Abstract
Background
Preterm birth (PTB) can be caused by different factors. The factors can be classified into different categories: socio demographic, obstetric, reproductive health, medical, behavioral and nutritional related. The objective of this review was identifying determinants of PTB among mothers who gave birth in East African countries.
Methods
We have searched the following electronic bibliographic databases: PubMed, Google scholar, Cochrane library, AJOL (African journal online). Cross sectional, case control and cohort study published in English were included. There was no restriction on publication period. Studies with no abstracts and or full texts, editorials, and qualitative in design were excluded. Funnel plot was used to check publication bias. I-squared statistic was used to check heterogeneity. Pooled analysis was done by using fixed and random effect model. The Joanna Briggs Critical Appraisal Tools for review and meta-analysis was used to check the study quality.
Results
A total of 58 studies with 134,801 participants were used to identify determinants of PTB. On pooled analysis, PTB was associated with age < 20 years (AOR 1.76, 95% CI: 1.33–2.32), birth interval less than 24 months (AOR 2.03, 95% CI 1.57–2.62), multiple pregnancy (AOR 3.44,95% CI: 3.02–3.91), < 4 antenatal care (ANC) visits (AOR 5.52, 95% CI: 4.32–7.05), and absence of ANC (AOR 5.77, 95% CI: 4.27–7.79). Other determinants of PTB included: Antepartum hemorrhage (APH) (AOR 4.90, 95% CI: 3.48–6.89), pregnancy induced hypertension (PIH) (AOR 3.10, 95% CI: 2.34–4.09), premature rupture of membrane (PROM) (AOR 5.90, 95% CI: 4.39–7.93), history of PTB (AOR 3.45, 95% CI: 2.72–4.38), and history of still birth/abortion (AOR 3.93, 95% CI: 2.70–5.70). Furthermore, Anemia (AOR 4.58, 95% CI: 2.63–7.96), HIV infection (AOR 2.59, 95% CI: 1.84–3.66), urinary tract infection (UTI) (AOR 5.27, 95% CI: 2.98–9.31), presence of vaginal discharge (AOR 5.33, 95% CI: 3.19–8.92), and malaria (AOR 3.08, 95% CI: 2.32–4.10) were significantly associated with PTB.
Conclusions
There are many determinants of PTB in East Africa. This review could provide policy makers, clinicians, and program officers to design intervention on preventing occurrence of PTB.
Similar content being viewed by others
Background
Preterm birth (PTB) is birth occurs between 20 weeks of pregnancy and 37 weeks of pregnancy. It is a concern because babies who are born too early may not be fully developed. They may be born with serious health problem. Some problems like cerebral palsy, can last a life time. Other problems like learning disabilities may appear later in childhood or even adulthood [1].
Each year 15 million babies in the world, more than one in 10 births is born too early. More than 1 million of those babies die shortly after birth; countless other suffer some type of life long physical, neurological or educational disabilities often at great cost to families and society [2]. The survival chances of the 15 million babies born preterm each year vary dramatically depending on where they are born. South Asia and sub-Saharan Africa account for half the world’s births, more than 60% of the world’s preterm babies and over 80% of the world’s 1.1 million deaths due to PTB complications. Around half of these babies are born at home. Even for those born in a health clinic or hospital, essential newborn care is often lacking. The risk of a neonatal death due to complications of PTB is at least 12 times higher for an African baby than for a European baby. Yet, more than three-quarters of PTB could be saved with feasible, cost-effective care, and further reductions are possible through intensive neonatal care [3].
PTB has multiple causes; therefore, solutions will not come through a single discovery but rather from an array of discoveries addressing multiple biological, clinical, and socio-behavioral risk factors. Age of mother [4], household income [5], educational status of mother [4, 6], place of residence and employment status [7] were associated with PTB. Many studies in different settings of the world revealed the contributing factors of PTB as physical activity [8], maternal cardiovascular disease [9], delivering by previous cesarean section [10], had history of miscarriage [11], and history of PTB [11,12,13]. The contributing factors for PTB also include pregnancy interval [14], body mass Indexes(BMII) [11], antenatal care(ANC) [12, 15, 16], multiple pregnancy [17], antepartum hemorrhage(APH) [15],urinary tract infections(UTI) [11], premature rupture of membrane(PROM) [15, 18], and pregnancy induced hypertension(PIH) [17, 19]. Moreover, marital status [12], polyhydramnios or oligohydramnios and genitourinary infections [20], periodontal disease [11], ascending infection (bacteriuria) [21] and exposure to intimate partner violence (IPV) [15, 22] are included in contributing factors of PTB.
So far different researches are done and published on determinants of PTB among mothers who gave birth in East Africa countries. However, the results of the studies were inconsistent, factors that had direct association in some studies may be inversely associated or had no association in other studies and vise-versa. Moreover, to the best of our knowledge, there is no pooled data on determinants of PTB in East Africa. Hence, this systematic review and meta-analysis was conducted to identify determinants of PTB among mothers who gave birth in East Africa countries. This will help to make conclusions based on best available scientific evidence. Moreover, the result of this review could support policy makers, clinicians, and programmers to design intervention on preventing PTB.
Methods and materials
Reporting
The report was written by using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)) guideline [23]. This review was registered in PROSPERO database (PROSPERO 2019: CRD42019127645).
Inclusion and exclusion criteria
Cross sectional, case control and cohort studies done in East African countries were included in this study. East African countries include (Sudan, South Sudan, Kenya, Uganda, Djibouti, Eritrea, Ethiopia, Somalia, Tanzania, Rwanda, Burundi, Comoros, Mauritius, Seychelles, Mozambique, Madagascar, Zambia, Malawi, Zimbabwe, Reunion, Mayotte) [24]. Studies reported the determinants of PTB and published in English were incorporated. There was no restriction on publication period. Studies with no abstracts and or full texts, editorials, and qualitative studies were excluded.
Searching strategy and information sources
We have searched the following electronic databases: PubMed, Google scholar, Cochrane library, AJOL (African journal online). Furthermore, we have searched bibliographies and contacted authors. There was no restriction on publication period. To conduct a search of the literature databases, we have used Boolean Logic, connectors “AND”, “OR” in combinations [25]. The search strategy for PubMed database was done as following: preterm OR “preterm birth” OR “premature birth”(MeSH terms) AND birth OR parturition OR newborn(MeSH terms) OR infant OR child AND Sudan OR South Sudan OR Kenya OR Uganda OR Djibouti OR Eretria OR Ethiopia OR Somalia OR Tanzania OR Rwanda OR Burundi OR Comoros OR Mauritius OR Seychelles OR Mozambique OR Madagascar OR Zambia OR Malawi OR Zimbabwe OR Reunion OR Mayotte. The search algorithm for other database was done by modifying search strategy used for PubMed.
Study selection
Studies retrieved from database were exported to reference note manager, endnote version 7 to remove duplicate studies. Title and abstract was screened by two reviewers. The full text of these potentially eligible studies was retrieved and independently assessed for eligibility by two review team members. Disagreement between them over the eligibility of particular studies was resolved through discussion with a third reviewer.
Risk of bias in individual studies
To evaluate the quality of the papers, the Joanna Briggs Critical Appraisal Tools for review and meta-analysis was used [26]. Two independent reviewers assessed the quality of the study. Differences was reconciled by a third reviewer.
The following items was used to appraise case control studies:(1) comparable groups, (2) cases and controls matched, (3) the same criteria used for identification, (4) exposure measured in a standard, valid and reliable way, (5) exposure measured in the same way for cases and controls, (6) confounding factors identified, (7) strategies to deal with confounding factors, (8) outcomes assessed in a standard, valid and reliable way for cases and controls, (9) The exposure period of interest long enough, and (10) appropriate statistical analysis. Cohort studies were appraised by using the following items:(1) the two groups similar and recruited from the same population, (2) the exposures measured similarly to assign people to both exposed and unexposed groups, (3) the exposure measured in a valid and reliable way,(4)confounding factors identified, (5) strategies to deal with confounding factors, (6) participants free of the outcome at the start of the study, (7) the outcomes measured in a valid and reliable way, (8) follow up time reported and sufficient to be long enough for outcomes to occur, (9) follow up complete, and if not, were the reasons to loss to follow up described and explored, (10) strategies to address incomplete follow up, and (11) appropriate statistical analysis. For cross sectional studies the following items were used to appraise the quality: (1) criteria for inclusion, (2) study subjects and the setting described, (3) exposure measured in a valid and reliable way, (4) standard criteria used for measurement, (5) confounding factors identified, (6) strategies to appropriate statistical analysis deal with confounding factors, (7) outcomes measured in a valid and reliable way, and (8) appropriate statistical analysis.
Studies scored 50% and above in the quality assessment indictors were considered as low risk and included in the analysis.
Data collection process
Two independent reviewers extracted data by using structured data extraction form. The name of the first author and year, country, study design, sample size, determinants of PTB, AOR (95% CI), events and total in experimental and control groups were extracted. Whenever variations of extracted data observed, the phase was repeated.
Outcome measurement
PTB was considered, when newborn born < 37 weeks [27].
Data analysis
To identify determinants of PTB, the analyses were divided in to six parts: Socio economic and demographic factors, reproductive health (RH), obstetric factors, medical condition, nutrition and behavioral factors. The Meta-analysis was done by using RevMan 5.3 software [28]. Heterogeneity of the studies was done by I-squared statistic (I2). A values of 25, 50, and 75% represented low, moderate, and high I2, respectively [29]. In this study I-squared value less than 50% was considered to interpret the combined effect size. Publication bias was checked by funnel plot. As the studies included in each outcome was less than 10, funnel plot was not presented [30]. Sensitivity analysis could investigate whether any indication of bias (such as different sizes of estimates from studies with individual participant data and from those without, or evidence of funnel plot asymmetry) remains when studies with individual participant data are standardized to match those lacking individual participant data [31]. We have conducted sensitivity analysis to see the effects of a single study on determinants of PTB. For small number of studies, it may be impossible to estimate the between studies variance with any precision. Therefore, we used fixed effect model for less than five studies and random effect model for five and above studies [32]. Pooled analysis was done using mantel-haenszel (M-H) statistical methods and effect measure was computed by odds ratio by using fixed and random effect model [30].
Results
Study characteristics
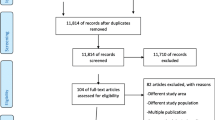
The search strategy retrieved 839 studies from databases and other sources. After duplication removed, 635 studies remained. Full text review was conducted for 100 studies and 58 studies with sample size of 134,801 participants were included to assess determinants of preterm birth (Fig. 1). The studies were included from 11 East African countries. Nineteen studies were from Tanzania [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51], 11 from Ethiopia [52,53,54,55,56,57,58,59,60,61,62,63], 6 from Kenya [5, 54, 64,65,66,67], 7 from Malawi [4, 6, 68,69,70,71,72], 3 from Sudan [10, 73, 74], 3 from Zambia [75,76,77],3 from Zimbabwe [78,79,80], 2 from Uganda [7, 81], 2 from Mozambique [82, 83], 1 from Rwanda [84], and 1 from Madagascar [85]. Twenty five studies were done by cohort study design [33, 35, 36, 39, 41, 43, 45,46,47,48,49,50, 59, 61, 65,66,67,68, 70, 75, 77, 80, 83,84,85], 9 studies by case control study design [7, 10, 37, 38, 53, 55, 57, 73, 79] and 24 studies by cross sectional study design [4,5,6, 34, 40, 42, 44, 51, 52, 54, 56, 58, 60, 62, 64, 69, 71, 72, 74, 76, 78, 81, 82] (Table 1).
Risk of bias within studies
Joanna Briggs Critical Appraisal Tools for review and meta-analysis for case control studies, cross sectional studies and cohort studies were used. We included studies that had low risk (Table 1).
Sensitivity analyses
On history of still birth, Feresu et al. 2004 [79], PIH, Deresa et al. 2018 and Abaraya et al.2018 [56, 57] and PROM, Ayebare et al. 2018 [7] had shown impact. On anemia, Abaraya et al.2018 and Remsi et al.2107 [57, 81] had revealed impact. On multiple pregnancy, Teklya et al. 2018 and Mahapula et al.2016 studies [38, 55] had brought out effects. Likewise, on ANC < 4 visits, Teklya et al. 2018 [55] and absence of ANC visits, Bekele et al. 2016 and Feresu 2004 et al. [63, 79] disclosed effects. Moreover, on HIV positive, Coley et al.2001 and Deressa et al.2018 [50, 56], and shorter pregnancy interval, Mahande et al. 2016 [39] showed effects. Besides, on UTI, Mahapula et al.2016 [38], malaria, Rempis et al.2017 [81], and obstetric complication Mekonen et al. 2018 [52] had shown impact. The abovementioned studies were become out of pooled analysis.
Factors associated with PTB
Socio economic and demographic factors
The pooled analysis of three studies identified that age less than 20 years increased the probability PTB (AOR 1.76; 95% CI: 1.33–2.32) [44, 64, 82] (Fig. 2). Age of sexual debut at 16–18 years (AOR 2.17; 95% CI: 1.2–3.8) and 18–30 years (AOR 1.99; 95% CI: 1.1–3.6) [48] was associated with PTB. Younger maternal age was protective for PTB (AOR 0.98; 95% CI: 0.96–1.00) [4].
Household income ≤ US$ 97.85 (AOR 2.7; 95% CI: 1.4–5.0) [5] and low income < 600 birr (AOR 2.6; 95% CI: 1.1, 6.6) [63] was significantly associated with PTB. Women with no education had higher probability to have PTB (AOR 3.50; 95% CI: 1.58–7.77) [6]. Higher maternal education was protective against PTB (AOR 0.70; 95% CI: 0.51–0.95) [4]. Delivery in rainy season increased the risk of PTB (AOR 3.93; 95% CI: 1.75–8.79) [6]. Maternal weight less than 50 kg (AOR 5.1; 95% CI: 1.7–15.9) [72] and weight gain < 1 kg increased the probability of having PTB (AOR 2.64; 95% CI: 1.39–5.02) [83] whereas weight gain during pregnancy (AOR 0.89; 95% CI 0.82–0.97) reduced the odds of PTB [69]. Later birth year was associated with lower PTB risk (AOR 0.35, 95% CI: 0.19–0.70) [4]. Female gender (AOR 1.24; 95% CI: 1.04–1.48) [4] and being adolescents (AOR 2.60, 95% CI: 1.16–5.78) [76] increased risk of PTB. Dwelling in rural area (AOR = 6.56; 95% CI: 2.64–16.10) and being unemployed (AOR 0.36; 95% CI: 0.15–0.86) [7] were associated with PTB. MUAC of mother 17–28.5 cm (ARR 0.95; 95% CI: 0.92, 0.99) [79] and less than 24 cm (AOR 2.6; 95% CI 1.1–6.1) [52] was significantly associated with PTB. Increasing in BMI (AOR 0.91, 95%, CI 0.85–0.97) [69] reduced the probability of PTB whereas BMI < 18.499 increased the risk of PTB (AOR 4.52, 95% CI: 2.39–9.27) [61]. Being primigravida was positively associated with PTB (AOR 2.3; 95% CI: (1.3–4.0) [71].
The effects of IPV on PTB were examined by three studies. Of three, two studies found positive association between IPV and PTB [35, 53] and one found no association [58]. Women exposed to IPV (AOR 2.5; 95% CI: 2.19–2.96) and physical violence (AOR 5.3; 95% CI: 3.95–7.09) during pregnancy were more likely to experience PTB [53]. Furthermore, women exposed to physical IPV (AOR 2.9; 95% CI: 1.3–6.5) and women with previous adverse pregnancy outcomes (AOR 4.5; 95% CI: 1.5–13.7) were more likely to experience PTB [35].
Reproductive health factors
The pooled effects of four studies illustrated that birth interval less than 24 months was positively associated with PTB (AOR 2.03; 95% CI: 1.57–2.62) [10, 57, 63, 74]. The pooled analyses of four studies (AOR 5.52; 95% CI: 4.32–7.05) [37, 38, 52, 72] and three studies (AOR 5.77, 95% CI 4.27–7.79) [7, 38, 57] showed that ANC visits < 4 and absence of ANC increased the risk of PTB, respectively. The pooled effects of two studies identified that multiple pregnancy was positively associated with PTB (AOR 3.44; 95% CI: 3.02–3.91) [57, 79] (Fig. 3). Infants conceived after longer inter-pregnancy interval (≥60 months) had more chance to be PTB (AOR 1.13, 95% CI: 1.02–1.24) [39]. Mothers who had no PMTCT (prevention mother to child treatment) intervention had higher risk of having PTB [61]. Absence of public prenatal care (AOR 2.1; 95% CI: 1.1–4.1) [38] and ANC visits < 5 (AOR 2.2, 95% CI 1.3–3.7) [71] increased the risk of PTB.
Obstetric complications
The pooled analysis of five studies disclosed that APH increased the risk of PTB (AOR 4.90; 95% CI: 3.48–6.89) [7, 37, 54, 57, 79]. The pooled effects of seven studies revealed that PIH was significantly associated with PTB (AOR 3.10; 95% CI: 2.34–4.09) [7, 46, 54, 55, 62, 64, 79]. The pooled analysis of four studies pointed out an association of PROM with PTB (AOR 5.90; 95% CI: 4.39–7.93) [52, 54, 57, 63]. In addition, obstetric complications was associated with PTB as depicted by the pooled analysis of three studies (AOR 3.48; 95% CI: 2.60–4.65) [38, 61, 63] (Fig. 4).
Fertility treatment before this pregnancy (AOR 7.0; 95% CI: 1.90–27.26), uterine pain in the current pregnancy (AOR 5.0; 95% CI: 1.7–14.35) and regular menstrual bleeding (AOR 5.8 (2.3–14.86) increased odds of PTB [37]. Cervical incompetence (AOR 11.6, 95% CI: 1.1–121.5) and polyhydramnios (AOR 8.3; 95% CI: 1.7–40.2) were reported by mothers who gave PTB [38]. History of cesarean delivery (AOR 5.4, 95% CI: 1.7–17.3] [10], history of either PTB or small baby (AOR 3.1; 95% CI 1.1–8.4) [60], history of a previous PTB (AOR 2.13; 95% CI: 1.19–3.80) [61] raised the probability of PTB.
The presence of chorio-amnionitis (AOR 3.8; 95% CI: 1.3–10.8) [72] and placenta praevia was correlated with PTB (ARR 3.30, 95% CI: 1.34, 8.14) [79]. The pooled analysis of four studies displayed that history of still birth/abortion was positively associated with PTB (AOR 3.93; 95% CI: 2.70–5.70) [10, 37, 57, 64]. The pooled effects of four studies displayed that history of PTB increased the odds of PTB (AOR 3.45; 95% CI: 2.72–4.38) [10, 46, 64, 69] (Fig. 2). Fetal distress (AOR 4.0; 95% CI: 1.9, 8.2) and birth defects (AOR 3.20; 95% CI: 1.22–8.32) increased the odds of PTB [55]. Low birth weight (RR 2.9; 95% CI: 2.3–3.6) and perinatal death (RR 2.5; 95% CI: 1.9–3.5) raised the risk of PTB [46].
Medical conditions
The pooled effects of two studies displayed that anemia was positively associated with PTB (AOR 4.58; 95% CI: 2.63–7.96) [52, 79]. HIV infection (AOR 2.59; 95% CI: 1.84–3.66) [5, 47, 62, 72, 84] and mothers who had started HAART before pregnancy was associated with PTB as identified by the pooled analysis of studies (AOR 1.68; 95% CI: 1.39–2.02) [41, 61]. Presence of malaria was associated with PTB as evidenced by the pooled analysis of four studies (AOR 3.08; 95% CI: 2.32–4.10) [42, 44, 79, 83]. The pooled analysis of two studies identified that UTI was significantly associated with PTB (AOR 5.27; 95% CI: 2.98–9.31) [37, 64] (Fig. 5). Presence of vaginal discharge increased the risk PTB as demonstrated by the pooled effects of three studies (AOR 5.33: 95% CI: 3.19–8.92) [10, 37, 38] (Fig. 2).
Women with unknown HIV status had moderately increased risks of having PTB (ARR 1.40; 95% CI: 1.23–1.59) [47]. Compared with women infected with HIV alone, primigravida with dual infection of HIV and malaria had an increased risk of delivering a PTB (AOR 3.4; 95% CI: 1.8–6.4) [86]. Baseline maternal CD4 level below 200/mm3 was significantly associated with PTB (AOR 5.37; 95% CI: 1.86–15.49) [61]. Presence of cord blood parasitemia was positively correlated with PTB (AOR 3.34; 95% CI: 1.26–8.82) [6]. The presence of maternal malaria increased the probability of having PTB (AOR 3.19, 95% CI: 1.9–5.2) [48]. Untreated bacterial vaginosis increased the probability of PTB (AOR 2.95, 95% CI: 1.3–6.6) [48].
TNF2 (Tumor Necrosis Factor) homozygosity was associated with PTB when compared with TNF1 homozygotes (RR 7.3, 95% CI: 2.85–18.9) and heterozygotes (RR 6.7, 95% CI: 2.0–23) [67]. Women with plasma levels of Chitinase-3-Like Protein-1(CHI3L1(AOR 2.82; 95% CI:1.56, 5.08)), C5a(AOR 1.94; 95% CI: 1.15–3.29), soluble Intercellular Adhesion Molecule-1(sICAM-1(AOR 1.91; 95% CI:1.12, 3.29), and Interleukin-18 Binding Protein(IL-18BP (AOR 2.60; 95% CI:1.47, 4.63) in the highest quartile had an increased risk of PTB compared with those in the lowest quartile. Women with Leptin (AOR 0.39; 95% CI: 0.20, 0.73) and Angiopoietin (Ang2 (AOR 0.48; 95% CI: 0.27, 0.85) in the highest quartile had a reduced risk of PTB compared with women in the lowest quartile [43].
Women with high-titer active syphilis were at the greatest risk of having PTB (ARR 6.1 (2.5–15.3) [49]. Presence of periodontal disease (AOR 2.32; 95%CI: 1.33–4.27) [34] and periodontitis (at least three sites from different teeth with clinical attachment loss greater than or equal to4 mm [85] was significantly associated with PTB. Maternal depression increased the probability of having PTB (ARR 4.13; 95% CI: 2.82–17.42) [65]. Hematocrit level < 33 (AOR 7.2; 95% CI: 3.1–16.8) [63] and presence of chronic illness (AOR 4.5; 95% CI: 2, 10.2) [63] were found to be significantly associated with PTB. Maternal parasitized red blood cells <=10(AOR 1.9; 95%CI: 1.1–3.4), > 10(AOR 3.2; 95% CI: 1.5–7.0) and perivillous fibrin deposition> 30 (AOR 2.1; 95% CI: 1.3–3.5) were associated with increased risk of premature delivery [51].
Nutritional factors
Women in the inadequate dietary diversity group had a higher risk for PTB (ARR 4.61; 95% CI: 2.31, 9.19) [59]. Median folic acid level 8.6 ng/mg (OR 0.64; 95% CI: 0.53–0.77) [73], higher calcium intake (RR 0.76; 95% CI: 0. 65–0.88) and dietary animal Fe (Iron) intake (RR 0.67; 95% CI: 0.51–0.90) reduced the probability of PTB [36].
Behavioral factors
Drank home brew during pregnancy reduced the probability of having PTB (ARR 0.75; 95% CI: 0.60, 0.93) [79]. Use of traditional medication in pregnancy (AOR 5.6; 95% CI: 2.1–14.87) [37] and quinine exposure in first trimester was associated with an increased risk of PTB (OR 2.6; 95% CI: 1.3–5.3) [45]. Women who did not take IPT (Intermittent preventive treatment) had higher chance to have PTB (AOR 21.0, 95% CI: 2.9–153.8) [72]. Use of at least 2 doses of SP (sulphadoxine-pyrimethamine) for IPT during pregnancy (AOR 0.1, 95% CI: 0.05–0.4) [42], two or more doses compared to 0–1 dose reduced preterm delivery (OR, 0.42; 95% CI: 0.27, 0.67) [75]. Among multigravida women, at least two or more doses of SP-IPTp SPIPTp (sulphadoxine-pyrimethamine for IPT remained significantly associated with protection from PTB (AOR 0.28, 95% CI: 0.13–0.60) [77]. Last SP time lapse ≤4 weeks reduced the PTB (AOR 0.38, 95% CI: 0.15, 0.97) [44].
Conceptual frame work
The conceptual framework of the study showing determinants of PTB in East Africa (Fig. 6).
Discussion
The objective of this systematic review and meta-analysis was identifying determinants of PTB in East Africa. Age of women less than 20 years was correlated with PTB. This is comparable with other studies [22, 87]. The increased risk PTB in younger age can be linked to the fact that their reproductive organs are not yet fully developed.
The current study depicted that history of still birth/abortion was significantly associated with PTB. This is related with systematic review and meta-analysis study [11, 88]. History of PTB was significantly associated with PTB. This is in agreement with other studies [11,12,13, 19, 89]. The reason for this could be the likelihood of having PTB with the women with prior spontaneous labor as well as those with inducing PTB rising. PROM was significantly associated with PTB. This is similar with systematic review and meta-analysis study [18].
Shorter pregnancy interval was significantly associated with PTB. This is in line with studies done in Egypt and other places [11, 14, 90]. This may be because mothers do not have time to recover from the physical stress and nutritional burden of the pregnancy.
Women who attended ANC < 4 times had higher probability to have PTB. This finding is comparable with systematic review and meta-analysis study conducted in Iran [16]. Likewise, the absence of ANC was significantly associated with PTB. The reason for this might be when women had no chance to attend ANC, she cannot be informed of early identification of risk factors associated with PTB. Consequently, the probability of having PTB will increase.
Having multiple pregnancy increased the probability of PTB. It is in line with other studies [17, 91].This may be due to overstretching of uterus and deciding to complete pregnancy before term. Furthermore, it may be due to spontaneous labor or PROM.
Maternal UTI was associated with PTB. This is in agreement with other studies [11, 15, 19]. UTI can weaken the membranes of the amniotic sac around the baby. This could lead to PROM and preterm labor [92]. Presence of malaria increased the risk of PTB. This is in line with other studies [15]. Presence of anemia was associated with PTB. This is in agreement with other study [15]. Anemia can decrease blood flow to placenta and this can causes placental insufficiency, finally results in PTB. Presence of vaginal discharge during pregnancy increased the chance of having PTB. This is alike with other study [37].
Women who were already on HAART preconception had higher probability to have PTB. This is in line with other studies [93, 94]. HIV positive women had more probability to give PTB than HIV negative women. This is in agreement with study done in South Africa [95]. Presence of periodontal disease was significantly associated with PTB as depicted systematic reviews of studies. Study done in Egypt showed similar result [11]. This may be due to periodontal can results in an increase of pro-inflammatory molecules that can directly or indirectly lead to uterine contractions and cervical dilatation.
From determinants of PTB, PROM [12, 13, 15, 19, 96, 97], APH [13, 15, 19], ANC status [12, 13, 19], PIH [13, 19, 97], History of PTB [11, 13, 19], maternal age [13, 15, 97] multiple pregnancy [13, 15, 96] was prevalent in other African countries. The possible reason for this could be poor management of maternal health problems, limited access to health facilities and low health seeking behavior of the community. Birth interval less than 24 months, age less than 20 years, ANC status, and anemia could be more quickly modified.
The strength of this study is that this review seems to be the first done in East Africa, indicating the various determinants of PTB from 58 studies. This study has the following limitations. The search strategy was limited to studies published in English language, this can cause reporting bias. Data were not found for 10 East African countries, this can cause representativeness problems. Thus, further studies on determinants of PTB should be conducted in all East African countries by using standard WHO definition of PTB.
Conclusions
There are many determinants of PTB in East Africa. The determinants can be categorized into socio economic and demographic factors, RH, obstetric complications, medical condition, and behavior related factors. This review could provide policy makers, clinicians, and program officers to design intervention on preventing the occurrence of PTB.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ANC:
-
Antenatal care
- APH:
-
Antepartum hemorrhage
- ART:
-
Anti-retroviral therapy
- BMI:
-
Body mass index
- HAART:
-
Highly active anti-retroviral therapy
- HIV:
-
Human immune deficiency virus
- IPT:
-
Intermittent preventive treatment
- IPTp-SP:
-
Intermittent preventive treatment of malaria in pregnancy
- IPV:
-
Intimate partner violence
- MUAC:
-
Mid-upper arm circumference
- PIH:
-
Pregnancy induced hypertension
- PMTCT:
-
Prevention mother to child transmission
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROM:
-
Premature rupture of membrane
- PTB:
-
Preterm birth
- RH:
-
Reproductive health
- TNF:
-
Tumor necrosis factor
- UTI:
-
Urinary tract infection
- WHO:
-
World health organization
References
Preterm and labor.The American college of obstetricians and gynecologists. Available in www.ocog.org. 2019.
The partnership for maternal, newborn and child health. Born too soon: the global action report on preterm birth, vol. 2018; 2018.
Born too soon. The global action report on preterm birth. March of Dimes, save the children, WHO. 2012.
Taha TE, Dadabhai SS, Rahman MH, Sun J, Kumwenda J, Kumwenda NI. Trends in birth weight and gestational age for infants born to HIV-infected, antiretroviral treatment-naive women in Malawi. Pediatr Infect Dis J. 2012;31(5):481–6.
Karki S. Preterm Birth among Kenyan Women.pdf>. washington: washington; 2016.
Sullivan AD, Nyirenda T, Cullinan T, Taylor T, Harlow SD, James SA, et al. Malaria infection during pregnancy: intrauterine growth retardation and preterm delivery in Malawi. J Infect Dis. 1999;179(6):1580–3.
Ayebare E, Ntuyo P, Malande OO, Nalwadda G. Maternal, reproductive and obstetric factors associated with preterm births in Mulago Hospital, Kampala, Uganda: a case control study. Pan Afr Med J. 2018;30:272.
Aune D, Schlesinger S, Henriksen T, Saugstad OD, Tonstad S. Physical activity and the risk of preterm birth: a systematic review and meta-analysis of epidemiological studies. BJOG. 2017;124:11.
Wu P, Gulati M, Kwok CS, Wong CW, Narain A, O’Brien S, et al. Preterm Delivery and Future Risk of Maternal Cardiovascular Disease:A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2018;7:1–29.
Alhaj AM, Radi EA, Adam I. Epidemiology of preterm birth in Omdurman Maternity hospital, Sudan. J Matern Fetal Neonatal Med. 2010;23(2):131–4.
Fyala E. Prevalence and Risk Factors of Spontaneous Preterm Birth. Med J Cairo Univ. 2016;84(1):5.
Iyoke C, Lawani L, Ezugwu E, Ilo K, Ilechukwu G, Asinobi I. Maternal risk factors for singleton preterm births and survival at the University of Nigeria Teaching Hospital, Enugu, Nigeria. Niger J Clin Pract. 2019;18(6):744–750.
Akintayo AA, Awoleke JO, Ogundare EO, Olatunya OS, Aduloju OP. Preterm births in a resource constrained setting: soci-obiologic risk factors and perinatal outcomes. Ghana Med J. 2015;49(4):251–257.
Wong L, Wilkes J, Korgenski K, Varner M, Manucka T. Risk factors associated with preterm birth after a prior term delivery. BJOG. 2015;123(7):1772.
Zini ME, Omo-Aghoja LO. Clinical and sociodemographic correlates of preterm deliveries in two tertiary hospitals in southern Nigeria. Ghana Med J. 2019;53(1):9.
Sharifi N, Dolatian M, Kazemi AFN, Pakzad R. The Relationship Between the Social Determinants of Health and Preterm Birth in Iran Based on the WHO Model: A Systematic Review and Meta-analysis. Int J Womens Health Reprod Sci. 2018;6(2):10.
Mulualem G, Wondim A, Woretaw A. The effect of pregnancy induced hypertension and multiple pregnancies on preterm birth in Ethiopia: a systematic review and meta-analysis. BMC Res Notes. 2019;12(91):1–7.
Phillips C, Velji Z, Hanly C, Metcalfe A. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open. 2017;2017:7.
Mokuolu OA, Suleiman B, Adesiyun O, Adeniyi A. Prevalence and determinants of pre-term deliveries in the University of Ilorin Teaching Hospital, Ilorin, Nigeria. Pediatric Reports. 2010;2:11–14.
Mahajan A, Magon S. Study of risk factors for preterm births in a teaching hospital: A prospective study. IJMDS. 2017;6(1):6 ● wwwijmdsorg ●.
Bianchi-Jassir F, Seale AC, Kohli-Lynch M, Lawn JE, Baker CJ, Bartlett L, et al. Preterm Birth Associated With Group B Streptococcus Maternal Colonization Worldwide: Systematic Review and Meta-analyses. CID. 2017;65(2):5133–5142.
Chen K-H, Chen I-C, Yang Y-C, Chen K-T. The trends and associated factors of preterm deliveries from 2001 to 2011 in Taiwan. Medicine. 2019;98(13):1–6.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoSmedicine. 2009;6(7):e1000097.
East african countries: https://en.wikipedia.org/wiki/Category:East_African_countries. Countries in the East Africa Region 2019.
Tuttle BD, Isenburg MV, Schardt C, Powers A. PubMed instruction for medical students: searching for a better way. Med Ref Serv Q. 2009;28(3):11.
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. Joanna Briggs Institute Reviewer’s Manual: The Joanna Briggs Institute; 2017. Available from https://reviewersmanualjoannabriggsorg/.
Preterm birth. http://www.who.int/news-room/fact-sheets/detail/preterm-birth. World health organization; 2018.
Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014.
Ioannidis J, Patsopoulos N, Evangelou E. Uncertainty in heterogeneity estimatesin meta-analysis. BMJ. 2007;335:914–918.
Cochrane hand book for systematic reviews of intervention version 5.1.0(updated march 2011): The cochrane collaboration; 2011.
Ahmed I, Sutton AJ, Riley D. Assessment of publication bias, selection bias, and unavailable data in meta-analyses using individual participant data: a database survey. BMJ. 2012;344:1–10.
Borenstein M, Hedges L, Rothstein H. Meta-Analysis: Fixed effect vs. random effects; 2007. p. 162.
Stephen G, Mgongo M, Hussein Hashim T, Katanga J, Stray-Pedersen B, Msuya SE. Anaemia in Pregnancy: Prevalence, Risk Factors, and Adverse Perinatal Outcomes in Northern Tanzania. Anemia. 2018;2018:1846280.
Gesase N, Miranda-Rius J, Brunet-Llobet L, Lahor-Soler E, Mahande MJ, Masenga G. The association between periodontal disease and adverse pregnancy outcomes in Northern Tanzania: a cross-sectional study. Afr Health Sci. 2018;18(3):601–11.
Sigalla GN, Mushi D, Meyrowitsch DW, Manongi R, Rogathi JJ, Gammeltoft T, et al. Intimate partner violence during pregnancy and its association with preterm birth and low birth weight in Tanzania: A prospective cohort study. PLoS One. 2017;12(2):e0172540.
Mosha D, Liu E, Hertzmark E, Chan G, Sudfeld C, Masanja H, et al. Dietary iron and calcium intakes during pregnancy are associated with lower risk of prematurity, stillbirth and neonatal mortality among women in Tanzania. Public Health Nutr. 2017;20(4):678–86.
Mahande JM. Risk Factors for Preterm Birth among Women Who Delivered Preterm Babies at Bugando Medical Centre, Tanzania. SOJ Gynecol Obstet Womens Health. 2017;3(2):7.
Mahapula FA, Kumpuni K, Mlay JP, Mrema TF. Risk factors associated with pre-term birth in Dar es Salaam, Tanzania: a case-control study. Tanzan J Health Res. 2016;18(1):8.
Mahande MJ, Obure J. Effect of interpregnancy interval on adverse pregnancy outcomes in northern Tanzania: a registry-based retrospective cohort study. BMC Pregnancy Childbirth. 2016;16(1):140.
Mahande AM, Mahande MJ. Prevalence of parasitic infections and associations with pregnancy complications and outcomes in northern Tanzania: a registry-based cross-sectional study. BMC Infect Dis. 2016;16:78.
Li N, Sando MM, Spiegelman D, Hertzmark E, Liu E, Sando D, et al. Antiretroviral Therapy in Relation to Birth Outcomes among HIV-infected Women: A Cohort Study. J Infect Dis. 2016;213(7):1057–64.
Ndeserua R, Juma A, Mosha D, Chilongola J. Risk factors for placental malaria and associated adverse pregnancy outcomes in Rufiji, Tanzania: a hospital based cross sectional study. Afr Health Sci. 2015;15(3):810–8.
McDonald CR, Darling AM, Conroy AL, Tran V, Cabrera A, Liles WC, et al. Inflammatory and Angiogenic Factors at Mid-Pregnancy Are Associated with Spontaneous Preterm Birth in a Cohort of Tanzanian Women. PLoS One. 2015;10(8):e0134619.
Mpogoro FJ, Matovelo D, Dosani A, Ngallaba S, Mugono M, Mazigo AHD. Uptake of intermittent preventive treatment with sulphadoxine-pyrimethamine for malaria during pregnancy and pregnancy outcomes: a cross-sectional study in Geita district,North-Western Tanzania. Malar J. 2014;13(455):14.
Mosha D, Mazuguni F, Mrema S, Sevene E, Abdulla S, Genton B. Safety of artemether-lumefantrine exposure in first trimester of pregnancy: an observational cohort. Malar J. 2014;13:197.
Mahande MJ, Daltveit AK, Obure J, Mmbaga BT, Masenga G, Manongi R, et al. Recurrence of preterm birth and perinatal mortality in northern Tanzania: registry-based cohort study. Tropical Med Int Health. 2013;18(8):962–7.
Habib NA, Daltveit AK, Bergsjo P, Shao J, Oneko O, Lie RT. Maternal HIV status and pregnancy outcomes in northeastern Tanzania: a registry-based study. Bjog. 2008;115(5):616–24.
Watson-Jones D, Weiss HA, Changalucha JM, Todd J, Gumodoka B, Bulmer J, et al. Adverse birth outcomes in United Republic of Tanzania--impact and prevention of maternal risk factors. Bull World Health Organ. 2007;85(1):9–18.
Watson-Jones D, Changalucha J, Gumodoka B, Weiss H, Rusizoka M, Ndeki L, et al. Syphilis in pregnancy in Tanzania. I. Impact of maternal syphilis on outcome of pregnancy. J Infect Dis. 2002;186(7):940–7.
Coley JL, Msamanga GI, Fawzi MC, Kaaya S, Hertzmark E, Kapiga S, et al. The association between maternal HIV-1 infection and pregnancy outcomes in Dar es Salaam, Tanzania. BJOG. 2001;108(11):1125–33.
Menendez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, Kahigwa E, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181(5):1740–5.
Mekonen DG, Yismaw AE, Nigussie TS, Ambaw WM. Proportion of Preterm birth and associated factors among mothers who gave birth in Debretabor town health institutions, northwest, Ethiopia. BMC Res Notes. 2019;12(2):1–6.
Berhanie E, Gebregziabher D, Berihu H, Gerezgiher A, Kidane G. Intimate partner violence during pregnancy and adverse birth outcomes: a case-control study. Reprod Health. 2019;16(1):22.
Wagura P, Wasunna A, Laving A, Wamalwa D, Ng'ang'a P. Prevalence and factors associated with preterm birth at kenyatta national hospital. Biomed Res Int. 2018;18(1):107.
Teklay G, Teshale T, Tasew H, Mariye T, Berihu H, Zeru T. Risk factors of preterm birth among mothers who gave birth in public hospitals of central zone, Tigray, Ethiopia: unmatched case–control study 2017/2018. BMC Res Notes. 2018;11(1):7.
Deressa AT, Cherie A, Belihu TM, Tasisa GG. Factors associated with spontaneous preterm birth in Addis Ababa public hospitals, Ethiopia: cross sectional study. BMC Pregnancy Childbirth. 2018;18(1):332.
Abaraya M, Seid SS, Ibro SA. Determinants of preterm birth at Jimma University Medical Center, southwest Ethiopia. Pediatric Health Med Ther. 2018;9:7.
Laelago T, Belachew T, Tamrat M. Effect of intimate partner violence on birth outcomes. Afr Health Sci. 2017;17(3):681–9.
Zerfu TA, Umeta M, Baye K. Dietary diversity during pregnancy is associated with reduced risk of maternal anemia, preterm delivery, and low birth weight in a prospective cohort study in rural Ethiopia. Am J Clin Nutr. 2016;103(6):1482–8.
Adane AA, Ayele TA, Ararsa LG, Bitew BD, Zeleke BM. Adverse birth outcomes among deliveries at Gondar University Hospital, Northwest Ethiopia. BMC Pregnancy Childbirth. 2014;14:90.
Kebede B, Andargie G, Gebeyehu A. Birth outcome and correlates of low birth weight and preterm delivery among infants born to HIV-infected women in public hospitals of Northwest Ethiopia. Health. 2013;05(07):25–34.
Gebreslasie K. Preterm Birth and Associated Factors among Mothers Who Gave Birth in Gondar Town Health Institutions: Hindawi; 2016.
Bekele T, Amanon A, Gebreslasie KZ. Preterm Birth and Associated Factors among Mothers Who gave Birth in Debremarkos Town Health Institutions, 2013 Institutional Based Cross Sectional Study. Gynecol Obstet. 2015;5(5):1–5.
Okube OT, Sambu LM. Determinants of Preterm Birth at the Postnatal Ward of Kenyatta National Hospital, Nairobi, Kenya. Open J Obstet Gynecol. 2017;7:16.
Mochache KK. Depression and Preterm Birth; kenyaA Prospective Cohort Study Carried Out Among Women Attending Antenatal clinic At Pumwani Maternity Hospital. Nairob: University of Nairob; 2016.
Ayisi JG, van Eijk AM, ter Kuile FO, Kolczak MS, Otieno JA, Misore AO, et al. The effect of dual infection with HIV and malaria on pregnancy outcome in western Kenya. Aids. 2003;17(4):585–94.
Aidoo M, McElroy PD, Kolczak MS, Terlouw DJ, ter Kuile FO, Nahlen B, et al. Tumor necrosis factor-alpha promoter variant 2 (TNF2) is associated with pre-term delivery, infant mortality, and malaria morbidity in western Kenya: Asembo Bay Cohort Project IX. Genet Epidemiol. 2001;21(3):201–11.
Chagomerana MB, Miller WC, Pence BW, Hosseinipour MC, Hoffman IF, Flick RJ, et al. PMTCT Option B+ Does Not Increase Preterm Birth Risk and May Prevent Extreme Prematurity: A Retrospective Cohort Study in Malawi. J Acquir Immune Defic Syndr. 2017;74(4):367–74.
Neilson JP. Factors associated with preterm, early preterm and late preterm birth in Malawi. PLoS One. 2014;9:e90128 (3 // () *Wellcome Trust*).
Turner AN, Tabbah S, Mwapasa V, Rogerson SJ, Meshnick SR, Ackerman WE, et al. Severity of maternal HIV-1 disease is associated with adverse birth outcomes in Malawian women: a cohort study. J Acquir Immune Defic Syndr. 2013;64(4):392–9.
Kalanda BF, Verhoeff FH, Chimsuku L, Harper G, Brabin BJ. Adverse birth outcomes in a malarious area. Epidemiol Infect. 2006;134(3):659–66.
Abrams ET, Milner DA Jr, Kwiek J, Mwapasa V, Kamwendo DD, Zeng D, et al. Risk factors and mechanisms of preterm delivery in Malawi. Am J Reprod Immunol. 2004;52(2):174–83.
Sharif ME, Mohamedain A, Ahmed AA, Nasr AM, Adam I. Folic acid level and preterm birth among Sudanese women. Matern Health Neonatol Perinatol. 2017;3(25):1–5.
Adam I, Ismail MH, Nasr AM, Prins MH, Smits LJ. Low birth weight, preterm birth and short interpregnancy interval in Sudan. J Matern Fetal Neonatal Med. 2009;22(11):1068–71.
Chico RM, Chaponda EB, Ariti C, Chandramohan D. Sulfadoxine-Pyrimethamine Exhibits Dose-Response Protection Against Adverse Birth Outcomes Related to Malaria and Sexually Transmitted and Reproductive Tract Infections. Clin Infect Dis. 2017;64(8):1043–51.
Kumwenda A, Vwalika B. Outcomes and Factors Associated with Adolescent Pregnancies at the University Teaching Hospital,Lusaka, Zambia. Med J Zambia. 2017;4:7.
Mace KE, Chalwe V, Katalenich BL, Nambozi M, Mubikayi L, Mulele CK, et al. Evaluation of sulphadoxine-pyrimethamine for intermittent preventive treatment of malaria in pregnancy: a retrospective birth outcomes study in Mansa, Zambia. Malar J. 2015;14:69.
Muti M, Tshimanga M, Notion GT, Bangure D, Chonzi P. Prevalence of pregnancy induced hypertension and pregnancy outcomes among women seeking maternity services in Harare, Zimbabwe. BMC Cardiovasc Disord. 2015;15:111.
Feresu SA, Harlow SD, Woelk GB. Risk factors for prematurity at Harare Maternity Hospital, Zimbabwe. Int J Epidemiol. 2004;33(6):1194–201.
Feresu SA, Harlow SD, Welch K, Gillespie BW. Incidence of and socio-demographic risk factors for stillbirth, preterm birth and low birthweight among Zimbabwean women. Paediatr Perinat Epidemiol. 2004;18(2):154–63.
Rempis EM, Schnack A, Decker S, Braun V, Rubaihayo J, Tumwesigye NM, et al. Option B+ for prevention of vertical HIV transmission has no influence on adverse birth outcomes in a cross-sectional cohort in Western Uganda. BMC Pregnancy Childbirth. 2017;17(1):82.
Arnaldo P, Rovira-Vallbona E, Langa JS, Salvador C, Guetens P, Chiheb D, et al. Uptake of intermittent preventive treatment and pregnancy outcomes: health facilities and community surveys in Chokwe district, southern Mozambique. Malar J. 2018;17(1):13.
Osman NB, Challis K, Cotiro M, Nordahl G, Bergstrom S. Perinatal outcome in an obstetric cohort of Mozambican women. J Trop Pediatr. 2001;47(1):30–8.
Leroy V, Ladner J, Nyiraziraje M, De Clercq A, Bazubagira A, Van de Perre P, et al. Effect of HIV-1 infection on pregnancy outcome in women in Kigali, Rwanda, 1992–1994. Pregnancy and HIV Study Group. AIDS. 1998;12(6):643–50.
Rakoto-Alson S, Tenenbaum H, Davideau JL. Periodontal diseases, preterm births, and low birth weight: findings from a homogeneous cohort of women in Madagascar. J Periodontol. 2010;81(2):205–13.
Ayisia JG, Eijk AMV, Kuile FOT, Kolczak MS, Otieno JA, Misore AO, et al. The effect of dual infection with HIV and malaria on pregnancy outcome in western Kenya. AIDS. 2003;17:10.
Ahankari A, Bapat S, Myles P, Fogarty A, Tata L. Factors associated with preterm delivery and low birth weight: a study from rural Maharashtra, India. F1000Research. 2017;6(72):12.
Malacova E, Regan A, Nassar N, Raynes-Greenow C, Leonard H, Srinivasjois R, et al. Title: Risk of stillbirth, preterm delivery and fetal growth restriction following exposure in previous birth: systematic review and meta-analysis. In: Health Sop, editor. . Australia: Curtin University. 2016. p. 23.
Delnord M, Blondel B, Prunet C, Zeitlin J. Are risk factors for preterm and early-term live singleton birth the same? A population-based study in France. BMJ Open. 2018;8(1):1–9.
Howard EJ, Harville E, Kissinger P, Xiong X. The Association Between Short Interpregnancy Interval and Preterm Birth in Louisiana: A Comparison of Methods. Matern Child Health J. 2015;17(5):13.
Sutan R, Mohamed NE, Mahdy ZA, Ishak S, Shamsuddin K, Idris IB, et al. A 5 year trend and predictors of preterm births in single referral centre of the Greater Kuala Lumpur,Malaysia. Int J Pregnancy Child Birth. 2018;4(6):196–201.
Devlieger R, Millar LK, Bryant-Greenwood G, Lewi L, Deprest JA. Fetal membrane healing after spontaneous and iatrogenic membrane rupture: a review of current evidence. Am J Obstet Gynecol. 2006;195:8.
Machado ES, Hofer CB, Costa TT, Nogueira SA, Oliveira RH, Abreu TF, et al. Pregnancy outcome in women infected with HIV-1 receiving com- bination antiretroviral therapy before versus after concep- tion. Sex Transm Infect. 2009;85(2):6.
Stringer EM, Kendall MA, Lockman S, Campbell TB, Nielsen-Saines K, Sawe F, et al. Pregnancy outcomes among HIV-infected women who conceived on antiretroviral therapy. PLoS One. 2018;13(7):12.
Naidooa M, Sartoriusb B, Tshimanga-Tshikalaa G. Maternal HIV infection and preterm delivery outcomes at an urban district hospital in KwaZulu-Natal 2011. South Afr J Infect Dis. 2016;31(1):4.
Demmouche A, Mai AH, Kaddouri MS, Ghani A, Rahmani S, Beddek F, et al. Etiology of Preterm Birth in Relizane Region (West of Algeria). J Nutr Food Sci. 2004;4(5):1–3.
Butali A, Ezeaka C, Ekhaguere O, Weathers N, Ladd J, Fajolu I, et al. Characteristics and risk factors of preterm births in a tertiary center in Lagos, Nigeria. Pan Afr Med J. 2016;24(1):1–8.
Acknowledgments
We would like to thank Mr. Terefe Gone for provision of internet access during the review process.
Funding
‘Not applicable in this section’.
Author information
Authors and Affiliations
Contributions
TL conceived, designed, coordinated, searched, analyzed, interpreted data and wrote the review and protocol. TY and GT screened studies, extracted data, and appraised the quality of studies and wrote the review. All authors’ read and approved the manuscript.
Authors’ information
TL is lecturer of reproductive health, he has master of public health in reproductive health.
TY is lecturer of epidemiology, he has master of public health in epidemiology.
GT is public health emergency officer, he has master in field epidemiology.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
“Not applicable in this section”.
Consent for publication
“Not applicable in this section”.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Laelago, T., Yohannes, T. & Tsige, G. Determinants of preterm birth among mothers who gave birth in East Africa: systematic review and meta-analysis. Ital J Pediatr 46, 10 (2020). https://doi.org/10.1186/s13052-020-0772-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13052-020-0772-1