Abstract
Background
The pathogenic variation of CASK gene can cause CASK related mental disorders. The main clinical manifestations are microcephaly with pontine and cerebellar hypoplasia, X-linked mental disorders with or without nystagmus and FG syndrome. The main pathogenic mechanism is the loss of function of related protein caused by variant. We reported a Chinese male newborn with a de novo variant in CASK gene.
Case presentation
We present an 18-day-old baby with growth retardation and brain hypoplasia. Whole-exome sequencing was performed, which detected a hemizygous missense variant c.764G > A of CASK gene. The variant changed the 255th amino acid from Arg to His. Software based bioinformatics analyses were conducted to infer its functional effect.
Conclusions
In this paper, a de novo variant of CASK gene was reported. Moreover, a detailed description of all the cases described in the literature is reported. CASK variants cause a variety of clinical phenotypes. Its diagnosis is difficult due to the lack of typical clinical symptoms. Genetic testing should be performed as early as possible if this disease is suspected. This case provides an important reference for the diagnosis and treatment of future cases.
Similar content being viewed by others
Background
CASK gene located in Xp11.4 [1] and is an important gene in mammals, which plays a very important role in metabolic regulation and affects the development of postnatal brain [2]. CASK gene variants cause a wide range of human phenotypes. The pathogenic variants can lead to CASK related mental disorders. It is reported that CASK gene variant can mainly lead to these phenotypes: severe intellectual disability, microcephaly with pontine and cerebellar hypoplasia (MICPCH, OMIM: 300,749) in women. In men, mild to severe X-related mental disorders were observed with or without nystagmus, microcephaly and other malformations, and FG syndrome. The varied clinical phenotypes depend on the types of variants [3,4,5].
CASK gene encodes calcium/ calmodulin dependent serine protein kinase, which belongs to the membrane associated guanosine kinase (MAGUK) scaffold protein family. MAGUK protein plays an important role in the ionic channel targeting, anchoring and signal transduction of synapses, as well as regulating neural activity. CASK is a special member of p55 subfamily and is the only MAGUK which contains the calcium/ calmodulin dependent kinase (CaMK) domain at its N-terminal. CASK protein contains five domains, including two L27 (Lin2, lin7) domains, one PDZ domain and one integrated SH3 and GUK domain [6].
The CASK disorder is rare. ZHANG Yi et al. [7] reported a case of Chinese children in 2019 which is the first one in China. Here, we reported the second case and identified a de novo variant c.764G > A (p. Arg255His) of CASK gene in China. Bioinformatics software were used to predict the effects of the missense variant on the function of the CASK protein. Additionally, we reviewed the previously reported cases of CASK gene variants from different ethnic groups (Table 1), which contained the nucleotide changes, amino acid changes and clinical phenotypes caused by gene variants [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23].
Case presentation
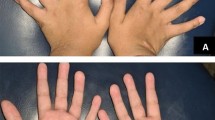
The patient was an 18-day-old male baby, gravida 3, para 1, born in full term with a birth weight of 2790 g. The condition of intrauterine distress was unknown, and the history of asphyxia was denied. Crying after birth but slightly weak. Apgar score was unknown. The couple denied the family genetic disease history. The patient was hospitalized in Tianjin Children's hospital mainly due to sucking weakness. When he was fed for the first time after birth, he was not willing to take the initiative to suck. He was fed with a spoon and could swallow. The infant rarely cries and cry weakly, with no fever and hoarseness, moaning and other symptoms. Admission examination: weight 2840 g, length 50 cm, head circumference 33 cm. The child's consciousness was weak and he occasionally had inspiratory laryngitis, hypotonia of the extremities. Holding reflex and embracing reflex were normal, the foraging reflex ( ±), sucking reflex ( ±). When he was crying, the corners of his mouth inclined to the left. His left nasolabial groove became slightly shallow along with right hand slightly hanging wrist, right foot slightly turned inward, and his right-hand pass-through palm. Laryngoscope showed that the arytenoid epiglottic folds on both sides were close to each other, and the mucosa was slightly tense. The cricoarytenoid joint were adducted, and the throat entrance was slightly blocked. Head Magnetic Resonance Imaging(MRI)showed that the bilateral frontal parietal lobes had slightly intense T1 and T2 signal shadows. The extracerebral space was widened, and the posterior angles of bilateral lateral ventricles were widened. Brainstem auditory evoked potential test showed deafness in the left ear and abnormality in the right brainstem. Ambulatory electroencephalogram examination and cerebrospinal fluid test were normal. Neuroelectrophysiological examination showed that there was no abnormality in facial nerve detection. After admission, the patient was given anti-infective treatment of Latamoxef disodium, expectorant therapy of ambroxol and other symptomatic treatment. Six days after hospitalization, there was no fever in the child and the supplementary feeding became better. The family members required to be discharged from the hospital.
Telephone follow-up at the age of 4–5 months, the symptoms of sucking weakness were slightly better than before. Later, spasm occurred at the age of 6 months and he was diagnosed epilepsy which was characterized by cyanosis of lips and clenching of both hands. After 9 months of oral medication with Sodium Valproate and Topiramate, there was no obvious improvement in condition. At present, the child was 14 months old, with a weight of 6000 g. He has microcephaly compared with children of the same age (family members did not measure the head circumference), accompanied by severe developmental delay and intellectual disability. He still can’t raise head, speak and walk.
The results of Whole-exome sequencing (WES) showed that there was a hemizygous missense variant c.764G > A in exon 8 of CASK gene in proband. The variant changed the 255th amino acid from Arg to His. Because of the gene is located on the X chromosome, the paternal sample of the child does not need to be detected. Sanger sequencing of the child showed that the variant was not detected in his mother (Fig. 1). The pathogenicity classification of variants by American College of Medical Genetics (ACMG) guidelines [41] indicated that c.764G > A (p. Arg255His) is of pathogenic. The variant was not included in HGMD, 1000 Genomes, gnomAD and ESP6500 public databases.
Prediction of functional effects of CASK variant showed the c.764G > A variant was possibly damaging (Fig. 2). Amino acid sequence alignment showed that the variant occurred at a highly conserved residue in CASK with surrounding amino acid residues being conserved between orthologs (Fig. 3). Protein structure 3D modeling was performed. It was shown that the variant (p. Arg255His) had a damaging effect on the CASK protein structure stability (Fig. 4).
Discussion and conclusions
CASK is widely distributed in different brain regions of mice. The insertion variant and targeted knockout of CASK gene cause the death of mice within 1–2 days after birth. The mice exhibit a cleft palate and apoptosis of thalamic cell increased. The research results indicate the important role of CASK gene in the nervous system [35]. In human fetal tissues, CASK is most expressed in brain, followed by kidney and lung, and the expression level of CASK in brain is 3–5 times higher than other organs [42].Although CASK is expressed in neurons, it is not limited to neurons. Studies have shown that CASK is widely present in basement membrane, lateral membrane or lateral basement membrane in different epithelial cells [45].
The structure of CASK suggests that CASK plays an important role in signal transduction, intercellular connection, cytoskeleton and binding to membrane proteins [33].CASK interacts with a variety of cell proteins and plays different roles according to the time and location of expression [37]. Firstly, it is involved in the formation of synapses and the interaction between synapses [46]. For example, CASK regulates axon growth and branch by interacting with Bcl11A [24]; Interaction between CASK and syndecan-2 regulates maturation of dendritic protein [25]. At presynaptic sites, CASK forms compound with MALS/Mint-1/Liprinα through its CaMK and L27A domains. This compound is involved in the organization of synaptic vesicles and regulates the release of neurotransmitters [26]. Secondly, CASK involves protein transport of NMDA glutamate receptor and synaptic target of N-type calcium channel. Through its PDZ and SH3 domains, CASK forms targeted interaction and regulation with neurexin-1 and ion channel synapses in a CDK5-dependent manner. Thirdly, CASK regulating gene expression and neurodevelopment. CASK can enter the nucleus and bind to a specific DNA sequence in the Tbr-1 complex. As a co-activator of Tbr-1, CASK induces the transcription of this sequence, so as to regulate the expression of genes related to the development of cerebral cortex, such as RELN [37]. Protein kinase A phosphorylation regulates the interaction between CASK and Tbr-1 and it is an important regulatory factor of CASK in the nucleus [27]. Y-P30 can control the nuclear localization of CASK in a cell adhesion molecule dependent manner [28]. CASK is involved in many cellular pathways, including mitochondrial, synaptic and protein metabolism. The dysfunction of these cells may be the basis of complex neurological diseases related to CASK dysfunction [29].
In 2008, Najm J et al. first reported the heterozygous deletion and variant of CASK gene in girls and boys with severe pontine and cerebellar hypoplasia [30]. Since then, 104 pathogenic variants of CASK gene have been identified through next generation sequence (Table 1). According to these publications, CASK variants cause a variety of clinical phenotypes. These cases shown that CASK gene does not have a hot variant site that causes pathogenic clinical phenotype. Inactivated variant is more common in female patients, and the clinical phenotype is more serious.
MICPCH is a rare X-linked disease, usually seen in women, characterized by neurodevelopmental delay, microcephaly, and pontocerebellar hypoplasia. The main clinical phenotypes of the disease are severe developmental delay or intellectual disability, microcephaly after birth, often accompanied by slow growth, language development disorders, axial muscle tone reduction with or without increased limb muscle tone, optic nerve hypoplasia and / or other eye abnormalities, such as nystagmus. Patients often have special facial phenotypes including microcephaly, protruding broad bridge and tip of nose, small nose or short nose, small jaw deformity, big ears, with varying degrees of pons and cerebellum hypoplasia and progressive aggravation, as well as hearing loss, epilepsy etc. [31, 32]. There are also some female patients without microcephaly and pontine dysplasia. Bozarth X et al. reported a case of early-onset infantile spasm caused by CASK frame deletion variant in a girl. Brain MRI showed focal supratentorial brain malformation. EEG showed peak rhythm disorder, but no MICPCH [34].
The relationship between genotype and phenotype of CASK variant is not clear. CASK inactivating variants appear to account for the majority of MICPCH cases and with severer phenotypes [36]. It is fatal to men in the prenatal or neonatal period. Najm J et al. reported a male child died at 2 weeks after birth. In addition to deletion or duplication variant, women with MICPCH phenotype also have heterozygous deletion variants, including nonsense, frameshift and splice site variants [30]. In general, CASK missense variant is common in boys with X-linked intellectual disability. The clinical phenotype is not very serious, and it is usually asymptomatic in girls. However, Laconte L E W et al. reported three women with CASK missense variant in heterozygote, and they have severe intellectual disability, microcephaly and hindbrain hypoplasia [38].
The child in our case was born with weakness sucking, decreased muscle tension of limbs, abnormal face, right hand and right foot deformity, deafness of left ear, epilepsy, microcephaly, serious developmental delay and mental disorder. The results of next generation sequencing showed that there was a hemizygote missense variant c.764gG > A, p. (Arg255His) in exon 8 of CASK gene in children. According to the classification of gene variation by ACMG, the variant could be classified as pathogenic. The patient was a male child with pathogenic missense variant. Compared with literature reports published, the missense variant is a de novo variant, and the clinical phenotype of the patient is consistent with the published cases.
MRI of CASK variant patients showed that the size of the corpus callosum was normal, the proportion of brain/ corpus callosum was low, and the area of brain, pons, midbrain, cerebellar vermis and hemispheres were reduced. Some studies have shown that MRI results of hypoplasia and normal or large corpus callosum in the middle and posterior brain of girls with microcephaly and neurodevelopmental delay should indicate the possibility of CASK variant, especially in the case of low brain / corpus callosum ratio [39].
In terms of disease diagnosis, WES is a powerful tool for the diagnosis of highly heterogeneous neurodevelopmental disorders [40]. Children with microcephaly will face lifelong psychomotor, cognitive and communication disorders. For this kind of children, their motor development is often delayed for several years, and they are far behind the children of the same age in intelligence and communication ability. These children usually have serious speech disorders. DeLuca SC et al. conducted intensive treatment on three girls with CASK gene heterozygote variant and MICPCH. Conducting targeted trials to improve fine and coarse motor skills, visual motor coordination, social and communication skills. Studies have shown that MICPCH children respond to intensive therapy aimed at improving function or independence [43]. The therapy can improve the life track and affect the quality of life. CASK is highly conserved in structure. LaConte LE et al. used a high-throughput imaging method to measure the misfolding tendency of CASK mutants, and proved that a chemical chaperone may be helpful to save the misfolding of CASK caused by missense variants. It providing a possibility for the treatment of structural variants in the future [44].
In summary, we reported a de novo variant of CASK gene. Moreover, a detailed description of all the cases described in the literature is reported. All published cases suggest that the variant of CASK can cause a variety of clinical phenotypes. Its diagnosis is difficult due to the lack of typical clinical symptoms. Genetic testing should be performed as early as possible if this disease is suspected. We believe that this case provides an important reference for the diagnosis and treatment of future cases.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- MICPCH:
-
Microcephaly with pontine and cerebellar hypoplasia
- MAGUK:
-
The membrane associated guanosine kinase
- CaMK:
-
The calcium/ calmodulin dependent kinase
- WES:
-
Whole-exome sequencing
- ACMG:
-
American College of Medical Genetics
- MRI:
-
Magnetic Resonance Imaging
References
Dimitratos SD, Stathakis DG, Nelson CA, Woods DF, Bryant PJ. The location of human CASK at Xp11.4 identifies this gene as a candidate for X-linked optic atrophy. Genomics. 1998;51(2):308–9. https://doi.org/10.1006/geno.1998.5404.
Srivastava S, McMillan R, Willis J, et al. X-linked intellectual disability gene CASK regulates postnatal brain growth in a non-cell autonomous manner. Acta Neuropathol Commun. 2016;4:30. https://doi.org/10.1186/s40478-016-0295-6.
Piluso G, D’Amico F, Saccone V, et al. A missense mutation in CASK causes FG syndrome in an Italian family. Am J Hum Genet. 2009;84(2):162–77. https://doi.org/10.1016/j.ajhg.2008.12.018.
Hackett A, Tarpey PS, Licata A, et al. CASK mutations are frequent in males and cause X-linked nystagmus and variable XLMR phenotypes. Eur J Hum Genet. 2010;18(5):544–52. https://doi.org/10.1038/ejhg.2009.220.
Hayashi S, Okamoto N, Chinen Y, et al. Novel intragenic duplications and mutations of CASK in patients with mental retardation and microcephaly with pontine and cerebellar hypoplasia (MICPCH). Hum Genet. 2012;131(1):99–110. https://doi.org/10.1007/s00439-011-1047-0.
Hsueh YP. Calcium/calmodulin-dependent serine protein kinase and mental retardation. Ann Neurol. 2009;66(4):438–43. https://doi.org/10.1002/ana.21755.
Yi Z, Yao Ruen Xu, Yufei HC, Jian W. Clinical characteristics of CASK gene mutation in a child. J Clin Pediatr. 2019;37(9):693–6. https://doi.org/10.3969/j.issn.1000-3606.2019.09.014.
Cristofoli F, Devriendt K, Davis EE, Van Esch H, Vermeesch JR. Novel CASK mutations in cases with syndromic microcephaly. Hum Mutat. 2018;39(7):993–1001. https://doi.org/10.1002/humu.23536.
Dunn P, Prigatano GP, Szelinger S, et al. A de novo splice site mutation in CASK causes FG syndrome-4 and congenital nystagmus. Am J Med Genet A. 2017;173(3):611–7. https://doi.org/10.1002/ajmg.a.38069.
Hayashi S, Mizuno S, Migita O, et al. The CASK gene harbored in a deletion detected by array-CGH as a potential candidate for a gene causative of X-linked dominant mental retardation. Am J Med Genet A. 2008;146A(16):2145–51. https://doi.org/10.1002/ajmg.a.32433.
LaConte L, Chavan V, DeLuca S, et al. An N-terminal heterozygous missense CASK mutation is associated with microcephaly and bilateral retinal dystrophy plus optic nerve atrophy. Am J Med Genet A. 2019;179(1):94–103. https://doi.org/10.1002/ajmg.a.60687.
Michaud JL, Lachance M, Hamdan FF, et al. The genetic landscape of infantile spasms. Hum Mol Genet. 2014;23(18):4846–58. https://doi.org/10.1093/hmg/ddu199.
Moog U, Bierhals T, Brand K, et al. Phenotypic and molecular insights into CASK-related disorders in males. Orphanet J Rare Dis. 2015;10:44. https://doi.org/10.1186/s13023-015-0256-3.
Murakami H, Kimura Y, Enomoto Y, et al. Discordant phenotype caused by CASK mutation in siblings with NF1. Hum Genome Var. 2019;6:20. https://doi.org/10.1038/s41439-019-0051-0.
Muthusamy B, Selvan L, Nguyen TT, et al. Next-Generation Sequencing Reveals Novel Mutations in X-linked Intellectual Disability. OMICS. 2017;21(5):295–303. https://doi.org/10.1089/omi.2017.0009.
Nakajiri T, Kobayashi K, Okamoto N, et al. Late-onset epileptic spasms in a female patient with a CASK mutation. Brain Dev. 2015;37(9):919–23. https://doi.org/10.1016/j.braindev.2015.02.007.
Popp B, Ekici AB, Thiel CT, et al. Exome Pool-Seq in neurodevelopmental disorders. Eur J Hum Genet. 2017;25(12):1364–76. https://doi.org/10.1038/s41431-017-0022-1.
Rivas L, Blanco Ó, Torreira C, Repáraz A, Melcón C, Amado A. Pontocerebellar hypoplasia secondary to CASK gene deletion: Case report. Rev Chil Pediatr. 2017;88(4):529–33. https://doi.org/10.4067/S0370-41062017000400014.
Saitsu H, Kato M, Osaka H, et al. CASK aberrations in male patients with Ohtahara syndrome and cerebellar hypoplasia. Epilepsia. 2012;53(8):1441–9. https://doi.org/10.1111/j.1528-1167.2012.03548.x.
Seto T, Hamazaki T, Nishigaki S, et al. A novel CASK mutation identified in siblings exhibiting developmental disorders with/without microcephaly. Intractable Rare Dis Res. 2017;6(3):177–82. https://doi.org/10.5582/irdr.2017.01031.
Takanashi J, Okamoto N, Yamamoto Y, et al. Clinical and radiological features of Japanese patients with a severe phenotype due to CASK mutations. Am J Med Genet A. 2012;158A(12):3112–8. https://doi.org/10.1002/ajmg.a.35640.
Tarpey PS, Smith R, Pleasance E, et al. A systematic, large-scale resequencing screen of X-chromosome coding exons in mental retardation. Nat Genet. 2009;41(5):535–43. https://doi.org/10.1038/ng.367.
Valayannopoulos V, Michot C, Rodriguez D, et al. Mutations of TSEN and CASK genes are prevalent in pontocerebellar hypoplasias type 2 and 4. Brain. 2012;135(Pt 1):e199; author reply e200. https://doi.org/10.1093/brain/awr108.
Kuo TY, Hong CJ, Chien HL, Hsueh YP. X-linked mental retardation gene CASK interacts with Bcl11A/CTIP1 and regulates axon branching and outgrowth. J Neurosci Res. 2010;88(11):2364–73. https://doi.org/10.1002/jnr.22407.
Hu HT, Shih PY, Shih YT, Hsueh YP. The involvement of neuron-specific factors in dendritic spinogenesis: molecular regulation and association with neurological disorders. Neural Plast. 2016;2016:5136286. https://doi.org/10.1155/2016/5136286.
Butz S, Okamoto M, Südhof TC. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell. 1998;94(6):773–82. https://doi.org/10.1016/s0092-8674(00)81736-5.
Huang TN, Chang HP, Hsueh YP. CASK phosphorylation by PKA regulates the protein-protein interactions of CASK and expression of the NMDAR2b gene. J Neurochem. 2010;112(6):1562–73. https://doi.org/10.1111/j.1471-4159.2010.06569.x.
Landgraf P, Mikhaylova M, Macharadze T, et al. Binding of Y-P30 to syndecan 2/3 regulates the nuclear localization of CASK. PLoS One. 2014;9(2):e85924. https://doi.org/10.1371/journal.pone.0085924.
Patel PA, Liang C, Arora A, et al. Haploinsufficiency of X-linked intellectual disability gene CASK induces post-transcriptional changes in synaptic and cellular metabolic pathways. Exp Neurol. 2020;329:113319. https://doi.org/10.1016/j.expneurol.2020.113319.
Najm J, Horn D, Wimplinger I, et al. Mutations of CASK cause an X-linked brain malformation phenotype with microcephaly and hypoplasia of the brainstem and cerebellum. Nat Genet. 2008;40(9):1065–7. https://doi.org/10.1038/ng.194.
Moog U, Kutsche K, Kortüm F, et al. Phenotypic spectrum associated with CASK loss-of-function mutations. J Med Genet. 2011;48(11):741–51. https://doi.org/10.1136/jmedgenet-2011-100218.
Nakamura K, Nishiyama K, Kodera H, et al. A de novo CASK mutation in pontocerebellar hypoplasia type 3 with early myoclonic epilepsy and tetralogy of Fallot. Brain Dev. 2014;36(3):272–3. https://doi.org/10.1016/j.braindev.2013.03.007.
Hata Y, Butz S, Sudhof TC. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16(8):2488–94. https://doi.org/10.1523/JNEUROSCI.16-08-02488.1996.
Bozarth X, Foss K, Mefford HC. A de novo in-frame deletion of CASK gene causes early onset infantile spasms and supratentorial cerebral malformation in a female patient. Am J Med Genet A. 2018;176(11):2425–9. https://doi.org/10.1002/ajmg.a.40429.
Atasoy D, Schoch S, Ho A, et al. Deletion of CASK in mice is lethal and impairs synaptic function. Proc Natl Acad Sci U S A. 2007;104(7):2525–30. https://doi.org/10.1073/pnas.0611003104.
Hayashi S, Uehara DT, Tanimoto K, et al. Comprehensive investigation of CASK mutations and other genetic etiologies in 41 patients with intellectual disability and microcephaly with pontine and cerebellar hypoplasia (MICPCH). PLoS One. 2017;12(8):e0181791. https://doi.org/10.1371/journal.pone.0181791.
Hsueh YP, Wang TF, Yang FC, Sheng M. Nuclear translocation and transcription regulation by the membrane-associated guanylate kinase CASK/LIN-2. Nature. 2000;404(6775):298–302. https://doi.org/10.1038/35005118.
LaConte L, Chavan V, Elias AF, et al. Two microcephaly-associated novel missense mutations in CASK specifically disrupt the CASK-neurexin interaction. Hum Genet. 2018;137(3):231–46. https://doi.org/10.1007/s00439-018-1874-3.
Takanashi J, Arai H, Nabatame S, et al. Neuroradiologic features of CASK mutations. AJNR Am J Neuroradiol. 2010;31(9):1619–22. https://doi.org/10.3174/ajnr.A2173.
Rump P, Jazayeri O, van Dijk-Bos KK, et al. Whole-exome sequencing is a powerful approach for establishing the etiological diagnosis in patients with intellectual disability and microcephaly. BMC Med Genomics. 2016;9:7. https://doi.org/10.1186/s12920-016-0167-8.
Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. 2015;17(5):405–24. https://doi.org/10.1038/gim.2015.30.
Stevenson D, Laverty HG, Wenwieser S, Douglas M, Wilson JB. Mapping and expression analysis of the human CASK gene. Mamm Genome. 2000;11(10):934–7. https://doi.org/10.1007/s003350010170.
DeLuca SC, Wallace DA, Trucks MR, Mukherjee K. A clinical series using intensive neurorehabilitation to promote functional motor and cognitive skills in three girls with CASK mutation. BMC Res Notes. 2017;10(1):743. https://doi.org/10.1186/s13104-017-3065-z.
LaConte LE, Chavan V, Mukherjee K. Identification and glycerol-induced correction of misfolding mutations in the X-linked mental retardation gene CASK. PLoS One. 2014;9(2):e88276. https://doi.org/10.1371/journal.pone.0088276.
Cohen AR, Woods DF, Marfatia SM, Walther Z, Chishti AH, Anderson JM. Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells. J Cell Biol. 1998;142(1):129–38. https://doi.org/10.1083/jcb.142.1.129.
Hsueh YP. The role of the MAGUK protein CASK in neural development and synaptic function. Curr Med Chem. 2006;13(16):1915–27. https://doi.org/10.2174/092986706777585040.
Acknowledgements
Not applicable.
Funding
The study was supported by the National Natural Science Foundation of China (grant number 81771589); the Program of Tianjin Science and Technology Plan (18ZXDBSY00170) and the Tianjin Health Bureau Science and Technology (2014KZ031). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
YL and JBS designed this study and data interpretation. YZ prepared the manuscript. YYN and YM presented the clinical information of the patient and performed literature review. JZ and XWX performed the bioinformatics analyses. FZ provided the clinical treatment and consultation for the patient. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Our article was published with the consent of the child’s parents and approved by the Ethics Committee of Tianjin Children's hospital.
Consent for publication
Written informed consent was obtained from the child’s parent for the publication of this case report, including any data contained within.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, Y., Nie, Y., Mu, Y. et al. A de novo variant in CASK gene causing intellectual disability and brain hypoplasia: a case report and literature review. Ital J Pediatr 48, 73 (2022). https://doi.org/10.1186/s13052-022-01248-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13052-022-01248-z