cancer patients in our study who carried a pathogenic BRCA1/2 non-founder mutation was older than 60 years of age, but in this case, family cancer history was positive in the study published by Couch et al., 3.1% of triple-negative breast cancer patients older than 60 years and only 1.4% with no family history of breast or ovarian cancer were diagnosed with BRCA1/2 pathogenic mutation [25]. Therefore, our study results support the current NCCN guidelines for screening all triple-negative breast cancer patients younger than 60 years of age.
In contrast, the application of the upper age limit for triple-negative breast cancer patients of 40 years (Swedish Breast cancer group criteria for screening for mutations in BRCA1 and BRCA2) would miss several BRCA-positive cases in our cohort [26].
Our small study showed the high accuracy of the Manchester scoring system for the prediction of pathogenic non-founder BRCA1/2 mutations in founder mutation-negative patients. Our finding is supported by several other studies performed on the validation of the Manchester scoring system in populations of UK, Germany and South East Asia [13, 27, 28]. However, larger numbers of cases are needed for comprehensive validation of these criteria in the population of Latvia.
Additionally, three out of eight patients tested negative for 26 breast and ovarian cancer susceptibility genes were HER2 positive. According to a recently published study, only 9% of BRCA1-related breast tumours and 13% of BRCA2-related breast tumours were HER2 positive [29]. HER2 positivity is also included in the Manchester scoring system as a BRCA1/2 probability decreasing factor [13].
Ovarian cancer in a personal or family history was documented in three out of seven patients who carried a pathogenic BRCA1/2 non-founder mutation. Additionally, in one case, unknown gynaecological cancer was reported in a paternal aunt. According to recent studies, the presence of ovarian cancer in personal or family history of pathogenic BRCA1 founder-negative breast cancer patients increases the possibility of carrying previously undetected pathogenic BRCA1/2 non-founder mutations [30, 31]. Recently, in a study published by Couch et al., ovarian cancer in family history was documented only in 1 of 54 pathogenic non-BRCA1/2 mutation carriers with triple-negative breast cancer [25].
In our study, no pathogenic mutations were detected in another 24 genes included in the panel. Some previously published studies demonstrated that the rate of pathogenic mutations in non-BRCA1/2 genes ranged from 2.9 to 9.3% [32,33,34,35].
Four of the 16 (25%) patients were identified to have a variant of unknown significance (VUS) in BRCA2, RAD50, CDH1 and MRE11. Unfortunately, due to an insufficient sample size in our study, we cannot elaborate upon those results.
Conclusion
A relatively high incidence of pathogenic non-founder BRCA1/2 mutations was observed among patients with triple-negative familial breast cancer in a founder population. The Manchester scoring system predicted the probability of non-founder pathogenic mutations with high accuracy.
Abbreviations
- ATM :
-
Ataxia-telangiectasia mutated
- BARD1 :
-
BRCA1 (Breast Cancer 1) Associated RING Domain 1 gene
- BLM :
-
Bloom’s syndrome gene
- BRCA1 :
-
Breast cancer susceptibility gene 1
- BRCA2 :
-
Breast cancer susceptibility gene 2
- BRIP1 :
-
BRCA1-interacting protein 1 gene
- CDH :
-
Cadherin-1 gene
- cDNA:
-
Complementary Deoxyribonucleic Acid
- CHEK2 :
-
Checkpoint kinase 2 gene
- DNA:
-
Deoxyribonucleic Acid
- EPCAM :
-
epithelial cell adhesion molecule gene
- ER:
-
Estrogen receptor
- FAM175A :
-
Family with sequence similarity 175A gene
- G2 :
-
Moderately differentiated
- G3:
-
Well differentiated
- HER2:
-
Human epidermal growth factor receptor 2
- MEN1 :
-
multiple endocrine neoplasia type 1
- MLH :
-
MutL homolog 1 gene
- MRE11A :
-
MRE11 meiotic recombination 11 homolog A gene
- MSH6 :
-
MutL homolog 6 gene
- MUTYH :
-
MutY DNA glycosylase
- NA:
-
Not applicable
- NBN :
-
Nibrin gene
- NCCN:
-
National Comprehensive Cancer Network
- PALB2 :
-
Partner and localizer of BRCA2 gene
- PAT:
-
Pathological
- PMS2 :
-
postmeiotic segregation increased 2
- PR:
-
Progesterone receptor
- PTEN :
-
Phosphatase and tensin homolog gene
- RAD50 :
-
Human homolog of S. cerevisiae RAD50 gene
- RAD51 :
-
RAD51 paralog D
- RAD51C :
-
RAD51 homolog C
- STK11 :
-
serine/threonine kinase 11 gene
- TP53 :
-
tumor protein p53 gene
- USA:
-
United States of America
- VUS:
-
Variant of uncertain significance
- XRCC2 :
-
X-ray repair cross-complementing protein 2
References
Oosterwijk JC, de Vries J, Mourits MJ, de Bock GH. Genetic testing and familial implications in breast-ovarian cancer families. Maturitas. 2014;78:252.
Walsh T, Casadei S, Lee MK, Pennil CC, Nord AS, Thornton AM, Roeb W, Agnew KJ, Stray SM, Wickramanayake A, Norquist B, Pennington KP, Garcia RL, King MC, Swisher EM. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A. 2011;108(44):18032.
Plakhins G, Irmejs A, Gardovskis A, Subatniece S, Rozite S, Bitina M, Keire G, Purkalne G, Teibe U, Trofimovics G, Miklasevics E, Gardovskis J. Genotype-phenotype correlations among BRCA1 4153delA and 5382insC mutation carriers from Latvia. BMC Med Genet. 2011;12:147.
Armstrong J, Toscano M, Kotchko N, Friedman S, Schwartz MD, Virgo KS, Lynch K, Andrews JE, Aguado Loi CX, Bauer JE, Casares C, Teten RT, Kondoff MR, Molina AD, Abdollahian M, Brand L, Walker GS, Sutphen R. American BRCA outcomes and utilization of testing (ABOUT) study: a pragmatic research model that incorporates personalized medicine/patient-centered outcomes in a real world setting. J Genet Couns. 2015;24:18–28.
Tihomirova L, Vaivade I, Fokina O, Peculis R, Mandrika I, Sinicka O, Stengrevics A, Krilova A, Keire G, Petrevics J, Eglitis J, Timofejevs M, Leja M. BRCA1 gene-related hereditary susceptibility to breast and ovarian cancer in Latvia. Adv Med Sci. 2014;59(1):114–9.
Frank TS, Deffenbaugh AM, Reid JE, Hulick M, Ward BE, Lingenfelter B, Gumpper KL, Scholl T, Tavtigian SV, Pruss DR, Critchfield GC. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol. 2002;20:1480.
Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol. 2015;26:1291.
Berzina D, Nakazawa-Miklasevica M, Zestkova J, Aksenoka K, Irmejs A, Gardovskis A, Kalniete D, Gardovskis J, Miklasevics E. BRCA1/2 mutation screening in high-risk breast/ovarian cancer families and sporadic cancer patient surveilling for hidden high-risk families. BMC Med Genet. 2013;14:61.
Paluch-Shimon S, Cardoso F, Sessa C, Balmana J, Cardoso MJ, Gilbert F, Senkus E. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO clinical practice guidelines for cancer prevention and screening. Ann Oncol. 2016;27(5):103.
Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355(6330):1152.
Tung N, Battelli C, Allen B, Kaldate R, Bhatnagar S, Bowles K, Timms K, Garber JE, Herold C, Ellisen L, Krejdovsky J, DeLeonardis K, Sedgwick K, Soltis K, Roa B, Wenstrup RJ, Hartman AR. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121:25.
Kuusisto KM, Bebel A, Vihinen M, Schleutker J, Sallinen SL. Screening for BRCA1, BRCA2, CHEK2, PALB2, BRIP1, RAD50, and CDH1 mutations in high-risk Finnish BRCA1/2-founder mutation-negative breast and/or ovarian cancer individuals. Breast Cancer Res. 2011;13(1):R20.
Evans DG, Harkness EF, Plaskocinska I, Wallace AJ, Clancy T, Woodward ER, Howell TA, Tischkowitz M, Lalloo F. Pathology update to the Manchester scoring system based on testing in over 4000 families. J Med Genet. 2017;54(10):674.
Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938.
Lakhani SR, Van De Vijver MJ, Jacquemier J, Anderson TJ, Osin PP, McGuffog L, Easton DF. The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol. 2002;20(9):2310.
Stefansson OA, Jonasson JG, Johannsson OT, Olafsdottir K, Steinarsdottir M, Valgeirsdottir S, Eyfjord JE. Genomic profiling of breast tumours in relation to BRCA abnormalities and phenotypes. Breast Cancer Res. 2009;11(4):R47.
Diaz LK, Cryns VL, Symmans WF, Sneige N. Triple negative breast carcinoma and the basal phenotype: from expression profiling to clinical practice. Adv Anat Pathol. 2007;14(6):419.
Palacios J, Honrado E, Osorio A, Cazorla A, Sarrió D, Barroso A, Rodríguez S, Cigudosa JC, Diez O, Alonso C, Lerma E, Dopazo J, Rivas C, Benitez. Phenotypic characterization of BRCA1 and BRCA2 tumors based in a tissue microarray study with 37 immunohistochemical markers. Breast Cancer Res Treat. 2005;90(1):5.
Young SR, Pilarski RT, Donenberg T, Shapiro C, Hammond LS, Miller J, Brooks KA, Cohen S, Tenenholz B, Desai D, Zandvakili I, Royer R, Li S, Narod SA. The prevalence of BRCA1 mutations among young women with triple-negative breast cancer. BMC Cancer. 2009;9:86.
Comen E, Davids M, Kirchhoff T, Hudis C, Offit K, Robson M. Relative contributions of BRCA1 and BRCA2 mutations to “triple-negative” breast cancer in Ashkenazi women. Breast Cancer Res Treat. 2011;129(1):185.
Robertson L, Hanson H, Seal S, Warren-Perry M, Hughes D, Howell I, Turnbull C, Houlston R, Shanley S, Butler S, Evans DG, Ross G, Eccles D, Tutt A, Rahman N, TNT Trial TMG; BCSC (UK). BRCA1 testing should be offered to individuals with triple-negative breast cancer diagnosed below 50 years. Br J Cancer. 2012;106(6):1234–8.
Phuah SY, Looi LM, Hassan N, Rhodes A, Dean S, Taib NA, Yip CH, Teo SH. Triple-negative breast cancer and PTEN (phosphatase and tensin homologue) loss are predictors of BRCA1 germline mutations in women with early-onset and familial breast cancer, but not in women with isolated late-onset breast cancer. Breast Cancer Res. 2012;14(6):R142.
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lønning PE, Brown PO, Børresen-Dale AL, Botstein D. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100(14):8418.
Richardson AL, Wang ZC, De Nicolo A, Lu X, Brown M, Miron A, Liao X, Iglehart JD, Livingston DM, Ganesan S. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9(2):121.
Couch FJ, Hart SN, Sharma P, Toland AE, Wang X, Miron P, Olson JE, Godwin AK, Pankratz VS, Olswold C, Slettedahl S, Hallberg E, Guidugli L, Davila JI, Beckmann MW, Janni W, Rack B, Ekici AB, Slamon DJ, Konstantopoulou I, Fostira F, Vratimos A, Fountzilas G, Pelttari LM, Tapper WJ, Durcan L, Cross SS, Pilarski R, Shapiro CL, Klemp J, Yao S, Garber J, Cox A, Brauch H, Ambrosone C, Nevanlinna H, Yannoukakos D, Slager SL, Vachon CM, Eccles DM, Fasching PA. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol. 2015;33(4):304.
Nilsson MP, Winter C, Kristoffersson U, Rehn M, Larsson C, Saal LH, Loman N. Efficacy versus effectiveness of clinical genetic testing criteria for BRCA1 and BRCA2 hereditary mutations in incident breast cancer. Familial Cancer. 2017;16(2):187.
Kast K, Schmutzler RK, Rhiem K, Kiechle M, Fischer C, Niederacher D, Arnold N, Grimm T, Speiser D, Schlegelberger B, Varga D, Horvath J, Beer M, Briest S, Meindl A, Engel C. Validation of the Manchester scoring system for predicting BRCA1/2 mutations in 9,390 families suspected of having hereditary breast and ovarian cancer. Int J Cancer. 2014;135(10):2352–61.
Chew W, Moorakonda RB, Courtney E, Soh H, Li ST, Chen Y, Shaw T, Allen JC, Evans DGR, Ngeow J. Evaluation of the relative effectiveness of the 2017 updated Manchester scoring system for predicting BRCA1/2 mutations in a southeast Asian country. J Med Genet. 2017;55:344–50. [Epub ahead of print]
Kuchenbaecker KB, Neuhausen SL, Robson M, Barrowdale D, McGuffog L, Mulligan AM, Andrulis IL, Spurdle AB, Schmidt MK, Schmutzler RK, Engel C, Wappenschmidt B, Nevanlinna H, Thomassen M, Southey M, Radice P, Ramus SJ, Domchek SM, Nathanson KL, Lee A, Healey S, Nussbaum RL, Rebbeck TR, Arun BK, James P, Karlan BY, Lester J, Cass I, Breast Cancer Family Registry, Terry MB, Daly MB, Goldgar DE, Buys SS, Janavicius R, Tihomirova L, Tung N, Dorfling CM, van Rensburg EJ, Steele L, v O Hansen T, Ejlertsen B, Gerdes AM, Nielsen FC, Dennis J, Cunningham J, Hart S, Slager S, Osorio A, Benitez J, Duran M, Weitzel JN, Tafur I, Hander M, Peterlongo P, Manoukian S, Peissel B, Roversi G, Scuvera G, Bonanni B, Mariani P, Volorio S, Dolcetti R, Varesco L, Papi L, Tibiletti MG, Giannini G, Fostira F, Konstantopoulou I, Garber J, Hamann U, Donaldson A, Brewer C, Foo C, Evans DG, Frost D, Eccles D, EMBRACE Study, Douglas F, Brady A, Cook J, Tischkowitz M, Adlard J, Barwell J, Ong KR, Walker L, Izatt L, Side LE, Kennedy MJ, Rogers MT, Porteous ME, Morrison PJ, Platte R, Eeles R, Davidson R, Hodgson S, Ellis S, Godwin AK, Rhiem K, Meindl A, Ditsch N, Arnold N, Plendl H, Niederacher D, Sutter C, Steinemann D, Bogdanova-Markov N, Kast K, Varon-Mateeva R, Wang-Gohrke S, Gehrig A, Markiefka B, Buecher B, Lefol C, Stoppa-Lyonnet D, Rouleau E, Prieur F, Damiola F, GEMO Study Collaborators, Barjhoux L, Faivre L, Longy M, Sevenet N, Sinilnikova OM, Mazoyer S, Bonadona V, Caux-Moncoutier V, Isaacs C, Van Maerken T, Claes K, Piedmonte M, Andrews L, Hays J, Rodriguez GC, Caldes T, de la Hoya M, Khan S, Hogervorst FB, Aalfs CM, de Lange JL, Meijers-Heijboer HE, van der Hout AH, Wijnen JT, van Roozendaal KE, Mensenkamp AR, van den Ouweland AM, van Deurzen CH, van der Luijt RB, HEBON, Olah E, Diez O, Lazaro C, Blanco I, Teulé A, Menendez M, Jakubowska A, Lubinski J, Cybulski C, Gronwald J, Jaworska-Bieniek K, Durda K, Arason A, Maugard C, Soucy P, Montagna M, Agata S, Teixeira MR, KConFab Investigators, Olswold C, Lindor N, Pankratz VS, Hallberg E, Wang X, Szabo CI, Vijai J, Jacobs L, Corines M, Lincoln A, Berger A, Fink-Retter A, Singer CF, Rappaport C, Kaulich DG, Pfeiler G, Tea MK, Phelan CM, Mai PL, Greene MH, Rennert G, Imyanitov EN, Glendon G, Toland AE, Bojesen A, Pedersen IS, Jensen UB, Caligo MA, Friedman E, Berger R, Laitman Y, Rantala J, Arver B, Loman N, Borg A, Ehrencrona H, Olopade OI, Simard J, Easton DF, Chenevix-Trench G, Offit K, Couch FJ, Antoniou AC. Associations of common breast cancer susceptibility alleles with risk of breast cancer subtypes in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2014;16(6):3416.
Azzollini J, Scuvera G, Bruno E, Pasanisi P, Zaffaroni D, Calvello M, Pasini B, Ripamonti CB, Colombo M, Pensotti V, Radice P, Peissel B, Manoukian S. Mutation detection rates associated with specific selection criteria for BRCA1/2 testing in 1854 high-risk families: a monocentric Italian study. Eur J Intern Med. 2016;32:65–71.
Lee JS, John EM. McGuire Breast and Ovarian cancer in relatives of cancer patients, with and without BRCA mutations. Cancer Epidemiol Biomarkers Prev. 2006;15:359.
Susswein LR, Marshall ML, Nusbaum R, Postula KJV, Weissman SM, Yackowski L, Vaccari EM, Bissonnette J, Booker JK, Laura Cremona M, Gibellini F, Murphy PD, Pineda-Alvarez DE, Pollevick GD, Zhixiong X, Richard G, Bale S, Klein RT, Hruska KS, Chung WK. Pathogenic and likely pathogenic variant prevalence among the first 10,000 patients referred for next-generation cancer panel testing. Genet Med. 2016;18(8):823.
Desmond A, Kurian AW, Gabree M, Mills MA, Anderson MJ, Kobayashi Y, Horick N, Yang S, Shannon KM, Tung N, Ford JM, Lincoln SE, Ellisen LW. Clinical Actionability of multigene panel testing for hereditary breast and ovarian Cancer risk assessment. JAMA Oncol. 2015;1(7):943.
Thompson ER, Rowley SM, Li N, McInerny S, Devereux L, Wong-Brown MW, Trainer AH, Mitchell G, Scott RJ, James PA, Campbell IG. Panel testing for familial breast Cancer: calibrating the tension between research and clinical care. J Clin Oncol. 2016;34(13):1455.
Schroeder C, Faust U, Sturm M, Hackmann K, Grundmann K, Harmuth F, Bosse K, Kehrer M, Benkert T, Klink B, Mackenroth L, Betcheva-Krajcir E, Wimberger P, Kast K, Heilig M, Nguyen HP, Riess O, Schröck E, Bauer P, Rump A. HBOC multi-gene panel testing: comparison of two sequencing centers. Breast Cancer Res Treat. 2015;152(1):129.
Funding
This work was supported by State Research Program “Biomedicine for the public health (BIOMEDICINE)” project 5 “Personalised cancer diagnostics and treatment effectiveness evaluation”.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
JM, AI and GT analyzed and interpreted the patient data regarding the disease. ES and GP analyzed and interpreted patient data regarding chemotherapy. EM and DB analyzed and interpreted genetic screening results. JM, AI, EM and JG were major contributors in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by a Central Medical Ethics Committee of Latvia. Written consent was obtained.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Maksimenko, J., Irmejs, A., Trofimovičs, G. et al. High frequency of pathogenic non-founder germline mutations in BRCA1 and BRCA2 in families with breast and ovarian cancer in a founder population. Hered Cancer Clin Pract 16, 12 (2018). https://doi.org/10.1186/s13053-018-0094-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13053-018-0094-0