Abstract
Tissue-based biopsy is the present main tool to explore the molecular landscape of cancer, but it also has many limits to be frequently executed, being too invasive with the risk of side effects. These limits and the ability of cancer to constantly evolve its genomic profile, have recently led to the need of a less invasive and more accurate alternative, such as liquid biopsy. By searching Circulating Tumor Cells and residues of their nucleic acids or other tumor products in body fluids, especially in blood, but also in urine, stools and saliva, liquid biopsy is becoming the future of clinical oncology. Despite the current lack of a standardization for its workflows, that makes it hard to be reproduced, liquid biopsy has already obtained promising results for cancer screening, diagnosis, prognosis, and risk of recurrence.
Through a more accessible molecular profiling of tumors, it could become easier to identify biomarkers predictive of response to treatment, such as EGFR mutations in non-small cell lung cancer and KRAS mutations in colorectal cancer, or Microsatellite Instability and Mismatch Repair as predictive markers of pembrolizumab response.
By monitoring circulating tumor DNA in longitudinal repeated sampling of blood we could also predict Minimal Residual Disease and the risk of recurrence in already radically resected patients.
In this review we will discuss about the current knowledge of limitations and strengths of the different forms of liquid biopsies for its inclusion in normal cancer management, with a brief nod to their newest biomarkers and its future implications.
Similar content being viewed by others
Introduction
Behind the pathogenesis of cancer, there are accumulating mutations of genes involved in different pathways of cell survival, proliferation, and differentiation. Thus, currently, the way to identify their molecular profile, with important diagnostic and prognostic implications, usually consists of the direct tissue sampling of the tumor or metastatic lesion.
However, tumors are highly heterogeneous and sampling in their entirety is challenging, starting from the ability of their molecular profile to evolve over time. Several critical issues came out from the use of tissue sampling to determine the genomic profile of solid tumors such as the molecular divergency of individual cancers and metastatic lesions even within a single patient, and the molecular alterations induced by the therapeutic stress exerted by targeted drugs on tumor cells. Tissue biopsy is invasive, and it cannot be frequently repeated to monitor current tumor dynamics or response to treatment [1].
In contrast, the need for more sensitive and less invasive techniques to determine the molecular landscape of cancers has led to the development of genetic and genomic tests based on body fluids, especially from blood samples.
Liquid biopsies present different advantages over standard diagnostic tissue biopsy (Fig. 1): they are minimally invasive, having a simpler and more convenient sample and fewer side effects for patients, and potentially leading to more accurate prediction of tumor incidence, progression, treatment response, and survival prognosis [2,3,4].
The primary marker analyzed through liquid biopsies are distinctive tumor-derived components: circulating tumor cells (CTCs), cancer cells that leave the primary tumor potentially invading other tissues through the bloodstream [5, 6]; cell-free DNA (cfDNA), that has already presented raised levels in the serum of cancer patients and was first described by Mandel and Metais in 1948 [7, 8]; circulating tumor DNA (ctDNA), a fraction of cfDNA that belongs to cancer and presents its mutations [9, 10], studied for its implications as a prognostic and predictive factor for patients and for cancer detection [11,12,13]; tumor-derived RNAs (i.e. mRNA and miRNA) [14, 15]; extracellular vesicle, such as exosomes, of recent interest [16].
Moreover, blood is not the only body fluid that can be analyzed by liquid biopsy, extending the sources of cancer-derived molecules to other fluids such as urine [17], saliva [18], and stools [19].
The development of a targeted approach to investigating ctDNA, which studies known genetic mutations located in specific genes, has led to important progress for targeted therapies, such as the ability to predict therapeutic response to the EGFR inhibition in lung cancer by analyzing specific mutations of this gene [11, 20, 21]. On the other hand, an untargeted approach, aiming to detect any unknown mutation through whole genome sequencing, can lead to the discovery of new biomarkers involved in cancer management and prognosis. Detection of ctDNA can also be relevant for the identification of minimal residual disease (MRD) even in the absence of clinical evidence in patients following curative treatment or surgery [22, 23].
Anyways, liquid biopsy still presents some issues that must be considered to improve the evidence of its clinical utility, especially due to the lack of standardization across workflows during the different phases of laboratory testing, from specimen collection to its analysis.
Herein, we provide a brief overview of the various advantages and the current limitations of liquid biopsy in the management of cancer. We will also discuss the old and newest biomarkers and techniques implicated in its utility in cancer diagnosis, prognosis, and monitoring of treatment response or recurrence, including several promising studies that recently came out to enlighten how liquid biopsy should be integrated even more in clinical practice.
Technical aspects: limits and perspectives (sampling, storage, technologies, PCR, NGS, CGP, etc.), structured reports
Liquid biopsy for cancer patients involves the isolation of circulating tumor cells, circulating tumor DNA, and other tumor-derived materials such as proteins and exosomes from patient blood samples. Circulating tumor DNA (ctDNA) represents promising biomarkers in cancer diseases. ctDNA can be isolated from many body fluids, such as blood, saliva, urine, ascites, bile, cerebrospinal fluids, and pleural effusion may be considered as a source of ctDNA [1].
Despite the advantages of liquid biopsy, the majority of assays still lack evidence of clinical utility and validity [24], with only four tests [25] obtaining approval from the Food and Drug Administration (FDA). One reason for this is that liquid biopsy assays often lack reproducibility [26] due to the absence of standardization across workflows. For clinical labs to successfully implement liquid biopsy, they need to develop easy-to-use, robust, and reproducible workflows [27] that include “standard operating procedures” across all phases of laboratory testing. Of particular interest is the standardization of pre-analytical workflows for liquid biopsy as assay outcome can be influenced by many different variables during this phase.
The pre-analytical phase of liquid biopsy (Table 1) includes all the steps prior to analysis such as specimen collection, stabilization, transport, enrichment, processing, and isolation and quality assessment of the analyte. The purpose of this workflow is to maintain the integrity of the sample following blood draw and prepare it for analysis [28]. The pre-analytical phase is arguably the most important part of liquid biopsy workflows as 46% to 68% of errors occur during this phase [29]. These errors can adversely affect data quality in the following phases and can result in incorrect treatment decisions [29].
Arechederra M et al. reviewed the literature comparing different methodological approaches for each step in the sample preparation process [28]. The sheer number of reports combined with the sometimes-contradictory impacts of different pre-analytical variables highlights the urgent need to standardize these procedures [24]. To standardize these aspects of the pre-analytical phase, researchers first need to understand their impact on sample integrity and the eventual success of liquid biopsy tests [30].
Blood withdrawal represents one of the best sources due to the very simple and minimally invasive way of sampling. Moreover, it can be repeated at different time points, giving the opportunity for real-time monitoring of the disease. Circulating Free DNA (cfDNA) are spread from both cancer and normal cells, but in cancer patients their concentrations are greater [31, 32]. Circulating tumor DNA (ctDNA) is part of the cfDNA deriving from the tumor mass.
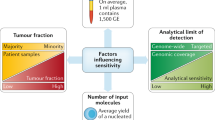
In cancer patients, a proportion of these cfDNA molecules also derive from the primary and secondary tumors. Although it was originally thought that the higher level of cfDNA in the blood of cancer patients might be a cancer biomarker itself, it has been since shown that many other conditions result in similar cfDNA increase. In this regard, important points must be considered: i) concentrations of cfDNA vary enormously between individuals and their physio-pathological conditions, being increased not only in advanced cancer patients but also in other scenarios including, autoimmune diseases, trauma, strenuous exercise, or pregnancy; ii) in most early stage cancers, the amount of cfDNA is very low, similar to healthy subjects [33]; iii) the fraction of ctDNA fragments in the total cfDNA is very small, varying from less than 0.01% to over 10% according to tumor burden [34] and tumor metabolism [35]; iv) different tumor types do not release the same amount of ctDNA, and, even in patients with the same disease, the concentration of ctDNA may vary consistently. In fact, Bettegowda et al. showed that most disease patients with metastatic cancers of the pancreas, bladder, colon, stomach, breast, liver, esophagus, and head and neck, as well as patients with neuroblastoma and melanoma, harbored detectable levels of ctDNA. In contrast, less than 50% of patients with metastatic cancers of the kidney, prostate, or thyroid harbored detectable ctDNA [36].
Many different pre-analytical aspects can lead to interlaboratory variability when performing liquid biopsy. These variables include i) the type of blood collection tubes used, ii) the storage conditions of the blood sample, iii) the time between blood collection and sample processing, iv) the blood processing protocol used, v) the extraction method used, vi) and the quality assessment method used. The impact of each of these variables depends on the liquid biopsy application [37,38,39].
Since blood is the most used source for ctDNA, plasma represent the matrix preferred in the majority of clinical trials and EDTA containing tubes are used for blood collection [37, 38, 40]. Using these tubes clotting is inhibited, and thus it is possible to recover plasma that represent the matrix of choice for ctDNA extraction. Actually, also serum can be used as a matrix to isolate ctDNA; indeed, it has been reported that the amount of ctDNA in serum can be 2–24 times higher than in plasma. This can be a consequence of the clotting process that causes white blood cells (WBCs) breaking, finally leading to the release of wild-type DNA. This contamination causes a further dilution of the tumor-specific DNA, making it even more difficult to detect.
Another important pre-analytical aspect is the time that elapses between the withdrawal and its processing for plasma recovery. Indeed, the more time passes, the more is the risk of WBCs lysis, leading again to ctDNA contamination with wild-type background DNA.
To prevent this increase in genomic DNA, blood samples stored in EDTA tubes that will be analyzed for circulating tumor DNA need to be processed within 6 h after the blood draw [41]. To overcome the inconvenience caused by this time restriction, there is a growing list of stabilizing reagents and dedicated blood collection tubes designed to preserve cell-free DNA profiles in whole blood [42]. These tubes prevent cell lysis, limiting contamination of the sample with genomic DNA. Blood samples for circulating tumor DNA analysis stored in specialized tubes can be kept at room temperature for a number of days before processing is needed [43].
While researchers have made progress in understanding how the type of tube used and storage conditions impact circulating tumor DNA analysis, no consensus on best practices has yet been reached [28]. There are also many other pre-analytical variables whose impacts on ctDNA analysis are unknown. Information on how these variables impact other applications for liquid biopsy, such as exosome analysis, remains unclear [24].
Another aspect to be considered is the high turnover ctDNA (15 min half-life), therefore some authors suggested to proceed with plasma preparation by centrifugation within 1 h after blood collection [40, 44].
Concerning sample processing, the complete removal of any cellular component is essential. For this goal, the best option is a two-step centrifugation at 1600 g for 10 min for plasma isolation [45]. According to this recommendation, Herrera et al. reported less concentration of cfDNA in plasma samples that were centrifuged twice compared with samples that were centrifuged only once (13 µg/l vs. 819 µg/l), revealing that cfDNA concentrations were contaminated with genomic DNA [46]. These observations confirm that the second centrifugation step is crucial for ctDNA analysis. Finally, it is well known that ctDNA integrity is better conserved as cfDNA extracts compared to plasma when samples are stored at -80 °C and avoiding freeze–thaw cycles [38].
As regard methods for ctDNA isolation, Sorber L et al. [47] have compared the efficiency of the most used kit, the QIAamp circulating nucleic acid kit (QIA), with four other cfDNA isolation kits: the PME free-circulating DNA Extraction Kit (PME), the Maxwell RSC ccfDNA Plasma Kit (RSC), the EpiQuick Circulating Cell-Free DNA Isolation Kit (EQ), and two consecutive versions of the NEXTprep-Mag cfDNA Isolation Kit (NpMV1/2). In the study, the detection of KRAS mutation and total cell-free DNA concentration were performed with droplet digital PCR, whereas real-time PCR was used to evaluate cfDNA integrity. They showed that QIA and the RSC kits displayed similar isolation efficiencies, whereas the yield generated by the PME and NpMV2 kits was significantly lower [47].ctDNA investigation can be achieved through two different analytical approaches: a targeted approach and an untargeted approach. The targeted approach relies on the possibility to analyze known genetic mutations that occur in hotspot region of specific genes with implications for therapy decisions. Among these methods, we can include real-time PCR, droplet digital PCR (ddPCR) and targeted next-generation sequencing (NGS).
In the untargeted approach, it is possible to investigate ctDNA without the knowledge of any specific mutations present in the primary tumor. This can be achieved through whole genome sequencing using NGS platforms. Nevertheless, this analysis is quite expensive and sometimes difficult to interpret; thus, it can be used for biomarkers discovery in the context of disease monitoring, detection of molecular resistance, and identification of new therapeutic targets. Despite whole genome sequencing, a more cost-effective method in the exome sequencing, which does not require prior knowledge of the genetic landscape of the tumor.
The main targeted approaches are real-time PCR, ddPCR and targeted NGS [48]. Real-time PCR represents the oldest technique and the power of this technique in detecting mutant allele at a very low frequency (< 1%) is limited, and therefore other more sophisticated methods have been developed. In ddPCR, the partitioning is obtained through an emulsion PCR, each generated droplets ideally represent a PCR reactor. At the end of the analysis, software allows to identify a positive or a negative signal indicating the presence or absence of a target sequence. Therefore, mutated ctDNA can be detected in a wide background of wild-type sequences. The ddPCR platforms now available are various, each of them with a more or less different workflow, but they all share a very high sensitivity (0.01%) [49].
NGS has revolutionized our approach to molecular testing, indeed we can analyze multiple genes and multiple patients at a time with a consistent reduction in time and money. Of great interest, there is the paper of Newman et al. that has developed cancer personalized profiling by deep sequencing (CAPP-Seq) (10.1038/nm.3519). CAPP-Seq method is able to detect ctDNA in 100% of patients with stage II–IV non–small-cell lung carcinoma and in 50% of patients with stage I. The diagnostic specificity was 96% for mutant allele fractions down to approximately 0.02% [50].
Several international organizations are working toward developing standards for liquid biopsy workflows. These organizations are either working directly to build these standards or are developing the infrastructure needed for data sharing across stakeholders to reach a consensus.
SPIDIA4P (https://www.spidia.eu/) is a continuation of SPIDIA, which tackled the standardization and improvement of pre-analytical procedures for in vitro diagnostics. The next phase of the initiative involves working to improve the global health care system by developing selected high-priority pre-analytical European Committee for Standardization (CEN) and International Organization for Standardization (ISO) standard documents. They are also looking to develop corresponding External Quality Assessment (EQA) schemes and implementation tools.
CANCER-ID (https://www.cancer-id.eu/) is a European consortium that is working to establish standard protocols for blood-based biomarkers. They are also working to clinically validate such biomarkers. This consortium is funded by the Innovative Medicines Initiative and is composed of 36 partners from 13 countries.
BloodPAC (https://www.bloodpac.org/) is an American initiative to accelerate the development, validation, and clinical use of liquid biopsy assays in order to better inform medical decisions so that patient outcomes can be improved. They have developed a collaborative infrastructure that allows for information sharing between stakeholders in the public, industry, academia, and regulatory agencies. They hope that information sharing, and evidence generation will help bring liquid biopsy into routine clinical practice.
An important step in the delivery of precision oncology to patients with lung cancer is the interpretation and reporting of variants in the clinical context [51]. Certain minimum requirements are needed for the reporting of molecular profiling results for all CAP-accredited laboratories [52]. These requirements cover assay methodology, basic clinical performance characteristics including clinical and analytical sensitivity and specificity, assay results, and interpretation. Recently, the ESMO Precision Medicine Working Group published recommendations (Table 2) on the use of circulating tumour DNA for patients with cancer [53].
All LB reports should contain date of sample acquisition, type of tubes used, timing of plasma separation, method and timing of ctDNA extraction. Moreover, treatment exposure (on/off treatment) at time of acquisition should be reflected.
Cases where gene variants are not detected must be reported as ‘non-informative’ or ‘not detected’, instead of ‘negative’. Indeed, ctDNA assays have an appreciable rate of discordance with tumour testing. Cases where a mutation is not detected may be interpreted as the variant not being present in the tumour, when in actuality, there was insufficient ctDNA in the specimen. Report communicates the potential for discordance in such cases.
Variant allele fractions (VAF) may provide information suggestive of possible germline origin, clonal relatedness of variants in the same panel and the potential for a false-positive result. ctDNA samples with low VAF variants can be the most challenging aspect of reliably reporting ctDNA results [54, 55]. Indeed, with the use of highly sensitive NGS approaches (LOD ∼0.5% or lower), somatic mutations within nonmalignant hematopoietic cells, known as clonal hematopoiesis, might represent a source of “biological noise” in cell-free DNA analyses.
Moreover, in patients with low disease burden or with bone or brain metastasis, circulating free DNA (cfDNA) quantities may be low. Moreover, some specific mutations can be under-representative of their frequency in tumors such as KRAS G12 [56]. It is unknown whether variants at low allele fractions are as responsive to targeted therapy as those at high allele fractions. Some studies indicated that low VAF oncogenic drivers respond to targeted therapy, which serves to emphasize the need for highly sensitive tests [57].
Variants in genes commonly implicated in clonal hematopoiesis of indeterminate potential (CHIP) should be flagged to caution the clinician about the potential non-tumour origin of these variants [58]. Clonal haematopoiesis is a common challenge for assays that include genes implicated in clonal haematopoiesis. Variant identified in ctDNA assay is assumed to be present in the tumour but is actually derived from leukocytes. Report should communicate the potential non-tumour origin of variants in genes commonly implicated in CHIP.
Targeted variant or regions examined by assay should be reported. This could range from a single variant for digital PCR assays (e.g. EGFR, c.2369C > T, p.T790M) to hundreds of genes for an expanded NGS-based panel. Assays are validated to detect and report specific types of variants (e.g., SNVs, small insertions/deletions, amplifications/copy number losses, gene fusions). Report should communicate which variant types are reported.
The limit of detection for each variant type should be determined and reported, ideally with an associated confidence interval. Some variant types are more difficult to detect with ctDNA assays. Report should communicate individual performance of different variant types. In cases where input plasma DNA is limiting, the reported sensitivity is adjusted, or a warning is inserted in the report.
Specific tumor variants identified should be classified as ‘actionable’ or “not”. Benign lesions can contain oncogenic variants. Identification of an oncogenic variant in ctDNA assays is not diagnostic of malignancy. As an example, BRAF V600E variant has been identified in plasma DNA from individuals with benign nevi [59]. Interpretation of ctDNA assays should be done in the context of tissue studies and other clinical information. To support classification, the Association for Molecular Pathology (AMP), American Society of Clinical Oncology (ASCO), and College of American Pathologists (CAP) jointly published a four-tiered system classification system for the interpretation and reporting of sequence variants in cancer [60]. The European Society for Medical Oncology (ESMO) also recommends the ESMO Scale for Clinical Actionability of Molecular Targets (ESCAT) variant classification guidelines, with subtle differences from the AMP/ASCO/CAP Guidelines [61].
Role of liquid biopsy in heredo-familiar tumors
The essential component of cancer risk assessment is the preventive oncology trough screening and early diagnosis [62]. About 5–10% of cancers have a hereditary component where specific and heritable pathogenic variants are clearly implicated in the genesis of the disease. Over 300 hereditary cancer susceptibility syndromes are reported [63], involving both families and individuals tested for mutation carriers [64].
Cancer predisposition-related genes may be classified into 3 groups based on penetrance: high (lifetime cancer risk: 50% or greater), moderate (lifetime cancer risk: 20% to 50% or a 2–fourfold increase above the general population risk), and low or unknown risk.
Currently, testing options for the identification of germinal mutation include single-gene testing and/or cancer panels. There are also two major categories of NGS cancer panels: cancer-site-specific panel testing and pan-cancer panel testing [63]. There are some screening methods proved to be useful for cancer prevention in high-risk phenotypes [65], as for breast, ovarian, pancreatic and colorectal cancer. However, limitations are based on low sensitivity and specificity and normally applicable to a single cancer type [62]. Despite the consolidated and progressive introduction of the genomic profiling in our daily practice in oncology by NGS and the advent of personalized oncology [63], minimally invasive approaches for the early diagnosis and the monitoring and prediction of the therapeutic response in cancer patients [66], are under intensive investigation, also in light of the intra and inter-tumor heterogeneity accompanied by dynamic biological changes and the sub-clonal genome architecture occurring over the time, which represent the most significant diagnostic challenge in the cancer field with unavoidable implication in clinic.
As a suggestion of possible germline origin, in a series of 1000 consecutive patients who underwent tissue NGS, 2.3% of patients were discovered to be carriers of a previously unrecognized germline mutation [67]. Although somatic and germline variants should be readily distinguished based on VAF, in a small subset of patients with high ctDNA burden this may not be possible and patients should be informed of the possibility that high-risk germline variants may be incidentally detected in a liquid biopsy. The informed consent should clarify whether the patient wants to be informed about these incidental findings. Reporting of potential germline variants should generally follow ESMO recommendations for germline-focused analysis of tumour-only sequencing [68]. Patients identified with a previously unrecognized germline mutation should be promptly referred for genetic counselling [52].
Specific features of hereditary cancer syndromes are related to higher frequency of classical genetic disorders, early clinically onset, and very likely potential risks to develop additional neoplasms.
Besides, a pool of genes with a certain degree of penetrance rather than a single genomic alteration, often influences the evolution of the disease. In this context, the investigation and the diagnostic validation of liquid biopsy likely finds its best application, as patients with inherited syndromes undoubtedly implies a narrower clinical surveillance [69].
For instance, the Lynch syndrome (LS, also known as hereditary non-polyposis colorectal cancer syndrome, HNPCC), which is inherited in an autosomal dominant pattern and accounting of the 3–5% of colorectal cancers, is caused by genomic mutations of the mismatch repair system (MMR), whose detection is a key step to screen this set of patients and possibly to combine the immunotherapy regimen.
Coherence of MMR phenotype between tumor tissue and cell free DNA (cfDNA) obtained through liquid biopsy, has been reported in subjects with LS [66]. To date, cfDNA obtained from liquid biopsies is suitable for detecting MMR mutations, microsatellite instability (MSI) and MLH1 promoter methylation status, and universal CRC markers.
There are also other biomarkers proposed for the LS screening, as blood sampling is not the only form of liquid biopsy providing ctDNA. Mutations in the telomerase reverse transcriptase (TERT) promoter and the fibroblast growth factor receptor 3 (FGFR3) are often found in LS. These alterations have been proposed as novel biomarkers of urothelial cancer (UC), the third most common cancer type in certain subsets of LS families and they are ideal candidates to be studied from ctDNA extracted from urine liquid biopsy. Bile is another source of ctDNA, as almost 4% of LS patients develop bile duct cancer [66].
Similarly, cell free DNA, found in patients with pancreatic cancer, has been demonstrated to possess a diagnostic/predictive significance: cfDNA is present at diagnosis in almost 50% of these patients with localized disease and that circulating tumor DNA may anticipate of 6.5 months potential recurrences [70]. This aspect is significant as almost 20% of prostate cancer cases show a familial origin history [71]. Other reports have shown that the detection rate of circulating DNA in pancreatic cancer, depends on the technique employed. When genomic alterations of a specific gene is sought (i.e. KRAS), a clear discrepancy between tissue and liquid biopsy is found [72], therefore suggesting that liquid biopsy requires the suitable technique in order to strengthen its diagnostic potential.
However, not only free DNA is currently investigated for inherited syndromes. Coherently, the novel concept of “circulome”, which entails miRNAs, mRNA, RNA, exosomes, extracellular vesicles (EV) and metabolites, has becoming a novel diagnostic strategy [73, 74]. The circulome can be considered the novel frontier of the liquid biopsy. The detection based more on a defined pool of molecules of cancer origin rather than relying on a single biomarker, is useful to design a more precise molecular scenario exhibited by the patient. For instance, the combination of the pathogenic variants of BRCA1/2 and high levels of two circulating proteins SPARC (Secreted protein acidic and rich in cysteine) and THBS1 (Thrombospondin 1), can be combined to distinguish women with ovarian cancer from those healthy and with wild type BRCA1/2 variants [75].
Thus, genomic and protein alterations are better integrated, allowing to reveal new insights on the heterogeneous facets of cancer. Bioinformatic algorithms and array analysis have been recently applied to the circulome, simplifying the predictive significance in hereditary cancers and overcoming the limitations of the small amount of soluble molecules and biomarkers often difficult to detect [72].
Circulating mRNA and miRNAs related to MMR can also be employed for the same purpose with an enhanced sensitivity and useful to stratify patients [66], therefore discriminating between patients with sporadic alterations of the MMR from those with LS. Notably, researchers are exploring differentially expressed miRNAs, which are more stable in the body fluids [76,77,78], but also their methylation status for follow ups or correlation to chemoresistance, therefore expanding the field of applicability in genetic-associated cancer disorders.
The epigenetic change such as methylation of circulating free tumor DNA, miRNAs or proteins is considered a key mechanism involved in the early tumorigenesis, therefore a useful screening and predictive tool [79]. The Circulating Cell-Free Genome Atlas Study (CCGA) based on the deep sequencing of methylation of circulating cell-free nucleic acids (cfNAs) is currently under attention for its potential to discriminate cancer vs non cancer (NCT02889978) [80].
Accordingly, the combined methylation analysis of both A disintegrin and metallopeptidase with ADAMTS1 (thrombospondin type 1 motif 1) reflects high sensitivity for cancer pancreatic diagnosis, increasing even more at higher stages of the tumor [81].
Moreover, EV have been studied in pancreatic cancer at early stages, by investigating the cargo of miRNAs, proteins and specific molecules such as the proteoglycan GPC1 (Glypican-1) found in serum of patients and revealed as a marker with high sensitivity of detection [82]. Despite this, we are still far from using EV as diagnostic/prognostic platform, given a wide range of biological variability among studies and technique employed [72].
Additional biological sources might implement the early detection of pancreatic cancer as demonstrated for driver genomic mutations of KRAS (G12V and G12D) found in pancreatic juice before malignancy is proven [83]. Notably, combining the detection of multiple genomic mutations with the size of mutated DNA fragments in the liquid biopsy and the stage of cancer, has been found useful to discriminate patients from healthy subjects.
However, several techniques are attempting to ameliorate the amplification, the mutational analysis or the methylation status of the small amount of free DNA in the blood. These are not limited to NGS-based systems but may include digital droplet PCR, and the inter-Alu-PCR or even nano-magnetic platforms [84] to enhance the sensitivity and reduce false negative samples. In addition, the detection of the mitochondrial DNA mutations in liquid biopsy seems to be a promising biomarker for the diagnosis of early colorectal cancer risk [85].
Sequencing-based technology combined with liquid biopsy (specifically with cell free DNA) such as the PapGene test, has been currently set up for screening of subjects with inherited predisposition to gynaecological cancers, LS and germline mutations in BRCA1, 2 or MMR system [86, 87], demonstrating that the diagnostic significance of the liquid biopsy can be strengthen by associating high throughput molecular platforms. Some clinical trials regarding liquid biopsy-based approaches in LS and breast cancer (detection of BRCA1 both in blood or circulating tumor cells of women with mutated TP53 mutation detection), are already completed (NCT02198092 and NCT02608346, respectively).
Other example of non-yet FDA approved combination of liquid biopsy with NGS is the Guardant360 (Guardant Health) and FoundationOne Liquid (Foundation Medicine), considered as companion diagnostic tests employed for prostate, breast, and ovarian cancers. There is evidence that the matching of NGS and liquid biopsy could help to improve the stratification of patients, attempting to understand who can really benefit from the targeted therapy expecially in advanced cancers, as demonstrated in metastatic breast cancer [88].
Liquid biopsy can also provide indications regarding potential actionable targets identified within multiple gene-based panels besides the canonical genomic mutations. For instance, alterations in ERS1 (Estrogen Receptor 1) gene, which is associated to oestrogen resistance, has been found in circulating tumor DNA of a cohort of patients with breast cancer [89]. Women with advanced hormone-receptor-positive and HER2 negative breast cancer eligible for therapy with alpelisib (active in patients with PIK3CA mutations), exhibit in the circulome (specifically in cDNA, EV and circulating tumor cells) PIK3CA mutations, mirroring the genomic alterations found in the corresponding cancer tissue [90].
A key question is how liquid biopsy can change the landscape of the therapy.
Role of liquid biopsy in minimal residual disease
Despite initial success of radical treatment of early-stage tumors, a substantial number of patients develops virtually incurable distant metastases during a variable period of time. Minimal Residual Disease, namely the presence of disseminated cells in the organism without clinical or radiological signs of disease, determines this fait accompli [91]. Neoadjuvant and adjuvant treatments have shown to improve long-term outcomes and are thus the standard of care for many tumors. However, those therapies are administered to every patient statistically considered to be at reasonable risk for distant recurrence in absence of tangible prove of cancer dissemination, thus most treated patients are exposed to toxicities without any benefit. The assessment of MRD by random sampling of organs trough tissue biopsy for all patients would obviously be unfeasible.
In this scenario, liquid biopsy is nowadays the most promising tool being implemented to unveil MRD, trough detection of shed circulating tumor products, like cells (CTCs) [92], DNA (ctDNA) [93] or RNA (ctRNA) [94]. Baseline and longitudinal repeated sampling of blood from radically resected patients could enable the detection of impending disease ahead of clinical and radiological methods and could be used to better define the real risk of relapse, helping the clinicians decide whether to start a treatment. Furthermore, the molecular characterization of circulating tumor material could be used to better define appropriate treatment. The relapse, especially for breast cancer, can happen years later from the dissection of primary tumor. However, tumors are made of cells bearing distinct molecular signatures. This inevitable heterogeneity is the result of the forces that initiate and promote normal cell transformation and represents the key feature that determines treatments failure [95]. Despite solid biopsy being feasible most of the time, they are invasive procedures and hardly repeatable in everyday clinical setting. Being a non-invasive and easily repeated tool, liquid biopsy is destined to help us keep pace with tumor evolution.
Nowadays the use of liquid biopsy to assess MRD has yet to enter in clinical practice (Table 3), but many studies have proven its ability to better define the prognosis of radically operated patients in a large number of solid tumors.
Prognostic and systemic treatment need definition
One of the major challenges in oncology is defining the population of radically resected patients that cannot be cured by surgery alone and that needs the administration of systemic therapy to eradicate the chances of relapse. A large and growing body of literature (Tables 4 and 5), has highlighted the grim prognostic value of MRD identified by liquid biopsy in patients that underwent surgery, pointing out a clearly positive correlation between the presence of residual tumor cells and the risk of relapse and death. Furthermore, clinical trials have initiated considering liquid biopsy as a tool to decide whether to start an adjuvant treatment, introducing a possible paradigm shift in everyday clinical practice.
Tie et al. assessed the role of ctDNA in defining stage II CRC prognosis and real need for adjuvant therapy [142]. Patients were randomly assigned to have treatment decisions guided by either ctDNA results or standard clinicopathological features. The results showed how ctDNA-guided decision for adjuvant treatment led to lower therapy administration (15% vs. 28% in the control group) without statistically significant differences in the 2-year RFS (93.5% and 92.4% in the control group).
Powles et al. evaluated ctDNA levels in patients enrolled in the IMvigor010 trial, that randomized patients to receive atezolizumab or observation after surgical resection for operable urothelial cancer [149]. The study did not show significant advantage in the active arm neither in DSF nor in OS [150]. However, when stratifying the patients based on the presence of ctDNA, improved disease-free survival and overall survival in the atezolizumab arm versus the observation arm was observed for ctDNA patients positive. For ctDNA negative patients, there was again no meaningful difference between arms.
These pioneering trials show that a liquid-biopsy-enhanced stratification of patients is possible and is likely to better select patients for active versus observational approaches. An increasing number of trials is ongoing to further develop this fundamental clinical question (NCT05411809; NCT04259944; NCT03748680; NCT04089631).
It is therefore possible that, in the future, adjuvant therapy will be escalated for ctDNA positive patients and standard or not administered at all for ctDNA negative patients. To further define the need for escalation of treatments in ctDNA positive patients, in the IDEA trial the presence of postoperative ctDNA was tested as a prognostic and predictive marker for prolonged adjuvant treatment duration [137]. ctDNA was confirmed as an independent prognostic marker and treatment for 6 months was superior to 3 months in both ctDNA negative and ctDNA positive patients. ctDNA positive patients treated 6 months had a similar prognosis to ctDNA negative patients treated 3 months. Trials with escalated treatment in ctDNA positive versus standard treatment in ctDNA negative resected patients are ongoing (NCT05062889; NCT04803539; NCT05427669).
Recurrence monitoring
Follow-up of radically resected patients is an integrated part of clinical oncology routine but evidence regarding the effectiveness of the different follow-up strategies varies substantially. The identification of relapse as soon as it presents, even in the absence of overt metastases, could maximize the changes of cure or at least delay complications related to the tumoral mass presence. Blood withdrawal is a guideline-included procedure for many tumors, especially those for which an oncological marker is recognized, thus the introduction of liquid biopsy would not pose a problem for patients. Despite few information is available regarding the prognostic relevance of liquid biopsy analyses focused on the surveillance of MRD through follow-up care studies, findings indicate that the detection of CTCs and ctDNA can provide evidence of metastatic relapse earlier than standard procedures.
To address this clinical question, Reinert et al. longitudinally analyzed ctDNA in a cohort of 125 stage I, II and III colon cancer [135]. Data showed that ctDNA-positive patients at postoperative day 30 had a higher recurrence rate compared with those who were ctDNA negative after surgery. Similarly, ctDNA positivity in patients treated with adjuvant chemotherapy was associated with a high risk of recurrence. Moreover, serial ctDNA analysis during surveillance after definitive treatment identified relapse with 88% sensitivity and 98% specificity. Interesting, ctDNA analyses revealed disease relapse up to 16.5 months ahead of standard-of-care computed tomography. These results clearly suggest that clinical applications of ctDNA in CRC could improve risk stratification, adjuvant chemotherapy monitoring and early relapse detection.
Similarly, Tarazona et al. performed a longitudinal evaluation of plasma ctDNA in 94 early CRC patients before and after the surgery [136]. Data showed that ctDNA presence, after surgery and during follow-up, were correlated with worse disease-free survival. In addition, ctDNA detection in patients after adjuvant chemotherapy was associated with early relapse. Detection of ctDNA had a median of 11.5-months lead time over radiological relapse suggesting the utility of ctDNA in identifying MRD and patients at high risk of disease recurrence.
The IMPROVE-IT2 (NCT04084249) is an ongoing trial that compare post-operative surveillance by ctDNA analysis or standard-of-care CT-scan in radically resected CRC patients [151]. The hypothesis is that combining ctDNA analysis and radiological assessments could improve the early detection of recurrent disease optimizing the postoperative treatment.
Liquid biopsy as a measure of response
Response to adjuvant therapy is impossible to assess with normal clinical and radiological exams, being the aim of the treatment to cure invisible MRD. Therefore, adjuvant treatment is administered, when possible, at its higher intensity, without the possibility to monitor the real effectiveness of the ongoing therapy. For patients that will eventually relapse, this means being exposed to toxicities that are sometimes fatal without any benefit. Furthermore, adjuvant regimens are always interrupted after a defined number of cycles, without real clue of the disease state at that point. All these limitations could be surpassed by MRD monitoring through liquid biopsy during and after treatment. We have already shown how monitoring ctDNA after adjuvant treatment can identify patients that convert to a negative status and are therefore at less risk of relapse from those that remain positive and have thus a worse prognosis.
Key findings come also from Henriksen et al., that investigated post-adjuvant chemotherapy ctDNA status in stage III colon cancer patients [141]. In particular, ctDNA presence was associated with disease recurrence postoperatively also in patients treated with adjuvant chemotherapy. Only patients who showed permanent clearance of ctDNA after adjuvant therapy did not relapse. Serial ctDNA analysis after the end of treatment was also predictive of disease recurrence suggesting that ctDNA assessment has a strong prognostic value.
For those patients in which ctDNA levels do not lower during and/or after treatment, if clinically feasible, one of those 3 options should be considered, given the proven grim association within ctDNA presence and relapse: switch of the treatment to another regimen, its prolongation or intensification, when possible, with addition of biomarker-based therapy in those patients with an actionable alteration.
The concept of a “second line adjuvant treatment” represents an absolute paradigm shift from today’s clinical practice. This approach, aimed to cure and not to palliate, presents obvious advantage for the patients, as the toxicities from therapies could be better tolerated without the burden of the metastatic disease. Furthermore, tumors are less resistant to therapies when the cells are isolated and scattered. Two trials (NCT04567420; NCT04985266) are currently investigating a second line adjuvant treatment for high-risk resected breast cancer patients currently undergoing hormonal treatment. Primary objective of the therapeutic randomized phase is to assess whether palbociclib plus fulvestrant improves relapse-free survival compared to standard of care adjuvant endocrine therapy in patients with detectable ctDNA in the plasma but without evidence of metastatic disease on imaging. Another trial (NCT05343013) is defining if TAS-102 treatment in resected colon cancer patients with positive ctDNA after completion of adjuvant chemotherapy treatment can determine a 6-month ctDNA clearance. In NCT04920032 trial, the percent of patients positive for ctDNA after 6 cycles or at least 3 months after starting second line adjuvant treatment will be used to estimate the efficacy of adjuvant trifluridine and TAS-102 in combination with irinotecan in patients with ctDNA positive colon adenocarcinoma after first line standard adjuvant treatment. The NCT05062889 trial aims to evaluate two different aspects in colon cancer resected patients: the escalation treatment for ctDNA positive patients (FOLFOXIRI vs FOLFOX/CAPOX in ctDNA negative) and the ctDNA clearance induced by TAS-102 in ctDNA positive patients after first line adjuvant therapy.
Patients’ treatment selection based on molecular alterations
Liquid biopsy-guided treatment based on molecular alterations is already consolidated clinical practice, especially for breast and lung cancers, in the metastatic settings [152, 153]. Several tests are already utilized and approved [153]. Guardant360 CDx test was FDA approved as a companion diagnostic for patients with EGFR-mutant NSCLC, with EGFR exon 20 insertion NSCLC and with KRAS G12C mutations NSCLC who may benefit from treatment with Osimertinib, Amivantamab and Sotorasib, respectively. Foundation Medicine’s FoundationOne Liquid CDx is approved as a companion diagnostic for the poly (ADP ribose) polymerase inhibitor rucaparib for the treatment of advanced metastatic prostate cancer and ovarian cancer with BRCA1/2 mutations, as a companion diagnostic to identify patients with BRCA1/2 mutations and/or ATM alterations in metastatic colorectal cancer for whom treatment with olaparib may be appropriate, to identify ALK rearrangements in patients with NSCLC eligible for treatment with alectinib as well as three tyrosine kinase inhibitors, including gefitinib, osimertinib, and erlotinib, approved for the first-line treatment of EGFR-mutant NSCLC, to assess TMB and MSI status in NSCLC and to identify mutations in the PIK3CA gene in patients with breast cancer eligible for treatment with alpelisib.
However, the introduction of blood molecular testing in the early setting is still in development and only few small trials are currently investing its role. One of such trials (NCT05079022) aims to assess the role of Furmonertinib, a third generation anti-EGFR, in EGFR-mutated radically resected stage I lung cancers, with the mutation being detected trough ctDNA analysis. The primary end point is the clearance of ctDNA at 6 months. Another study (NCT05388149) plans to escalate therapy in Her2-positive, radically resected with residual invasive disease following prior neoadjuvant trastuzumab (± pertuzumab)-based chemotherapy, breast cancer patients with the addition of Neratinib to TDM-1, if ctDNA is detected in plasma. The primary endpoint is again the clearance of ctDNA. As shown, clearance of ctDNA demonstrated to increase survival in radically resected patients after adjuvant treatment, but it’s validity as a surrogate endpoint for overall survival has still to be proven.
As tissue-based analysis for detection of molecular disease have already entered the clinical practice, for example for guiding anti-EGFR adjuvant treatment in NSCLC or anti-BRCA adjuvant treatment in breast cancer, the possibility of tracking the emergence of resistance mutations to a given treatment by liquid biopsy is becoming more and more appealing.
Role of Liquid biopsy in agnostic indications
Recently, some drugs have been approved regardless of the primary tumour type, but solely on the basis of fundamental molecular abnormalities driving the processes of carcinogenesis and disease progression. This innovative approach of precision medicine led to the first agnostic approvals of oncology drugs [154] (Tables 6 and 7).
In the last years, scientific research has focused on identifying biomarkers predictive of response to immunotherapy. The deficiency of DNA mismatch repair (dMMR) and MSI were among the first biomarkers used as expressing tumour mutability. Based on the results of five independent clinical trials (Keynote-016, Keynote-164, Keynote-012, Keynote-028, and Keynote-158), pembrolizumab received its first FDA approval for the treatment of adult and paediatric patients with unresectable or metastatic solid tumours, MSI-High (MSI-H) or dMMR, progressing after standard treatments and lacking other treatment options [167, 168].
Furthermore, in 2020 the FDA expanded the approval of pembrolizumab to include unresectable or metastatic tumors with high tumor mutational burden that have progressed following prior treatment and that have no satisfactory alternative therapy options. The FDA also approved the FoundationOneCDx assay as a companion diagnostic test for pembrolizumab [169].
The neurotrophic receptor tyrosine kinase (NTRK) genes, including NTRK1, NTRK2 and NTRK3, are key regulators of neuronal and embryonic development. NTRK rearrangements were shown to be able to drive oncogenesis, independently of histology [170, 171]. Indeed, NTRK fusions were detected in several type of solid tumors, such us, lung, breast, pancreatic, colon and thyroid [172]. On the basis of a combined analysis of three clinical trials, NCT02122913, NCT02637687 and NCT02576431, which included cancer patients with fusion in one of the three known NTRK genes, larotrectinib was the first FDA-approved molecule in November 2018 for adult and paediatric patients with NTRK fusions solid tumours [173]. The second TRK and ROS1 inhibitor molecule was Entrectinib, approved in August 2019, as an additional therapeutic option for NTRK fusion-positive tumours [174, 175].
BRAF is a gene encoding for a member of the Raf family, which plays a central role in many cell proliferation and differentiation processes through the MAP kinase (MAPK) pathway [176].
Mutated BRAF gene may be a key oncogenic driver in promoting carcinogenesis and tumour progression [177].
The Cancer Genome Atlas (TCGA) has identified BRAF mutations in many tumour types, especially melanomas, thyroid cancers, lung cancers. However, this mutation could also occurs in rare histological tumour types [178], such as diffuse gliomas, cholangiocarcinoma, hairy cell leukaemia, multiple myeloma and Langerhans cell histiocytosis [179].
In August 2022, the FDA approved the combination of dabrafenib (Tafinlar) and trametinib (Mekinist) for adult and paediatric patients (6 years of age or older) with unresectable or metastatic BRAF V600E-mutant solid tumours that have progressed after previous treatment and in the absence of other satisfactory treatment options.
This approval stems from efficacy and safety results obtained in recent studies including several solid tumours: ROAR (NCT02034110), NCI-MATCH (NCT02465060), and the CTMT212X2101 study (NCT02124772) in 36 paediatric patients.
The ROAR study included patients with high-grade glioma, biliary tract cancer, low-grade glioma, small bowel adenocarcinoma, gastrointestinal stromal tumour and anaplastic thyroid cancer. The NCI-MATCH trial included patients with BRAF V600E-positive solid tumours (excluding melanoma, thyroid carcinoma and colorectal carcinoma), while the paediatric trial included patients with refractory or recurrent low or high grade glioma. Overall, the objective response rate (ORR) was 41% among the 131 adult patients (95% CI, 33%-50%) [180,181,182,183].
The determination of tumor genomic profile requires analysis of tumour DNA by tissue biopsy. However, tumour biopsies, to date considered the gold standard in molecular tumour characterisation, have some important limitations. Liquid biopsy, on the other hand, is a non-invasive and easily repeatable diagnostic technique that can capture genomic heterogeneity within the patient and during therapy and represents a promising and innovative approach that could greatly facilitate access to agnostic therapies for more patients [1].
Although clinical biopsy overcomes some of the many limitations of standard tissue biopsy, it struggles to officially enter standard clinical practice. To date, liquid biopsy, using qPCR, has been approved by FDA and EMA for the detection of EGFR mutations in non-small cell lung cancer (NSCLC) and Kras mutations in colorectal cancer (CRC) [184,185,186]. Furthermore, liquid biopsy is recommended in the determination of resistance mechanisms in advanced NSCLC, in particular the T790M resistance mutation [187, 188].
Liquid biopsy has also shown promise in the agnostic indication of therapy, although still not officially approved and recommended by clinical practice guidelines compared to standard tissue biopsy.
Recently, the predictive value of TMB assessed on liquid biopsy (bTMB) was investigated in 2 different prospective studies. Both these studies showed that high TMB assessed on peripheral blood in patients with advanced NSCLC correlated with better outcomes during immunotherapy [155, 156]; in particular the phase 2 B-FIRST trial reported a greater overall response rate and a trend toward better Progression Free Survival (PFS) and Overall Survival (OS) in patients with high bTMB treated with atezolizumab.
However, the technique for determining TMB on peripheral blood is not yet standardised and therefore not officially recommended in clinical practice.
Tissue biopsy also remains the gold standard in the determination of MSI/dMMR, assessed by immunohistochemistry or molecular assays. However, liquid biopsy could also overcome important limitations in this field, especially intratumour heterogeneity, within the single disease site or between different disease sites (primary tumour and metastases) [189]. Indeed, the use of liquid biopsy could allow a rapid expansion of treatment options in patients with various solid tumours. A high degree of concordance between tissue-based MSI determination and MSI determination based on circulating tumour DNA has been reported in the literature [190, 191]. NGS is capable of analysing microsatellites at thousands of loci simultaneously and, at the same time, can assess the mutational profiling in targeted regions. It has been shown to determine both MSI and TMB status, achieving excellent sensitivity [192]. Among the NGS-based approaches, the Guardant360® CDx (Guardant Health, Redwood city, CA, USA) and the liquid CDx FoundationOne® (Foundation Medicine, Cambridge, MA, USA). Medicine, Cambridge, MA, USA) are FDA-approved blood-based diagnostic tests and are considered suitable for the determination of MSI on peripheral blood samples [157]. It has been shown that the Guardant360® CDx has an overall accuracy of 98.4% and a higher concordance between MSI on cell free DNA (cfDNA), tissue PCR and NGS than immunohistochemistry [158].
For the determination of NTRK rearrangements various tissue analysis techniques have been employed over the years, including NGS, immunochemestry and fluorescent in situ hybridization (FISH) [193].
The possibility of using liquid biopsy in the evaluation of NTRK fusions could ensure fast access to specific drugs for many patients, even in the case of insufficient or inadequate tumour tissue. Some plasma-based NGS have demonstrated in the literature a high degree of concordance with tissue genomic tests, although, actually, the potential of liquid biopsy in identifying NTRK fusions is largely unknown [159, 194].
Recently, a retrospective study reviewed ctDNA analysis data obtained with the Guardant360 cfDNA assay in patients with advanced solid tumours. The study showed that the presence of NTRK1 fusions in ctDNA was confirmed on tissue analysis in 88% of cases [160]. In view of the accessibility of two specific drugs for this molecular target, the potential of liquid biopsy should be explored in the detection of NTRK rearrangements to improve the identification of patients who may benefit from NTRK-specific treatments.
In light of the recent approval of Dabrafenib-Trametinib therapy in BRAF mutated neoplasms, liquid biopsy would represent an innovative approach that would also facilitate access to this treatment option for many neoplasms. However, most of the published literature on the clinical use of liquid biopsy to detect patients with BRAF mutation concerns maily mCRC, melanoma and NSCLC, while few data are available on less frequent types of cancer. Gonzales-Cao et al. reported the results of quantitative PCR analysis conducted in 92 serum and plasma samples from lung, colon and melanoma archives with paired tumour tissue, succeeding in detecting and quantifying BRAFV600E in mixed samples with a specificity of 100% and a sensitivity of 57.7% [161, 162]. Moreover, the RASANC study led to the approval of Idylla (Biocartis, Inc., Jersey City, NJ), a real time PCR-based assay for the detection of KRAS, NRAS and BRAF in metastatic colon cancer. The multicentre prospective study RASANC (NCT02502656), which included 98 patients with metastatic colon cancer, retrospectively assessed for the presence of ctDNA mutations in KRAS, NRAS and BRAF using the fully automated Idylla platform, showed an overall concordance between Idylla and NGS for BRAF of 99.5% [163, 164].
On the other hand, a recent systematic review comparing liquid biopsy and tissue biopsy with NGS analysis in NSCLC, showed that for BRAF mutation the positive percent agreement was inferior to 60%, probably due to the small size of cases [165]. Recently, in a small study it was possible to detect a BRAF V600E mutation in the plasma of 4/5 patients with BRAF V600E mutant brain tumors (both gliomas and brain metastasis) confirmed by ddPCR assay. Definitely, the method of analysis of Braf mutation in liquid biopsy would deserve further investigation in patients with different types of solid tumours [166].
The role of liquid biopsy has been extensively investigated in detecting PIK3CA-mutated breast tumors. Tumors carrying PIK3CA mutations may be sensitive to PIK3CA inhibitor drugs, although it is far from being considered a driver mutation proper. On 24 May 2019, the Food and Drug Administration approved alpelisib (PIQRAY, Novartis Pharmaceuticals Corporation) in combination with fulvestrant in metastatic/advanced, hormone receptor-positive, HER2-negative breast cancers carrying PI3CA mutation, after progression from a first-line endocrine therapy. The therascreen® PIK3CA RGQ PCR Kit diagnostic test, (QIAGEN Manchester, Ltd.), has also been approved to detect patients with PIK3CA mutations, which can be performed either on tumour tissue samples and/or in circulating tumour DNA (ctDNA) in plasma [195].
The phase 3 SOLAR-1 study led to the approval of this drug in breast cancer: median PFS was superior in the experimental arm, 11.0 months (95% CI: 7.5, 14.5) compared to 5.7 months (95% CI: 3.7, 7.4) in the control arm (HR 0.65; 95% CI: 0.50, 0.85; p = 0.001). In contrast, the median OS was 39.3 months (34.1–44.9) in the alpelisib-fulvestrant arm versus 31.4 months in patients of placebo-fulvestrant arm (P = 0.15) without reaching statistical significance, but, anyway, supporting the benefit of the combination in this PIK3CA-mutated patient population [195, 196]. In a phase Ia study (NCT01219699), alpelisib demonstrated tolerable safety and encouraging preliminary activity in patients with PIK3CA-mutant solid tumours, suggesting a rationale for its use alone or in combination with other drugs in the treatment of PIK3CA-mutant solid tumours [197].
A further Phase Ib, multicentre, open-label study recruited patients with advanced solid tumours and evaluated the combination of alpelisib and paclitaxel. Unfortunately, the safety profile was found to be of concern in patients with advanced solid tumours, and the study was terminated [198]. Further studies are needed to assess whether alpelisib may have an agnostic indication in solid tumours carrying the PI3KCA mutation.
Liquid biopsy represents an innovative approach that, in the era of agnostic therapies, would allow a rapid, minimally invasive and easily repeatable assessment of the genomic tumor profile. Further trials to validate and standardise analysis techniques in solid tumours are urgently needed to expand the use of liquid biopsy in clinical practice. Indeed, liquid biopsy could have a fundamental impact on a patient's oncological history in at least 2 situations: 1) at the time of diagnosis, in patients with insufficient tumour tissue for genomic profiling or inaccessibility of the tumour site to be biopsied 2) at the disease progression, to detect acquired resistance mechanisms. In both cases, an improved detection rate of molecular targets, eligible for agnostic therapies, could be achieved.
Role of liquid biopsy in monitoring the dynamics of CGP during anticancer therapies: the role of genomic reprofiling
Despite the multiple applications of liquid biopsy Comprehensive Genome Profile (CGP), most of the evidence concern metastatic setting and in particular the analysis of ctDNA rather than CTC or extracellular vesicles whose results today would seem less informative [199]. Several experiences in the most burdening disease (CRC, BC, and NSCLC) attest to the high agreement (> 80%) [200,201,202] in genomic profiling through tissue or liquid biopsy [203,204,205] (Table 8). Among the numerous fields of application through the patient journey, CRC liquid biopsy application was conceived in primary anti EGFR moAbs primary resistance linked to mutant RAS and BRAF status. Hence, NGS retrospective analysis of 92 patients from the CAPRI-GOIM [206] study using tissue and liquid biopsy showed similar PFS and OS comparing K-RAS exon 2 WT and RAS mutant patients [207]. Liquid biopsy in a prospective trial was useful for predicting emerging resistance genetic variants on several genes during treatment with anti-EGFR MoAbs as well as better prognosis for those patients with circulating wild-type biomarkers [208], although results in a similar context from other trials such as the phase III ASPECCT [209] suggested a less severe prognosis for mCRC patients treated first with Panitumumab developing emerging circulating mutations in RAS/BRAF pathway. In this regard, considering RAS mutations, BEAMing liquid biopsy showed better diagnostic accuracy than the tissue one (BEAMing and NGS) in a small series including paired tissue and liquid samples to detect rising resistance mutations (57.1% vs 7.1% and 9.5%, respectively, p = 0.008) [210] suggesting its specific utility in highlighting subclones under selective pressure during treatments with anti-EGFR. Therefore, these consistent results have been investigated on other genes involved in growing resistance such as HER-2, BRAF, or MET [211,212,213,214,215]. Recently, as a matter of course, liquid biopsy profiling has been the rationale for the development of rechallenge strategies. CRICKET trial [216] constitutes the proof-of-concept study in this setting, although in a small series of patients. In particular, investigators enrolled tissue confirmed RAS/BRAF WT mCRC population in which, of the 28 patients studied with ctDNA, only RAS/BRAF WT achieved a partial response with a strategy of anti-EGFR reintroduction. The most recent biomarker-driven CHRONOS trial [217] has strengthened these results by proposing the rechallenge strategy only to RAS/BRAF WT patients achieving a RECIST response and at least a 50% reduction in RAS ctDNA mutant fraction before receiving anti-EGFR retreatment. Confirmatory data of the phase II CAPRI-2 study (NCT05312398) evaluating the rechallenge with Cetuximab plus Irinotecan in mCRC patients harboring a RAS/BRAF mutant status after a first-line anti-EGFR first-line regimen are awaited.
In the last two decades, oncogene-addicted NSCLC patients did experience a therapeutic revolution linked to the introduction of tyrosine kinase inhibitors (TKIs) and their combinations aiming to overcome primary and secondary resistance growing up [218]. However, this scenario is rapidly changing due to emerging resistance (on-target, off-target bypass pathways, and histological transformations) [219,220,221,222] following treatment with 3rd generation EGFR-TKI as a second or first-line option following the results of AURA-3 [223] and FLAURA trials in mNSCLC patients carrying an EGFR sensitizing mutation. In this way, several new drugs have been tested in combination with upfront Osimertinib to overcome acquired resistance, mainly due to -MET (about 15%) genomic alterations. As for EGFR inhibitor TKIs, studies with ALK-TKIs demonstrate a profound variety of resistance mechanisms [224,225,226] which differ according to I, II, or III generation molecules. In particular, Shaw et al. [225] showed that the use of Lorlatinib, a 3rd generation ALK-TKI, produced almost identical ORR when evaluated in tissue or plasma (69% vs 62%) samples. However, several factors can undermine the diagnostic accuracy of liquid biopsy CGP affecting ctDNA levels. On the one hand, biological and pathological factors, such as tumor burden, anatomical site (intrathoracic vs extrathoracic), histology (adenocarcinoma vs squamous), proliferative index, necrosis, and the type of fluid investigated [36, 227]; on the other hand, a series of scientific shreds of evidence have shown that quite resistances to TKIs, not only EGFR-linked, are polyclonal and monoclonal and this would affect the disease biological evolution among different patients [228, 229]. In recent years, international scientific societies receipt liquid biopsy and NGS profiling as useful tools to provide clinically valuable information throughout the patient's therapeutic pathway [52, 53, 230, 231] to be included as a complementary opportunity for tissue biopsy. In the NILE study [232], although only 18% of patients received complete genotyping across the 8 advanced NSCLC guideline recommended biomarkers, liquid biopsy genomic profiling on 282 increased sensitivity (80%) for any of them. Interestingly, for EGFR, ALK, ROS-1, and BRAF the concordance and positive predictive value rates of tissue-plasma analyses were 98.2% and 100%, respectively. Furthermore, LB profiling increased the tissue diagnostic ability by about 48% with also a turnaround time (9 vs 15 days) benefit, supporting a plasma-first approach. Similarly, the phase II/III BFAST study [34, 100, 233] in the ALK + naïve cohort recently showed an intriguing high ORR (87.45 by INV and 92% by IRF) to ALK-targeted therapy after blood-based testing, when compared with data from the ALEX study (71.7%) [234]. These results can be explained by the inability of the tissue analysis to overcome issues related to both intratumoral and intrapatient heterogeneity. Likewise, data showed various limits of tissue biopsy to capture the subclonal population of tumor cells with distinct alterations as well as to intercept the single lesion-specific alterations [235]. Remarkably, not all patients are susceptible to new tissue sampling for disease reprofiling. In this regard, Remon J et al. in the APPLE trial (ESMO Annual Congress 2022) support the serial monitoring of the T790M mutation through LB sampling in a cohort of advanced NSCLC patients undergoing upfront gefitinib and Osimertinib. In particular, preliminary results of arm B (plasma-guided GefitinibrOsimertinib sequence) versus arm C (imaging-guided GefitinibOsimertinib sequence) underline that LB can detect a biochemical progression before radiological evaluation in 17% of cases with a 10% improvement in 18-month interim OS rate benefit (87% vs 77%). Although the analysis of ctDNA poses numerous challenges related to its highly variable fraction, fragmentation, and half-life, Aggrawal C. et al. (30325992) demonstrated in a prospective cohort sub-analysis of 67 NSCLC mNSCLC patients investigated with a 73-gene NGS platform that plasma-based biomarkers with low-allele frequency may respond to targeted therapy by achieving an overall disease control rate of 85.7%. Liquid biopsy CGP could also provide an important contribution to understanding the kinetics of the antitumor response. In this context, Mack PC et al. showed that EGFR ctDNA clearance after 60 days of EGFR-TKI and anti-EGFR-MoAb combination regimen correlated with substantial improvement in PFS and OS in a cohort of advanced NSCLC underwent a 73-gene blood-based NGS panel suggesting a role of LB in determining novel pharmacodynamic predictive biomarkers of response/resistance to targeted agents [236].
Emerging data support the use of genomic profiling by LB also in breast cancer both to determine the emergence of resistance and for dynamic monitoring during therapy, in particular, those based on hormone therapy. An analysis of the phase III PALOMA-3 study by O'Leary et al. [237, 238], comparing the combination of Fulvestrant + Palbociclib vs Fulvestrant + Placebo, 14 patients underwent paired ctDNA exome analysis showing biological signs of clonal evolution in 85% of cases with new emerging mutations both in all cohorts (PIK3CA, ESR1) or only in the Palbociclib combination arm (RB1) emphasizing a subclonal complexity of hormone-responsive breast cancer. In particular, the ESR1 Y537S mutation appears to be the major driver of resistance to Fulvestrant. The phase 2a PLASMA-MATCH platform multiarm study [239] showed the opportunity of ctDNA testing to select patients for a personalized approach. In this study, Turner NC et al. did enroll advanced breast cancer patients already treated with > = 2 hormone therapy options to perform a plasma-based NGS analysis to be divided into 4 parallel treatment groups according to mutational status (ESR1 mutations, HER2 mutations; AKT1 mutations and estrogen receptor-positive; AKT1 mutations and estrogen receptor-negative or PTEN mutation) in order to receive a tailored plasma-guided treatment. Results confirm a sufficient number of objective responses in cohorts B (HER-2 mutation, 5/20) and C (AKT1/ER + , 4/18) to further explore this scheme supporting its inclusion in future clinical practice. This evidence, bearing the polyclonal heterogeneity toward ER + breast cancer evolution, attests to the potential benefit of liquid biopsy CGP to capture different disease progression patterns expressing both polyclonal ESR1 and MAPK mutations significantly affecting survival outcomes or to distinguish between clonally dominant or sub-clonal variants [240] helping in the interpretation of tumor heterogeneity through the creation of genomic signatures related to the different histological profiles of breast cancer. Besides, the level of ctDNA in the plasma should be potentially useful for the monitoring of disease. A close relationship has been highlighted between ORR and the decrease/increase of ctDNA levels during disease response/progression [241] offering the opportunity to optimize treatment customization using combinatory regimens. This is supported by recent evidence that demonstrates the importance of testing the early ctDNA dynamics to select patients who underwent rapid disease progression [238, 241]. What remains to be established is the best time to optimize a liquid biopsy CGP approach [242] during the disease as well as interventional studies focused on catching plasma-based early dynamic changes.
Role of liquid biopsy in immunotherapy: limits and perspectives
Despite the durable, long-lasting responses for some patients with advanced solid tumors, the clinical benefit of Immune-checkpoint inhibitors (ICIs) is still limited to selected patients, as a result of primary or acquired resistance to therapy [243].
One of the major challenges in the field of cancer immunotherapy is the development of a robust and dynamic predictive biomarker for optimal patient selection [244]. These extensive efforts in biomarker research have led to biomarker-based, tissue-agnostic, approvals of ICIs for the treatment of patients whose tumors harbor microsatellite instability (MSI) or high tumor mutation burden (TMB) [245]. However, the currently available biomarkers, often rely on tumor tissue samples, such as elevated tumor PD-L1 expression in the tumor microenvironment (TME) [246, 247], the tissue TMB (tTMB) [248, 249], and others, have been unable to accurately identify the subset of cancer patients who benefit from these therapies. The plastic, dynamic, and multifactorial interaction of the tumor and host immune system under immunotherapy, makes the response to ICIs and its prediction a complex and winding process.
Following the promising results in targeted therapies, an increasing number of clinical studies are investigating the potential use of liquid biopsy to improve our ability to select the patients who are likely to respond to immunotherapy-based therapy [250] (Table 9).
Liquid biopsy is emerging as a minimally invasive, cost-effective and dynamic approach to assessing the landscape of intratumoral heterogeneity and longitudinal tumor evolution during ICI treatment [245]. Different targets were actively studied using liquid biopsy. Some examples are the evaluation of PD-L1 expression on Circulating Tumor Cells (CTCs) [265,266,267], the T-cell receptor (TCR) repertoire isolated from patients’ blood [268,269,270], and the circulating plasma or serum proteins, such as the soluble PD-L1 and PD-1 [271]. Recent findings indicate that the soluble forms of immune checkpoints can be detected in the peripheral blood [272, 273] and a correlation between baseline concentrations with clinical response was recently described in several cancer types [274,275,276]. However, the cell-free DNA (cfDNA), and their tumor-derived fraction (ctDNA), are currently the most advanced and studied approaches to liquid biopsy in the context of cancer immunotherapy. Particularly, the global quantification and kinetics of cfDNA/ctDNA during ICI treatment in the metastatic setting, the ctDNA-based assessment of blood TMB (bTMB) and blood MSI (bMSI) are mostly explored for patient selection [277].
The blood-based analysis of TMB and its role as a predictive biomarker of ICI response was retrospectively investigated in several clinical trials with promising findings. The POPLAR, OAK and MYSTIC trials included patients with metastatic NSCLC [155, 256]. In patients treated with atezolizumab versus docetaxel within the POPLAR and OAK trials, a high bTMB with a TMB cut-off of 16 mutations/Mb was associated with improved PFS and OS [155]. Subsequently, in the MYSTIC trial, that compared durvalumab and tremelimumab versus chemotherapy, a high bTMB (bTMB > 20 mutations/Mb) showed improved clinical outcomes [256]. Despite the promising results in retrospective trials, prospective studies in NSCLC have not confirmed the utility of bTMB to predict ICI response. In the phase 2 B-F1RST trial of atezolizumab monotherapy [156], and the phase 3 BFAST Trial of atezolizumab versus chemotherapy [252], a high bTMB using predefined bTMB thresholds of > 16 mutations/Mb, showed an increased ORR, further improved with higher bTMB thresholds. However, significant differences in PFS between high and low bTMB patients were not shown. Similarly, in the phase 3 NEPTUNE trial of durvalumab and tremelimumab versus chemotherapy, bTMB > 20 mutations/Mb fails to predict a clinical benefit [255].
Similar to bTMB, the predictive value of blood MSI has been investigated in patients treated with ICIs, using panel NGS or droplet digital PCR. The blood-based assessment of MSI, detected in ctDNA, was highly concordant with tissue-based testing and in predicted PFS in patients treated with ICIs [158, 167, 259]. However, the predictive role of bMSI for ICI therapeutic response has not been adequately investigated in prospective studies. For this reason, additional analyses and prospective validation are required to further explore the validity of bMSI for determining tumor MSI status and its predictive value.
Other potential innovative applications of liquid biopsy in the context of immunotherapy are the minimal residual disease (MRD) detection in the adjuvant/neoadjuvant setting, and the longitudinal response monitoring through ctDNA assessment during ICI treatment in the metastatic disease [245]. In the postoperative setting, ctDNA-based MRD detection may provide a useful tool to identify high-risk patients and to adequately select the subgroup for adjuvant treatment. To date, the utility of post-operative ctDNA detection is under investigation in several studies. In the IMvigor010 trial on urothelial carcinoma, the ctDNA detection after surgery showed improved outcomes in terms of disease-free survival (DFS) and OS in the atezolizumab group compared to the observation group of patients [149].
In the neoadjuvant setting, the association between ctDNA clearance and tumor response has been explored in patients with NSCLC [278]. In phase 3 CheckMate-816 trial, the patients with stage IB to IIIA resectable NSCLC were treated with nivolumab plus platinum-based chemotherapy or platinum-based chemotherapy alone, followed by resection [278]. Although a prospective validation is warranted, the data suggest that the pretreatment levels of ctDNA and the clearance during neoadjuvant treatment may be an early predictor of disease relapse after surgery [278].
Finally, in addition to the pre-treatment assessment of ctDNA as a predictive factor of ICI response, the longitudinal monitoring of ctDNA dynamics as an early predictor of tumor responsiveness is an area of active clinical research in the metastatic setting. Several studies support the ctDNA dynamic detection during the ICI treatment, highlighting how the “on-treatment” increased ctDNA levels is often related to progressive disease. On the other hand we have to consider that plasma genotyping demonstrated negative prognostic value of TP53 mutations appearance and negative predictive value of KRAS/STK11 and KRAS/STK11/TP53 co-mutations. Moreover, another potential source of false positive results is the possible contamination of hematopoietic or smoke-induced mutation that could compromise the predictive value of TMB count in liquid biopsy [261,262,263, 279].
Currently, advancing technologies and the recent promising clinical data in the era of immunotherapy make liquid biopsy a rapidly evolving field. However, several barriers still limit the transfer of liquid biopsy into clinical practice. Beyond the known analytical and clinical validation framework, and the clinical need for a perspective and robust validation of findings, one major challenge exists.
Anticancer immunity is a dynamic, complex, and context-dependent process. Thus, the plasticity of the immune system under immunotherapy, makes limited the validity of liquid biopsy when a single target is studied. Probably, only combinatorial strategies will able to capture the complexity of the continuously evolving tumor immune microenvironment, to precisely predict the response or resistance to immunotherapy [280].
Role of liquid biopsy in analyses of vesicular genome
Over the last decade, apart from ctDNA, other members of the growing liquid biopsy “family” such as EVs or specific subtypes of EVs (namely, exosomes), have increasingly aroused considerable interest as a valuable biosource of cancer biomarkers [281,282,283,284].
From a historical perspective, EVs used to be considered lipid-rich particles isolated from cell culture supernatants and physiological fluids while only serving as disposal of cellular waste products [285]. To date, a growing body of evidence defined EVs as nanoscale-sized particles that, even if released under physiological and pathological conditions in the body fluids from almost all living lipid bilayer cells, seemed to be involved in cell-to-cell communication, promoting cross-talk between cancer cells within the tumor microenvironment while mediating tumor response and progression [286]. In this vein, emerging preclinical and clinical data supported the investigation of their use as either a compelling diagnostic tool or even a delivery approach for therapeutic purposes [287].
Following the minimal requirements released by the International Society of Extracellular vesicles (ISEV), EVs should be subclassified according to physical characteristics (size and density), biochemical composition, descriptions of conditions or cell of origin [288]. Although the biogenesis pathway remains far from clear with no wide consensus established yet, it is acknowledged that exosomes seemed to be generated by the fusion of multivesicular bodies in the late endosome whereas larger microparticles/microvesicles revealed to share a plasma membrane-derived origin [289]. The exploration of such EVs has increasingly been implemented in the cancer research field owing to their cell-specific cargo containing either proteins or nucleic acids, playing a crucial role in the intercellular exchange of genetic information [290].
Although being recovered from different other biofluids, the preferred source for EV isolation is blood plasma since serum might harbor further EVs additionally released during the clot formation [291, 292]. Compared to ctDNA and circulating tumor cells (CTCs), the presence of large and stable amounts of circulating EVs certainly represent major advantages, despite the high variability in diagnostic assays and clinical datasets [293]. Independently from the underlying mechanism of origin, EVs could be numerically easier to obtain than CTCs [294] while being more stable and representative than ctDNA in depicting the parental biological cargo [295].
Besides enclosing both protein-coding and non-coding RNAs, EVs also express proteins on their surface that proved to be useful for prognostication and therapy monitoring, supporting the clinical implementation of these analytes as relevant carriers of tumor genome in different cancer settings [296]. In this vein, plasma EV-associated molecules (such as DNA and non-coding RNAs) and proteins (mainly PD-L1) have been widely investigated as biomarkers for predicting therapeutic response [297]. Namely, in patients with advanced NSCLC undergoing immunotherapy, dynamic changes of plasma EV PD-L1 were significantly associated with survival, recently underlining even a better prediction for durable response than tissue PD-L1 [298]. Likewise, EV-associated miRNAs and long non-coding RNAs have received global attention in the longitudinal monitoring of systemic treatments in melanoma, breast and prostate cancer [299,300,301]. Further, a dynamic increase in plasma-derived EV KRAS or EGFR mutations seemed to be reliably suggestive of disease progression in pancreatic and lung cancer, respectively [302, 303].
However, the lack of harmonization of the different isolation and characterization techniques along with the low purity of circulating tumor-derived EVs critically affected the broader use of such promising biomarkers for functional research, further limiting the future implementation in the clinical practice [288, 304].
In this fascinating scenario, a multi-omic strategy combining EV information on either the DNA, RNA or protein level with the other liquid biopsy analytes might more comprehensively inform the molecular profile of patients with cancer while tailoring the most personalized therapeutic approach.
Role of liquid biospy in analyses of other biological fluids (saliva, urine, fecal)
Although the majority of liquid biopsy research has focused on blood- based biomarkers, a plethoraof alternative sources of cancer-derived molecules such as circulating tumor DNA (ctDNA) and circulating microRNAs are now emerging [305,306,307]. In this section, we discuss existing evidence supporting the utility of analyzing non-blood biological fluids including urine, saliva and stool to identify potential diagnostic, prognostic and predictive biomarkers.
Role of liquid biopsy in urinary samples
Several evidence suggested the potential clinical use of urine as a source of liquid biopsy for cancer diagnosis, disease monitoring and prediction of relapse (Table 10). ctDNA represents the most promising biomarkers in urine sample. It comprises of two distinct fractions: transrenal tumour DNA (trtDNA), which originates from plasma and enters the urine through glomerular filtration; urinary cell- free DNA (ucfDNA) which derives from cells shedding directly from the urinary tract [308]. trtDNA is, therefore, limited in size (typically < 250 bp) by virtue of undergoing renal filtration, while ucfDNA can be of larger molecular weight.
The potential clinical use of trtDNA was mainly investigated in patients with non- small- cell lung cancer (NSCLC), testing for alterations in EGFR, including the T790M mutation and KRAS [17, 309, 328]. In a cohort of 63 patients from the Tiger- X trial the analysis of trtDNA demonstrated a detection sensitivity of EGFR specific mutations similar to that observed in plasma and tissue providing the early evidence of concordance between trtDNA and tissue EGFR status [17]. Interesting, combined analysis of urinary and plasma ctDNA improved the detection of all T790M mutations compared with those detected with tissue-only, suggesting a potential synergistic effect of combining different liquid biopsy methods [17]. Similarly, mutant KRAS DNA within urine specimens and primary tissue biopsies showed high levels of concordance [309, 310]. The potential clinical utility of trtDNA analysis is emerged also in colorectal cancer (CRC) where KRAS and BRAF mutation profile detected in urine overlapped with matched tumor tissue and plasma [312]. Liquid biopsy of trtDNA proved to be a high sensitive early detection method in CRC, breast and hepatocellular cancer [233, 313, 314, 329]. Indeed, KRAS mutations have been detected in urine samples of patients with stage I CRC despite the lower levels of ctDNA in the early disease [313]. Moreover, a longitudinal analysis of trtDNA concentration in early breast cancer patients showed that it could be a sensitive method for the monitoring of disease and the prediction of relapse [314]. Similarly, in patients with hepatocellular carcinoma, trtDNA has been detected prior to radiological evidence of disease recurrence suggesting its potential utility to complement imaging technique [233].
Tumors that occur within the urinary tract such as bladder, prostate and renal cell cancers (RCC) can release DNA fragments directly into urine as ucfDNA. A pilot study of bladder cancer patients demonstrated that specific gene mutation panel in urine had a great diagnostic potential for identifying cancer from hematuria patients [315]. Similarly, ucfDNA resulted a diagnostic method more sensitive than ctDNA in identifying cancer-associated genomic alterations in patients with suspected urothelial carcinoma [316]. Based on these promising results, a specific multiplex PCR- based assay, UroSEEK, has been developed for the early detection of urothelial carcinoma. In 570 patients at risk for bladder cancer, UroSEEK alone identified 83% of patients who went on to be diagnosed with bladder cancer, this sensitivity increasing to 95% when combined with urinary cytology [317]. Another recent high-throughput sequencing method for detection of urine tumor, called CAPP-Seq, proved to be not only a promising method of early cancer detection but also for monitoring disease progression or recurrence in patients with urothelial carcinomas [318]. Studies in RCC patients found less ctDNA than other tumour types and limited overlap between the plasma and urine ctDNA content [330]. However, the analysis of DNA methylome of ucfDNA showed a high level of sensitivity in early RCC detection [319]. A pilot study in prostate cancer and healthy volunteers revealed that ucfDNA might provide a more accurate alternative to serum prostate-specific antigen (PSA) for the early diagnosis of cancer [320]. Moreover, emerging evidence reported that urine could be a sensitive tool for the study of prostate cancer epigenetic alterations [331].
In summary, urinary ctDNA analysis provides results that are highly concordant and potentially complementary to those obtained from tissue and plasma ctDNA sequencing. In addition, the concentration of ctDNA in urine is higher than ctDNA from plasma since it derived from renal cell and urothelial carcinomas that occur within the urinary tract. Thus, in these tumors, the sensitivity of urinary ctDNA in cancer detection and/or recurrence is often greater compared to blood ctDNA and tumor tissue. Despite these advantages, certain critical issues in urinary ctDNA tests are emerged reducing their clinical development. Firstly, trtDNA content is limited by glomerular filtration and the rate of filtration can be highly variable and influenced by anticancer therapy. Secondly, ctDNA yield can vary by time since previous void: for example lower trtDNA yields are obtained from samples < 1.5 h after a previous void [332]. Finally, the methods of preservation and analysis of urinary ctDNA are not yet standardized and require further implementations.
In addition to urinary ctDNA, mRNA [264, 333], long non coding RNA (lncRNA) [334], miRNAs, PIWI-interacting RNA (piRNA) [335], and circular RNAs (circRNAs) [336], have been identified in urine samples as potential biomarkers in urological cancers. In particular, specific urinary lncRNAs, such as Prostate Cancer Antigen (PCA3), provide diagnostic and prognostic information better than PSA [321,322,323,324]. In this regards, Intelliscore test, a commercial exosome-based assay, was included in the National Comprehensive Cancer Network (NCCN) guidelines for prostate cancer early detection [325]. Another test, SelctMDx, based on the overexpression of two mRNA, DLX1 (distal-less homeo-box 1), HOXC6 (homeo-box C6), was recently developed as diagnostic tool in prostate cancer [326]. Urinary lncRNA proved to be useful biomarker also for bladder cancer detection [311, 327].
The most advantage of urinary liquid biopsy is the nature entirely noninvasively of samples that can be obtained within the patient’s home, without the need for venesection or the presence of health-care professional specialists. In addition, the entirely non-invasive sampling enables longitudinal analysis at different timepoints, without the need for hospital visits providing a unique benefit for patients with urological cancers. Moreover, urine can be collected in large volumes, which solves one of the major problems with tissue or blood-based liquid biopsy that are often limited by the quantity and the number of samples.
Role of liquid biopsy in salivary samples
Saliva contains cells, proteins and nucleic acids and represents an alternative source of liquid biopsy [337]. Similar to urinary ctDNA, salivary ctDNA (sctDNA) mainly originates from local tumors such as head and neck squamous cell carcinomas (HNSCC), but it can derive also from distant malignancies through the blood across the mucosal membrane [338]. Several evidence reported that sctDNA could be a useful diagnostic tool for identifying patients with HNSCC [339] (Table 11). In oropharyngeal squamous cell carcinoma (OSSC), combined analysis of HPV-16 in plasma and saliva increased the sensitivity of identifying HPV-16–positive patients. Interesting, HPV DNA presence and concentration in saliva were correlated with disease recurrence and survival [340]. Similarly, in another study, salivary HPV DNA was correlated with tumor burden and predictive of treatment response [341]. A study that pooled different HNSCC tumor types showed that sctDNA is ideal for the assessment of the oral cavity cancers, while the combination analysis of plasma and saliva ctDNA is necessary to increase the sensitivity for diagnosis and prognosis of oropharynx, hypopharynx and larynx tumors [18]. The role of saliva-based liquid biopsy was also investigated in NSCLC, where a high concordance of EGFR mutations was found in sctDNA and plasma ctDNA [342]. However, saliva might not be a suitable sample for NSCLC diagnostics due to the low ctDNA concentrations entering the saliva from plasma [342]. In addition, sctDNA fragments are ultrashort (40–60 bp), thus conventional PCR techniques failed in assessing EGFR mutations in saliva. In this regard, novel and more sensitive technologies, such as the electric field- induced release and measurement (EFIRM) assay, are developed to detect EGFR alterations in sctDNA [343,344,345]. Currently, it represents the optimal method to analyze saliva samples from patients with malignancies other than HNSCC [346].
In addition to ctDNA, the potential diagnostic and prognostic role of salivary circulating tumor RNA (ctRNA) has also been investigated in HNSCC patients [360]. Interesting, several studies showed that salivary mRNA might be a potential biomarker for early detection and prognosis in HNSCC [347,348,349,350]. Similarly, specific salivary miRNA signatures were found in HNSCC patients suggesting their potential use in early detection [351,352,353]; other studies demonstrated the utility of saliva miRNAs as biomarkers also in predicting therapeutic response [354, 355, 361]. LncRNAs and circRNAs also showed a potential diagnostic and prognostic value in HNSCC [356,357,358], but additional research are needed to confirm these results. A recent multicenter study identified a saliva-derived exosomal small RNA signature for esophageal squamous cell carcinoma diagnosis, prognosis, and particularly, prediction of response to adjuvant therapy [359]. Finally, recent studies have been linked non-genome-based markers with the OSCC occurrence such as salivary metabolites and oral microbiome [362, 363].
In summary, similar to urine sample, saliva is another body fluid that can be non- invasively obtained without restrictions on sampling location and without the presence of a health-care professional. The ease of sampling enables longitudinal evaluation at multiple timepoints useful for monitoring treatment response and disease recurrence. In addition, sctDNA demonstrated to be a suitable diagnostic and prognostic tool in cancers of the oral cavity, while it provides useful information in combination with other techniques for assessing tumors of the oropharynx, hypopharynx and larynx. The major disadvantages are the low ctDNA concentrations and the limited fragment size that requires more advanced detection technologies such as EFIRM platform. Regarding salivary ctRNA, the main limitation is the risk of RNA degradation due to the presence of RNases in the saliva that could increase the false-positive and false-negative detection rates.
Role of liquid biopsy in stool samples
The role of stool DNA as diagnostic biomarker for CRC is currently under investigation based on the evidence that early-stage colorectal lesions develop predominantly within the mucosa with epithelial shedding of DNA into the lumen of the colon (Table 12). In particular, a fecal DNA panel consisted of 21 mutations in KRAS, adenomatous polyposis coli and p53 tumor-suppressor genes showed a high sensitivity for detection of CRC compared to fecal immunochemistry and occult blood testing [364]. These promising results led to the development and approval by FDA of the first stool-based colorectal screening test (Cologuard) that detects the presence of specific cancer-associated DNA mutations [365, 366]. Although this assay is more sensitive compared to a commonly used occult blood testing, this technique is less cost- effective than the alternatives and might not be applicable for large- scale screening programs [367].
Stool DNA analysis proved its potential diagnostic utility also in patients with other tumour types particularly in pancreatic cancer that has a poor prognosis mainly due to delayed diagnosis. In particular, the analysis of KRAS mutations detected in stool samples of pancreatic patients showed a high concordance with those identified in the resected carcinomas [368]. In addition to CRC, the analysis of DNA mutations in stool specimens of gastric cancer patients demonstrating its potential application for early cancer detection [369]. Increasing evidence supports the role of the gut microbiota in the responsiveness and toxicities to immune- checkpoint inhibitors suggesting the utility of stool DNA beyond the detection of tumour DNA [251, 370,371,372,373,374]. Indeed, microbiome composition identified through the analysis of 16S ribosomal DNA in stool samples could act as a predictive biomarker to select patients who might benefit from immunotherapy.
Different studies have explored individual miRNAs, miRNA panels, or a combination of fecal miRNAs with fecal hemoglobin for CRC early detection [375,376,377,378,379,380,381]. The analysis of diagnostic performance indicators reported AUCs, sensitivities, and specificities ranging from 0.64 to 0.97, 15% to 97%, and 38% to 100%, respectively [383]. Fecal miRNAs have several advantages such as high stability and reproducibility that make them promising biomarker for CRC screening.
Although few studies have investigated the role of stool lncRNAs as potential diagnostic biomarker, some evidence reported a potential utility of cancer-related lncRNA panels to identify and distinguish CRC patients from healthy individuals [382].
In summary, the physical proximity to CRC may facilitate the detection of tumor DNA providing an optimal diagnostic tool. In this regard, stool DNA is already used for CRC screening and provides information on the genomic profiles of other tumour types such as pancreatic and gastric cancer. The major limitation is the low ctDNA component (around 0.01% of the total DNA content of stool) due to the high presence of microbial DNA [384]. Patient aversion to providing fecal samples represents another limitation which might hinder the adoption of stool liquid biopsy [385, 386].
Conclusions
Cancer research has reached very important and advanced achievements in the last decades, by extending patient’s life and improving the quality of life for a major part of these pathologies, especially due to the development of targeted therapy, but still much more must be done.
Liquid biopsy has already revolutionized clinical practice in oncology, but it still has great hidden potential to participate in this struggle, which must be expressed by providing evidence-based guidelines for the procedure and improving the technology of this technique to maintain the integrity of the sample, by extending the cohorts of patients in its studies and the knowledge of the implications of its new biomarkers.
Several new studies and ctDNA-based trials have emerged in the last decade to investigate and expand the application of liquid biopsy in cancer management, and the promising results obtained until now indicate that it could have more important implications in different aspects of clinical practice in oncology, from diagnosis to the selection of targeted therapy and the monitoring of its effect, passing by the stratification of patients based on cancer risk and the detection of MRD.
Thus, further studies should focus also on confirming the clinical applicability of blood-based molecular profiling for CGP to improve patient outcomes.
Availability of data and materials
All the data generated is included within the manuscript and its supplementary files.
References
Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol settembre. 2017;14(9):531–48.
Poulet G, Massias J, Taly V. Liquid Biopsy: General Concepts. Acta Cytol. 2019;63(6):449–55.
Pantel K, Alix-Panabières C. Real-time liquid biopsy in cancer patients: fact or fiction? Cancer Res. 2013;73(21):6384–8.
Zhu JW, Charkhchi P, Akbari MR. Potential clinical utility of liquid biopsies in ovarian cancer. Mol Cancer. 2022;21(1):114.
Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XHF, Norton L, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139(7):1315–26.
Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4(6):448–56.
Leon SA, Shapiro B, Sklaroff DM, Yaros MJ. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37(3):646–50.
Mandel P, Metais P. Nuclear acids in human blood plasma. C R Seances Soc Biol Fil. 1948;142(3–4):241–3.
van der Pol Y, Mouliere F. Toward the early detection of cancer by decoding the epigenetic and environmental fingerprints of cell-free DNA. Cancer Cell. 2019;36(4):350–68.
Sorenson GD, Pribish DM, Valone FH, Memoli VA, Bzik DJ, Yao SL. Soluble normal and mutated DNA sequences from single-copy genes in human blood. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 1994;3(1):67–71.
Kimura H, Kasahara K, Kawaishi M, Kunitoh H, Tamura T, Holloway B, et al. Detection of epidermal growth factor receptor mutations in serum as a predictor of the response to gefitinib in patients with non-small-cell lung cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2006;12(13):3915–21.
Swisher EM, Wollan M, Mahtani SM, Willner JB, Garcia R, Goff BA, et al. Tumor-specific p53 sequences in blood and peritoneal fluid of women with epithelial ovarian cancer. Am J Obstet Gynecol settembre. 2005;193(3 Pt 1):662–7.
Sozzi G, Musso K, Ratcliffe C, Goldstraw P, Pierotti MA, Pastorino U. Detection of microsatellite alterations in plasma DNA of non-small cell lung cancer patients: a prospect for early diagnosis. Clin Cancer Res Off J Am Assoc Cancer Res. 1999;5(10):2689–92.
Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110(1):13–21.
Stevens GL, Scheer WD, Levine EA. Detection of tyrosinase mRNA from the blood of melanoma patients. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 1996;5(4):293–6.
Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19(2):43–51.
Reckamp KL, Melnikova VO, Karlovich C, Sequist LV, Camidge DR, Wakelee H, et al. A Highly Sensitive and Quantitative Test Platform for Detection of NSCLC EGFR Mutations in Urine and Plasma. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2016;11(10):1690–700.
Wang Y, Springer S, Mulvey CL, Silliman N, Schaefer J, Sausen M, et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci Transl Med. 2015;7(293):293ra104.
Diehl F, Schmidt K, Durkee KH, Moore KJ, Goodman SN, Shuber AP, et al. Analysis of mutations in DNA isolated from plasma and stool of colorectal cancer patients. Gastroenterology. 2008;135(2):489–98.
Kuang Y, Rogers A, Yeap BY, Wang L, Makrigiorgos M, Vetrand K, et al. Noninvasive detection of EGFR T790M in gefitinib or erlotinib resistant non-small cell lung cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2009;15(8):2630–6.
Qian X, Liu J, Sun Y, Wang M, Lei H, Luo G, et al. Circulating cell-free DNA has a high degree of specificity to detect exon 19 deletions and the single-point substitution mutation L858R in non-small cell lung cancer. Oncotarget. 2016;7(20):29154–65.
Tie J, Wang Y, Tomasetti C, Li L, Springer S, Kinde I, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8(346):346ra92.
Garcia-Murillas I, Schiavon G, Weigelt B, Ng C, Hrebien S, Cutts RJ, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med. 2015;7(302):302ra133.
Salvianti F, Gelmini S, Costanza F, Mancini I, Sonnati G, Simi L, et al. The pre-analytical phase of the liquid biopsy. New Biotechnol. 2020;55:19–29.
Chan HT, Chin YM, Low SK. Circulating tumor DNA-based genomic profiling assays in adult solid tumors for precision oncology: recent advancements and future challenges. Cancers. 2022;14(13):3275.
Geeurickx E, Hendrix A. Targets, pitfalls and reference materials for liquid biopsy tests in cancer diagnostics. Mol Aspects Med. 2020;72:100828.
Neumann MHD, Bender S, Krahn T, Schlange T. ctDNA and CTCs in liquid biopsy - current status and where we need to progress. Comput Struct Biotechnol J. 2018;16:190–5.
Arechederra M, Ávila MA, Berasain C. Liquid biopsy for cancer management: a revolutionary but still limited new tool for precision medicine. Adv Lab Med Av En Med Lab. 1 settembre 2020 [citato 18 febbraio 2023];1(3). Disponibile su: https://www.degruyter.com/document/doi/10.1515/almed-2020-0009/html
Green SF. The cost of poor blood specimen quality and errors in preanalytical processes. Clin Biochem. 2013;46(13–14):1175–9.
Tsui DWY, Blumenthal GM, Philip R, Barrett JC, Montagut C, Bramlett K, et al. Development, validation, and regulatory considerations for a liquid biopsy test. Clin Chem. 2020;66(3):408–14.
Delgado PO, Alves BCA, Gehrke F de S, Kuniyoshi RK, Wroclavski ML, Del Giglio A, et al. Characterization of cell-free circulating DNA in plasma in patients with prostate cancer. Tumour Biol J Int Soc Oncodevelopmental Biol Med. 2013;34(2):983–6.
Hashad D, Sorour A, Ghazal A, Talaat I. Free circulating tumor DNA as a diagnostic marker for breast cancer. J Clin Lab Anal. 2012;26(6):467–72.
Bettegowda C, Sausen M, Leary R, Kinde I, Agrawal N, Bartlett B, et al. Detection of circulating tumor dna in early and late stage human malignancies. Neuro-Oncol. 2014;16(suppl_3):iii7.
Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14(9):985–90.
Morbelli S, Alama A, Ferrarazzo G, Coco S, Genova C, Rijavec E, et al. Circulating Tumor DNA Reflects Tumor Metabolism Rather Than Tumor Burden in Chemotherapy-Naive Patients with Advanced Non-Small Cell Lung Cancer: 18F-FDG PET/CT Study. J Nucl Med Off Publ Soc Nucl Med. 2017;58(11):1764–9.
Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra24.
Umetani N, Kim J, Hiramatsu S, Reber HA, Hines OJ, Bilchik AJ, et al. Increased integrity of free circulating DNA in sera of patients with colorectal or periampullary cancer: direct quantitative PCR for ALU repeats. Clin Chem. 2006;52(6):1062–9.
Chan KCA, Yeung SW, Lui WB, Rainer TH, Lo YMD. Effects of preanalytical factors on the molecular size of cell-free DNA in blood. Clin Chem aprile. 2005;51(4):781–4.
Swinkels DW, Wiegerinck E, Steegers EAP, de Kok JB. Effects of blood-processing protocols on cell-free DNA quantification in plasma. Clin Chem marzo. 2003;49(3):525–6.
Zapico A, Grassa A, Martínez E, Menéndez M, Cortés Prieto J. Endometrial resection and preoperative LH-RH agonists: a prospective 5-year trial. Eur J Obstet Gynecol Reprod Biol. 2005;119(1):114–8.
Merker JD, Oxnard GR, Compton C, Diehn M, Hurley P, Lazar AJ, et al. Circulating tumor DNA analysis in patients with cancer: American society of clinical oncology and college of American pathologists joint review. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(16):1631–41.
Grölz D, Hauch S, Schlumpberger M, Guenther K, Voss T, Sprenger-Haussels M, et al. Liquid biopsy preservation solutions for standardized pre-analytical workflows-venous whole blood and plasma. Curr Pathobiol Rep. 2018;6(4):275–86.
Sorber L, Zwaenepoel K, Jacobs J, De Winne K, Van Casteren K, Augustus E, et al. Specialized blood collection tubes for liquid biopsy: improving the pre-analytical conditions. Mol Diagn Ther. 2020;24(1):113–24.
Markus H, Contente-Cuomo T, Farooq M, Liang WS, Borad MJ, Sivakumar S, et al. Evaluation of pre-analytical factors affecting plasma DNA analysis. Sci Rep. 2018;8(1):7375.
El Messaoudi S, Rolet F, Mouliere F, Thierry AR. Circulating cell free DNA: Preanalytical considerations. Clin Chim Acta Int J Clin Chem. 2013;424:222–30.
Herrera LJ, Raja S, Gooding WE, El-Hefnawy T, Kelly L, Luketich JD, et al. Quantitative analysis of circulating plasma DNA as a tumor marker in thoracic malignancies. Clin Chem. 2005;51(1):113–8.
Sorber L, Zwaenepoel K, Deschoolmeester V, Roeyen G, Lardon F, Rolfo C, et al. A Comparison of Cell-Free DNA Isolation Kits: Isolation and Quantification of Cell-Free DNA in Plasma. J Mol Diagn JMD. 2017;19(1):162–8.
Bidard FC, Madic J, Mariani P, Piperno-Neumann S, Rampanou A, Servois V, et al. Detection rate and prognostic value of circulating tumor cells and circulating tumor DNA in metastatic uveal melanoma. Int J Cancer. 2014;134(5):1207–13.
Sorber L, Zwaenepoel K, Deschoolmeester V, Van Schil PEY, Van Meerbeeck J, Lardon F, et al. Circulating cell-free nucleic acids and platelets as a liquid biopsy in the provision of personalized therapy for lung cancer patients. Lung Cancer Amst Neth. 2017;107:100–7.
Newman AM, Bratman SV, To J, Wynne JF, Eclov NCW, Modlin LA, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014;20(5):548–54.
Malone ER, Oliva M, Sabatini PJB, Stockley TL, Siu LL. Molecular profiling for precision cancer therapies. Genome Med. 2020;12(1):8.
Rolfo C, Mack PC, Scagliotti GV, Baas P, Barlesi F, Bivona TG, et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2018;13(9):1248–68.
Pascual J, Attard G, Bidard FC, Curigliano G, De Mattos-Arruda L, Diehn M, et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: a report from the ESMO precision medicine working group. Ann Oncol Off J Eur Soc Med Oncol. 2022;33(8):750–68.
Chan HT, Chin YM, Nakamura Y, Low SK. Clonal hematopoiesis in liquid biopsy: from biological noise to valuable clinical implications. Cancers. 2020;12(8):2277.
Mizuno K, Akamatsu S, Sumiyoshi T, Wong JH, Fujita M, Maejima K, et al. eVIDENCE: a practical variant filtering for low-frequency variants detection in cell-free DNA. Sci Rep. 2019;9(1):15017.
Chen I, Raymond VM, Geis JA, Collisson EA, Jensen BV, Hermann KL, et al. Ultrasensitive plasma ctDNA KRAS assay for detection, prognosis, and assessment of therapeutic response in patients with unresectable pancreatic ductal adenocarcinoma. Oncotarget. 2017;8(58):97769–86.
Jacobs MT, Mohindra NA, Shantzer L, Chen IL, Phull H, Mitchell W, et al. Use of low-frequency driver mutations detected by cell-free circulating tumor DNA to guide targeted therapy in non–small-cell lung cancer: a multicenter case series. JCO Precis Oncol. 2018;2:1–10.
Razavi P, Li BT, Brown DN, Jung B, Hubbell E, Shen R, et al. High-intensity sequencing reveals the sources of plasma circulating cell-free DNA variants. Nat Med. 2019;25(12):1928–37.
Tsao SCH, Weiss J, Hudson C, Christophi C, Cebon J, Behren A, et al. Monitoring response to therapy in melanoma by quantifying circulating tumour DNA with droplet digital PCR for BRAF and NRAS mutations. Sci Rep. 2015;5:11198.
Lindeman NI, Cagle PT, Aisner DL, Arcila ME, Beasley MB, Bernicker EH, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the college of American pathologists, the international association for the study of lung cancer, and the association for molecular pathology. Arch Pathol Lab Med. 2018;142(3):321–46.
Mateo J, Chakravarty D, Dienstmann R, Jezdic S, Gonzalez-Perez A, Lopez-Bigas N, et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol Off J Eur Soc Med Oncol. 2018;29(9):1895–902.
Chen M, Zhao H. Next-generation sequencing in liquid biopsy: cancer screening and early detection. Hum Genomics. 2019;13(1):34.
Rodríguez N, Viñal D, Rodríguez-Cobos J, De Castro J, Domínguez G. Genomic profiling in oncology clinical practice. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex. 2020;22(9):1430–9.
Garber JE, Offit K. Hereditary cancer predisposition syndromes. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(2):276–92.
Detection, Prevention, and Risk Reduction [Internet]. NCCN. [citato 26 marzo 2023]. Disponibile su: https://www.nccn.org/guidelines/category_2
Buglyó G, Styk J, Pös O, Csók Á, Repiska V, Soltész B, et al. Liquid biopsy as a source of nucleic acid biomarkers in the diagnosis and management of lynch syndrome. Int J Mol Sci. 2022;23(8):4284.
Meric-Bernstam F, Brusco L, Daniels M, Wathoo C, Bailey AM, Strong L, et al. Incidental germline variants in 1000 advanced cancers on a prospective somatic genomic profiling protocol. Ann Oncol Off J Eur Soc Med Oncol. 2016;27(5):795–800.
Mandelker D, Donoghue M, Talukdar S, Bandlamudi C, Srinivasan P, Vivek M, et al. Germline-focussed analysis of tumour-only sequencing: recommendations from the ESMO precision medicine working group. Ann Oncol Off J Eur Soc Med Oncol. 2019;30(8):1221–31.
Paramathas S, Guha T, Pugh TJ, Malkin D, Villani A. Considerations for the use of circulating tumor DNA sequencing as a screening tool in cancer predisposition syndromes. Pediatr Blood Cancer. 2020;67(12):e28758.
Sausen M, Phallen J, Adleff V, Jones S, Leary RJ, Barrett MT, et al. Clinical implications of genomic alterations in the tumour and circulation of pancreatic cancer patients. Nat Commun. 2015;6:7686.
Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10(2):63–89.
Hou J, Li X, Xie KP. Coupled liquid biopsy and bioinformatics for pancreatic cancer early detection and precision prognostication. Mol Cancer. 2021;20(1):34.
Kanikarla-Marie P, Lam M, Menter DG, Kopetz S. Platelets, circulating tumor cells, and the circulome. Cancer Metastasis Rev. 2017;36(2):235–48.
De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol Sci. 2019;40(3):172–86.
Ahn HS, Ho JY, Yu J, Yeom J, Lee S, Hur SY, et al. Plasma protein biomarkers associated with higher ovarian cancer risk in BRCA1/2 carriers. Cancers. 2021;13(10):2300.
Bordin A, Chirivì M, Pagano F, Milan M, Iuliano M, Scaccia E, et al. Human platelet lysate-derived extracellular vesicles enhance angiogenesis through miR-126. Cell Prolif. 2022;55(11):e13312.
Angelini F, Pagano F, Bordin A, Milan M, Chimenti I, Peruzzi M, et al. The Impact of environmental factors in influencing epigenetics related to oxidative states in the cardiovascular system. Oxid Med Cell. 2017;2017:2712751.
Tito C, De Falco E, Rosa P, Iaiza A, Fazi F, Petrozza V, et al. Circulating micrornas from the molecular mechanisms to clinical biomarkers: a focus on the clear cell renal cell carcinoma. Genes. 2021;12(8):1154.
Kerachian MA, Azghandi M, Mozaffari-Jovin S, Thierry AR. Guidelines for pre-analytical conditions for assessing the methylation of circulating cell-free DNA. Clin Epigenetics. 2021;13(1):193.
Liu MC, Oxnard GR, Klein EA, Swanton C, Seiden MV, CCGA Consortium. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol Off J Eur Soc Med Oncol. 2020;31(6):745–59.
Yang J, Xu R, Wang C, Qiu J, Ren B, You L. Early screening and diagnosis strategies of pancreatic cancer: a comprehensive review. Cancer Commun Lond Engl. 2021;41(12):1257–74.
Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523(7559):177–82.
Okada T, Iwano H, Ono Y, Karasaki H, Sato T, Yamada M, et al. Utility of «liquid biopsy» using pancreatic juice for early detection of pancreatic cancer. Endosc Int Open. 2018;6(12):E1454–61.
Liu Y, Cheng L, Wang G, Lv J, He Y, Shao PL, et al. A nano-magnetic size selective cfDNA extraction platform for liquid biopsy with enhanced precision. J Chromatogr B Analyt Technol Biomed Life Sci. 2022;1199:123236.
Li X, Wang Q, Wang R. Roles of exosome genomic DNA in colorectal cancer. Front Pharmacol. 2022;13:923232.
Wang Y, Li L, Douville C, Cohen JD, Yen TT, Kinde I, et al. Evaluation of liquid from the Papanicolaou test and other liquid biopsies for the detection of endometrial and ovarian cancers. Sci Transl Med. 2018;10(433):eaap8793.
Zhong Y, Xu F, Wu J, Schubert J, Li MM. Application of next generation sequencing in laboratory medicine. Ann Lab Med. 2021;41(1):25–43.
Precision medicine improves outcomes in metastatic breast cancer. Nature. 7 settembre 2022 [citato 22 febbraio 2023]; Disponibile su: https://www.nature.com/articles/d41586-022-02276-9
Guttery DS, Page K, Hills A, Woodley L, Marchese SD, Rghebi B, et al. Noninvasive detection of activating estrogen receptor 1 (ESR1) mutations in estrogen receptor-positive metastatic breast cancer. Clin Chem. 2015;61(7):974–82.
Cardinali B, De Luca G, Tasso R, Coco S, Garuti A, Buzzatti G, et al. Targeting PIK3CA actionable mutations in the circulome: a proof of concept in metastatic breast cancer. Int J Mol Sci. 2022;23(11):6320.
Pantel K, Alix-Panabières C. Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat Rev Clin Oncol. 2019;16(7):409–24.
Rushton AJ, Nteliopoulos G, Shaw JA, Coombes RC. A review of circulating tumour cell enrichment technologies. Cancers. 2021;13(5):970.
Moding EJ, Nabet BY, Alizadeh AA, Diehn M. Detecting liquid remnants of solid tumors: circulating tumor DNA minimal residual disease. Cancer Discov. 2021;11(12):2968–86.
Ishiba T, Hoffmann AC, Usher J, Elshimali Y, Sturdevant T, Dang M, et al. Frequencies and expression levels of programmed death ligand 1 (PD-L1) in circulating tumor RNA (ctRNA) in various cancer types. Biochem Biophys Res Commun. 2018;500(3):621–5.
Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15(2):81–94.
Bidard FC, Mathiot C, Delaloge S, Brain E, Giachetti S, de Cremoux P, et al. Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann Oncol Off J Eur Soc Med Oncol. 2010;21(4):729–33.
Rack B, Schindlbeck C, Jückstock J, Andergassen U, Hepp P, Zwingers T, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. 2014;106(5).
Janni WJ, Rack B, Terstappen LWMM, Pierga JY, Taran FA, Fehm T, et al. Pooled analysis of the prognostic relevance of circulating tumor cells in primary breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2016;22(10):2583–93.
Riethdorf S, Müller V, Loibl S, Nekljudova V, Weber K, Huober J, et al. Prognostic impact of circulating tumor cells for breast cancer patients treated in the neoadjuvant «geparquattro» trial. Clin Cancer Res Off J Am Assoc Cancer Res. 2017;23(18):5384–93.
Bidard FC, Michiels S, Riethdorf S, Mueller V, Esserman LJ, Lucci A, et al. Circulating tumor cells in breast cancer patients treated by neoadjuvant chemotherapy: a meta-analysis. J Natl Cancer Inst. 2018;110(6):560–7.
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–21.
Goodman CR, Seagle BLL, Friedl TWP, Rack B, Lato K, Fink V, et al. Association of circulating tumor cell status with benefit of radiotherapy and survival in early-stage breast cancer. JAMA Oncol. 2018;4(8):e180163.
Trapp E, Janni W, Schindlbeck C, Jückstock J, Andergassen U, de Gregorio A, et al. Presence of circulating tumor cells in high-risk early breast cancer during follow-up and prognosis. J Natl Cancer Inst. 2019;111(4):380–7.
Bidard FC, Jacot W, Kiavue N, Dureau S, Kadi A, Brain E, et al. Efficacy of circulating tumor cell count-driven vs clinician-driven first-line therapy choice in hormone receptor-positive, ERBB2-negative metastatic breast cancer: The STIC CTC randomized clinical trial. JAMA Oncol. 2021;7(1):34–41.
Matikas A, Kotsakis A, Apostolaki S, Politaki H, Perraki M, Kalbakis K, et al. Detection of circulating tumour cells before and following adjuvant chemotherapy and long-term prognosis of early breast cancer. Br J Cancer. 2022;126(11):1563–9.
van Dalum G, Stam GJ, Scholten LFA, Mastboom WJB, Vermes I, Tibbe AGJ, et al. Importance of circulating tumor cells in newly diagnosed colorectal cancer. Int J Oncol. 2015;46(3):1361–8.
Hinz S, Hendricks A, Wittig A, Schafmayer C, Tepel J, Kalthoff H, et al. Detection of circulating tumor cells with CK20 RT-PCR is an independent negative prognostic marker in colon cancer patients - a prospective study. BMC Cancer. 2017;17(1):53.
Dizdar L, Fluegen G, van Dalum G, Honisch E, Neves RP, Niederacher D, et al. Detection of circulating tumor cells in colorectal cancer patients using the GILUPI Cell Collector: results from a prospective, single-center study. Mol Oncol. 2019;13(7):1548–58.
Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(12):1556–63.
Hou JM, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(5):525–32.
Dorsey JF, Kao GD, MacArthur KM, Ju M, Steinmetz D, Wileyto EP, et al. Tracking viable circulating tumor cells (CTCs) in the peripheral blood of non-small cell lung cancer (NSCLC) patients undergoing definitive radiation therapy: pilot study results. Cancer. 2015;121(1):139–49.
Chinniah C, Aguarin L, Cheng P, Decesaris C, Cutillo A, Berman AT, et al. Early detection of recurrence in patients with locally advanced non-small-cell lung cancer via circulating tumor cell analysis. Clin Lung Cancer. 2019;20(5):384-390.e2.
Frick MA, Feigenberg SJ, Jean-Baptiste SR, Aguarin LA, Mendes A, Chinniah C, et al. Circulating tumor cells are associated with recurrent disease in patients with early-stage non-small cell lung cancer treated with stereotactic body radiotherapy. Clin Cancer Res Off J Am Assoc Cancer Res. 2020;26(10):2372–80.
Kuske A, Gorges TM, Tennstedt P, Tiebel AK, Pompe R, Preißer F, et al. Improved detection of circulating tumor cells in non-metastatic high-risk prostate cancer patients. Sci Rep. 2016;6(1):39736.
Salami SS, Singhal U, Spratt DE, Palapattu GS, Hollenbeck BK, Schonhoft JD, et al. Circulating Tumor Cells as a Predictor of Treatment Response in Clinically Localized Prostate Cancer. JCO Precis Oncol. 2019;3.
Rink M, Chun FK, Dahlem R, Soave A, Minner S, Hansen J, et al. Prognostic role and HER2 expression of circulating tumor cells in peripheral blood of patients prior to radical cystectomy: a prospective study. Eur Urol. 2012;61(4):810–7.
Gazzaniga P, Gradilone A, de Berardinis E, Busetto GM, Raimondi C, Gandini O, et al. Prognostic value of circulating tumor cells in nonmuscle invasive bladder cancer: a Cell Search analysis. Ann Oncol. 2012;23(9):2352–6.
Gazzaniga P, de Berardinis E, Raimondi C, Gradilone A, Busetto GM, De Falco E, et al. Circulating tumor cells detection has independent prognostic impact in high-risk non-muscle invasive bladder cancer. Int J Cancer. 2014;135(8):1978–82.
Nicolazzo C, Busetto GM, Gradilone A, Sperduti I, Del Giudice F, Loreni F, et al. Circulating tumor cells identify patients with super-high-risk non-muscle-invasive bladder cancer: updated outcome analysis of a prospective single-center trial. Oncologist. 2019;24(5):612–6.
Busetto GM, Ferro M, Del Giudice F, Antonini G, Chung BI, Sperduti I, et al. The prognostic role of circulating tumor cells (CTC) in high-risk non–muscle-invasive bladder cancer. Clin Genitourin Cancer. 2017;15(4):e661–6.
Abrahamsson J, Aaltonen K, Engilbertsson H, Liedberg F, Patschan O, Rydén L, et al. Circulating tumor cells in patients with advanced urothelial carcinoma of the bladder: Association with tumor stage, lymph node metastases, FDG-PET findings, and survival. Urol Oncol Semin Orig Investig. 2017;35(10):606.e9-606.e16.
Soave A, Riethdorf S, Dahlem R, von Amsberg G, Minner S, Weisbach L, et al. A nonrandomized, prospective, clinical study on the impact of circulating tumor cells on outcomes of urothelial carcinoma of the bladder patients treated with radical cystectomy with or without adjuvant chemotherapy. Int J Cancer. 2017;140(2):381–9.
Beije N, de Kruijff IE, de Jong JJ, Klaver SO, de Vries P, Jacobs RAL, et al. Circulating tumour cells to drive the use of neoadjuvant chemotherapy in patients with muscle-invasive bladder cancer. ESMO Open. 2022;7(2):100416.
Olsson E, Winter C, George A, Chen Y, Howlin J, Tang MHE, et al. Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease. EMBO Mol Med. 2015;7(8):1034–47.
Chen YH, Hancock BA, Solzak JP, Brinza D, Scafe C, Miller KD, et al. Next-generation sequencing of circulating tumor DNA to predict recurrence in triple-negative breast cancer patients with residual disease after neoadjuvant chemotherapy. Npj Breast Cancer. 2017;3(1):24.
Riva F, Bidard FC, Houy A, Saliou A, Madic J, Rampanou A, et al. Patient-specific circulating tumor DNA detection during neoadjuvant chemotherapy in triple-negative breast cancer. Clin Chem marzo. 2017;63(3):691–9.
McDonald BR, Contente-Cuomo T, Sammut SJ, Odenheimer-Bergman A, Ernst B, Perdigones N, et al. Personalized circulating tumor DNA analysis to detect residual disease after neoadjuvant therapy in breast cancer. Sci Transl Med. 2019;11(504).
Coombes RC, Page K, Salari R, Hastings RK, Armstrong A, Ahmed S, et al. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res Off J Am Assoc Cancer Res. 2019;25(14):4255–63.
Garcia-Murillas I, Chopra N, Comino-Méndez I, Beaney M, Tovey H, Cutts RJ, et al. Assessment of molecular relapse detection in early-stage breast cancer. JAMA Oncol. 2019;5(10):1473–8.
Parsons HA, Rhoades J, Reed SC, Gydush G, Ram P, Exman P, et al. Sensitive detection of minimal residual disease in patients treated for early-stage breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2020;26(11):2556–64.
Magbanua MJM, Swigart LB, Wu HT, Hirst GL, Yau C, Wolf DM, et al. Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival. Ann Oncol Off J Eur Soc Med Oncol. 2021;32(2):229–39.
Lipsyc-Sharf M, de Bruin EC, Santos K, McEwen R, Stetson D, Patel A, et al. Circulating tumor DNA and late recurrence in high-risk hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2022;40(22):2408–19.
Ng SB, Chua C, Ng M, Gan A, Poon PSY, Teo M, et al. Individualised multiplexed circulating tumour DNA assays for monitoring of tumour presence in patients after colorectal cancer surgery. Sci Rep. 2017;7(1):40737.
Schøler LV, Reinert T, Ørntoft MBW, Kassentoft CG, Árnadóttir SS, Vang S, et al. Clinical implications of monitoring circulating tumor DNA in patients with colorectal cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2017;23(18):5437–45.
Reinert T, Henriksen TV, Christensen E, Sharma S, Salari R, Sethi H, et al. Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncol. 2019;5(8):1124–31.
Tarazona N, Gimeno-Valiente F, Gambardella V, Zuñiga S, Rentero-Garrido P, Huerta M, et al. Targeted next-generation sequencing of circulating-tumor DNA for tracking minimal residual disease in localized colon cancer. Ann Oncol Off J Eur Soc Med Oncol. 2019;30(11):1804–12.
Taieb J, Taly V, Vernerey D, Bourreau C, Bennouna J, Faroux R, et al. LBA30_PR - Analysis of circulating tumour DNA (ctDNA) from patients enrolled in the IDEA-FRANCE phase III trial: prognostic and predictive value for adjuvant treatment duration. Ann Oncol. 2019;30:v867.
Tie J, Cohen JD, Lo SN, Wang Y, Li L, Christie M, et al. Prognostic significance of postsurgery circulating tumor DNA in nonmetastatic colorectal cancer: Individual patient pooled analysis of three cohort studies. Int J Cancer. 2021;148(4):1014–26.
Parikh AR, Van Seventer EE, Siravegna G, Hartwig AV, Jaimovich A, He Y, et al. Minimal residual disease detection using a plasma-only circulating tumor DNA assay in patients with colorectal cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2021;27(20):5586–94.
Vidal J, Casadevall D, Bellosillo B, Pericay C, Garcia-Carbonero R, Losa F, et al. Clinical impact of presurgery circulating tumor DNA after total neoadjuvant treatment in locally advanced rectal cancer: a biomarker study from the GEMCAD 1402 trial. Clin Cancer Res Off J Am Assoc Cancer Res. 2021;27(10):2890–8.
Henriksen TV, Tarazona N, Frydendahl A, Reinert T, Gimeno-Valiente F, Carbonell-Asins JA, et al. Circulating tumor DNA in stage III colorectal cancer, beyond minimal residual disease detection, toward assessment of adjuvant therapy efficacy and clinical behavior of recurrences. Clin Cancer Res Off J Am Assoc Cancer Res. 2022;28(3):507–17.
Tie J, Cohen JD, Lahouel K, Lo SN, Wang Y, Kosmider S, et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N Engl J Med. 2022;386(24):2261–72.
Abbosh C, Birkbak NJ, Wilson GA, Jamal-Hanjani M, Constantin T, Salari R, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017;545(7655):446–51.
Chaudhuri AA, Chabon JJ, Lovejoy AF, Newman AM, Stehr H, Azad TD, et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov. 2017;7(12):1394–403.
Chen K, Zhao H, Shi Y, Yang F, Wang LT, Kang G, et al. Perioperative dynamic changes in circulating tumor DNA in patients with lung cancer (DYNAMIC). Clin Cancer Res Off J Am Assoc Cancer Res. 2019;25(23):7058–67.
Xia L, Mei J, Kang R, Deng S, Chen Y, Yang Y, et al. Perioperative ctDNA-based molecular residual disease detection for non-small cell lung cancer: a prospective multicenter cohort study (LUNGCA-1). Clin Cancer Res Off J Am Assoc Cancer Res. 2022;28(15):3308–17.
Gale D, Heider K, Ruiz-Valdepenas A, Hackinger S, Perry M, Marsico G, et al. Residual ctDNA after treatment predicts early relapse in patients with early-stage non-small cell lung cancer. Ann Oncol Off J Eur Soc Med Oncol. 2022;33(5):500–10.
Lau E, McCoy P, Reeves F, Chow K, Clarkson M, Kwan EM, et al. Detection of ctDNA in plasma of patients with clinically localised prostate cancer is associated with rapid disease progression. Genome Med agosto. 2020;12(1):72.
Powles T, Assaf ZJ, Davarpanah N, Banchereau R, Szabados BE, Yuen KC, et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature. 2021;595(7867):432–7.
Bellmunt J, Hussain M, Gschwend JE, Albers P, Oudard S, Castellano D, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(4):525–37.
Nors J, Henriksen TV, Gotschalck KA, Juul T, Søgaard J, Iversen LH, et al. IMPROVE-IT2: implementing noninvasive circulating tumor DNA analysis to optimize the operative and postoperative treatment for patients with colorectal cancer - intervention trial 2. Study protocol. Vol. 59, Acta oncologica (Stockholm, Sweden). England; 2020. p. 336–41.
Kwapisz D. The first liquid biopsy test approved. Is it a new era of mutation testing for non-small cell lung cancer? Ann Transl Med. 2017;5(3):46.
Ignatiadis M, Sledge GW, Jeffrey SS. Liquid biopsy enters the clinic - implementation issues and future challenges. Nat Rev Clin Oncol. 2021;18(5):297–312.
Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site - when a biomarker defines the indication. N Engl J Med. 2017;377(15):1409–12.
Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018;24(9):1441–8.
Kim ES, Velcheti V, Mekhail T, Yun C, Shagan SM, Hu S, et al. Blood-based tumor mutational burden as a biomarker for atezolizumab in non-small cell lung cancer: the phase 2 B-F1RST trial. Nat Med. 2022;28(5):939–45.
Ignatiadis M, Sledge GW, Jeffrey SS. Liquid biopsy enters the clinic - implementation issues and future challenges. Nat Rev Clin Oncol. 2021;18(5):297–312.
Willis J, Lefterova MI, Artyomenko A, Kasi PM, Nakamura Y, Mody K, et al. Validation of microsatellite instability detection using a comprehensive plasma-based genotyping panel. Clin Cancer Res Off J Am Assoc Cancer Res. 2019;25(23):7035–45.
Odegaard JI, Vincent JJ, Mortimer S, Vowles JV, Ulrich BC, Banks KC, et al. Validation of a plasma-based comprehensive cancer genotyping assay utilizing orthogonal tissue- and plasma-based methodologies. Clin Cancer Res Off J Am Assoc Cancer Res. 2018;24(15):3539–49.
Rolfo C, Drilon A, Hong D, McCoach C, Dowlati A, Lin JJ, et al. NTRK1 Fusions identified by non-invasive plasma next-generation sequencing (NGS) across 9 cancer types. Br J Cancer. 2022;126(3):514–20.
Gonzalez-Cao M, Mayo-de-Las-Casas C, Molina-Vila MA, De Mattos-Arruda L, Muñoz-Couselo E, Manzano JL, et al. BRAF mutation analysis in circulating free tumor DNA of melanoma patients treated with BRAF inhibitors. Melanoma Res. 2015;25(6):486–95.
El Messaoudi S, Mouliere F, Du Manoir S, Bascoul-Mollevi C, Gillet B, Nouaille M, et al. Circulating DNA as a strong multimarker prognostic tool for metastatic colorectal cancer patient management care. Clin Cancer Res Off J Am Assoc Cancer Res. 2016;22(12):3067–77.
Jacobs B, Claes B, Bachet JB, Bouche O, Sablon E, Maertens GG, et al. Evaluation of a fully automated extended RAS-BRAF test on prospectively collected plasma samples from patients with metastatic colorectal cancer. J Clin Oncol. 2017;35(15_suppl):e15127–e15127.
Bachet JB, Bouche O, Taïeb J, Dubreuil O, Garcia ML, Meurisse A, et al. RAS mutations concordance in circulating tumor DNA (ctDNA) and tissue in metastatic colorectal cancer (mCRC): RASANC, an AGEO prospective multicenter study. J Clin Oncol. 2017;35(15_suppl):11509–11509.
Esagian SM, Grigoriadou GΙ, Nikas IP, Boikou V, Sadow PM, Won JK, et al. Comparison of liquid-based to tissue-based biopsy analysis by targeted next generation sequencing in advanced non-small cell lung cancer: a comprehensive systematic review. J Cancer Res Clin Oncol. 2020;146(8):2051–66.
Kang KM, Muralidharan K, Yekula A, Small JL, Rosh ZS, Jones PS, et al. Blood-based detection of BRAF V600E in gliomas and brain tumor metastasis. Cancers. 2021;13(6):1227.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20.
Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res Off J Am Assoc Cancer Res. 2019;25(13):3753–8.
Marcus L, Fashoyin-Aje LA, Donoghue M, Yuan M, Rodriguez L, Gallagher PS, et al. FDA Approval Summary: Pembrolizumab for the Treatment of Tumor Mutational Burden-High Solid Tumors. Clin Cancer Res Off J Am Assoc Cancer Res. 2021;27(17):4685–9.
Martin-Zanca D, Hughes SH, Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature. 1986;319(6056):743–8.
Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open. 2016;1(2):e000023.
Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15(12):731–47.
Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731–9.
Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21(2):271–82.
Rolfo C. NTRK gene fusions: a rough diamond ready to sparkle. Lancet Oncol. 2020;21(4):472–4.
Leicht DT, Balan V, Kaplun A, Singh-Gupta V, Kaplun L, Dobson M, et al. Raf kinases: function, regulation and role in human cancer. Biochim Biophys Acta. 2007;1773(8):1196–212.
Zaman A, Wu W, Bivona TG. Targeting oncogenic BRAF: past, present, and future. Cancers. 2019;11(8):1197.
Cohn AL, Day BM, Abhyankar S, McKenna E, Riehl T, Puzanov I. BRAFV600 mutations in solid tumors, other than metastatic melanoma and papillary thyroid cancer, or multiple myeloma: a screening study. OncoTargets Ther. 2017;10:965–71.
Oneal PA, Kwitkowski V, Luo L, Shen YL, Subramaniam S, Shord S, et al. FDA approval summary: vemurafenib for the treatment of patients with Erdheim-Chester disease with the BRAFV600 mutation. Oncologist. 2018;23(12):1520–4.
Bernocchi O, Sirico M, Corona SP, Strina C, Milani M, Cappelletti MR, et al. Tumor type agnostic therapy carrying BRAF mutation: case reports and review of literature. Pharm Basel Switz. 2021;14(2):159.
Mullard A. BRAF plus MEK inhibitor combo secures tumour-agnostic FDA approval. Nat Rev Drug Discov. 2022;21(8):548.
Subbiah V, Lassen U, Élez E, Italiano A, Curigliano G, Javle M, et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020;21(9):1234–43.
Wen PY, Stein A, van den Bent M, De Greve J, Wick A, de Vos FYFL, et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutant low-grade and high-grade glioma (ROAR): a multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol. 2022;23(1):53–64.
Jenkins S, Yang JCH, Ramalingam SS, Yu K, Patel S, Weston S, et al. Plasma ctDNA analysis for detection of the EGFR T790M mutation in patients with advanced non-small cell lung cancer. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2017;12(7):1061–70.
Schmiegel W, Scott RJ, Dooley S, Lewis W, Meldrum CJ, Pockney P, et al. Blood-based detection of RAS mutations to guide anti-EGFR therapy in colorectal cancer patients: concordance of results from circulating tumor DNA and tissue-based RAS testing. Mol Oncol. 2017;11(2):208–19.
Vidal J, Muinelo L, Dalmases A, Jones F, Edelstein D, Iglesias M, et al. Plasma ctDNA RAS mutation analysis for the diagnosis and treatment monitoring of metastatic colorectal cancer patients. Ann Oncol Off J Eur Soc Med Oncol. 2017;28(6):1325–32.
Kalemkerian GP, Narula N, Kennedy EB, Biermann WA, Donington J, Leighl NB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American society of clinical oncology endorsement of the college of american pathologists/international association for the study of lung cancer/association for molecular pathology clinical practice guideline update. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(9):911–9.
Majem M, Juan O, Insa A, Reguart N, Trigo JM, Carcereny E, et al. SEOM clinical guidelines for the treatment of non-small cell lung cancer (2018). Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex. 2019;21(1):3–17.
Ceccon C, Angerilli V, Rasola C, Procaccio L, Sabbadin M, Bergamo F, et al. Microsatellite instable colorectal adenocarcinoma diagnostics: the advent of liquid biopsy approaches. Front Oncol. 2022;12:930108.
Cescon DW, Bratman SV, Chan SM, Siu LL. Circulating tumor DNA and liquid biopsy in oncology. Nat Cancer. 2020;1(3):276–90.
Keller L, Belloum Y, Wikman H, Pantel K. Clinical relevance of blood-based ctDNA analysis: mutation detection and beyond. Br J Cancer. 2021;124(2):345–58.
Yu F, Makrigiorgos A, Leong KW, Makrigiorgos GM. Sensitive detection of microsatellite instability in tissues and liquid biopsies: Recent developments and updates. Comput Struct Biotechnol J. 2021;19:4931–40.
Russo A, Lopes AR, Scilla K, Mehra R, Adamo V, Oliveira J, et al. NTRK and NRG1 gene fusions in advanced non-small cell lung cancer (NSCLC). Precis Cancer Med. 2020 [citato 24 febbraio 2023];3(0). Disponibile su: https://pcm.amegroups.com/article/view/5551
Clark TA, Chung JH, Kennedy M, Hughes JD, Chennagiri N, Lieber DS, et al. Analytical validation of a hybrid capture-based next-generation sequencing clinical assay for genomic profiling of cell-free circulating tumor DNA. J Mol Diagn JMD. 2018;20(5):686–702.
André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380(20):1929–40.
André F, Ciruelos EM, Juric D, Loibl S, Campone M, Mayer IA, et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol Off J Eur Soc Med Oncol. 2021;32(2):208–17.
Juric D, Rodon J, Tabernero J, Janku F, Burris HA, Schellens JHM, et al. Phosphatidylinositol 3-Kinase α-selective inhibition with alpelisib (BYL719) in PIK3CA-Altered solid tumors: results from the first-in-human study. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(13):1291–9.
Rodon J, Curigliano G, Delord JP, Harb W, Azaro A, Han Y, et al. A Phase Ib, open-label, dose-finding study of alpelisib in combination with paclitaxel in patients with advanced solid tumors. Oncotarget. 2018;9(60):31709–18.
Rolfo C, Cardona AF, Cristofanilli M, Paz-Ares L, Diaz Mochon JJ, Duran I, et al. Challenges and opportunities of cfDNA analysis implementation in clinical practice: Perspective of the International Society of Liquid Biopsy (ISLB). Crit Rev Oncol Hematol. 2020;151:102978.
Siravegna G, Mussolin B, Buscarino M, Corti G, Cassingena A, Crisafulli G, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21(7):795–801.
García-Foncillas J, Alba E, Aranda E, Díaz-Rubio E, López-López R, Tabernero J, et al. Incorporating BEAMing technology as a liquid biopsy into clinical practice for the management of colorectal cancer patients: an expert taskforce review. Ann Oncol Off J Eur Soc Med Oncol. 2017;28(12):2943–9.
Bachet JB, Bouché O, Taieb J, Dubreuil O, Garcia ML, Meurisse A, et al. RAS mutation analysis in circulating tumor DNA from patients with metastatic colorectal cancer: the AGEO RASANC prospective multicenter study. Ann Oncol Off J Eur Soc Med Oncol. 2018;29(5):1211–9.
McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. 2017;168(4):613–28.
Onidani K, Shoji H, Kakizaki T, Yoshimoto S, Okaya S, Miura N, et al. Monitoring of cancer patients via next-generation sequencing of patient-derived circulating tumor cells and tumor DNA. Cancer Sci. 2019;110(8):2590–9.
Osumi H, Shinozaki E, Yamaguchi K, Zembutsu H. Early change in circulating tumor DNA as a potential predictor of response to chemotherapy in patients with metastatic colorectal cancer. Sci Rep. 2019;9(1):17358.
Ciardiello F, Normanno N, Martinelli E, Troiani T, Pisconti S, Cardone C, et al. Cetuximab continuation after first progression in metastatic colorectal cancer (CAPRI-GOIM): a randomized phase II trial of FOLFOX plus cetuximab versus FOLFOX. Ann Oncol Off J Eur Soc Med Oncol. 2016;27(6):1055–61.
Normanno N, Esposito Abate R, Lambiase M, Forgione L, Cardone C, Iannaccone A, et al. RAS testing of liquid biopsy correlates with the outcome of metastatic colorectal cancer patients treated with first-line FOLFIRI plus cetuximab in the CAPRI-GOIM trial. Ann Oncol Off J Eur Soc Med Oncol. 2018;29(1):112–8.
Thierry AR, El Messaoudi S, Mollevi C, Raoul JL, Guimbaud R, Pezet D, et al. Clinical utility of circulating DNA analysis for rapid detection of actionable mutations to select metastatic colorectal patients for anti-EGFR treatment. Ann Oncol Off J Eur Soc Med Oncol. 2017;28(9):2149–59.
Price TJ, Peeters M, Kim TW, Li J, Cascinu S, Ruff P, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 2014;15(6):569–79.
Siena S, Sartore-Bianchi A, Garcia-Carbonero R, Karthaus M, Smith D, Tabernero J, et al. Dynamic molecular analysis and clinical correlates of tumor evolution within a phase II trial of panitumumab-based therapy in metastatic colorectal cancer. Ann Oncol Off J Eur Soc Med Oncol. 2018;29(1):119–26.
Pietrantonio F, Vernieri C, Siravegna G, Mennitto A, Berenato R, Perrone F, et al. Heterogeneity of Acquired Resistance to Anti-EGFR Monoclonal Antibodies in Patients with Metastatic Colorectal Cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2017;23(10):2414–22.
Schrock AB, Pavlick D, Klempner SJ, Chung JH, Forcier B, Welsh A, et al. Hybrid capture-based genomic profiling of circulating tumor DNA from patients with advanced cancers of the gastrointestinal tract or anus. Clin Cancer Res Off J Am Assoc Cancer Res. 2018;24(8):1881–90.
Siravegna G, Sartore-Bianchi A, Nagy RJ, Raghav K, Odegaard JI, Lanman RB, et al. Plasma HER2 (ERBB2) copy number predicts response to HER2-targeted therapy in metastatic colorectal cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2019;25(10):3046–53.
Siravegna G, Lazzari L, Crisafulli G, Sartore-Bianchi A, Mussolin B, Cassingena A, et al. Radiologic and genomic evolution of individual metastases during HER2 blockade in colorectal cancer. Cancer Cell. 2018;34(1):148-162.e7.
Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. 2019;381(17):1632–43.
Cremolini C, Rossini D, Dell’Aquila E, Lonardi S, Conca E, Del Re M, et al. Rechallenge for patients With RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol. 2019;5(3):343–50.
Sartore-Bianchi A, Pietrantonio F, Lonardi S, Mussolin B, Rua F, Crisafulli G, et al. Circulating tumor DNA to guide rechallenge with panitumumab in metastatic colorectal cancer: the phase 2 CHRONOS trial. Nat Med. 2022;28(8):1612–8.
Listì A, Barraco N, Bono M, Insalaco L, Castellana L, Cutaia S, et al. Immuno-targeted combinations in oncogene-addicted non-small cell lung cancer. Transl Cancer Res. 2019;8(Suppl 1):S55-63.
Fenizia F, De Luca A, Pasquale R, Sacco A, Forgione L, Lambiase M, et al. EGFR mutations in lung cancer: from tissue testing to liquid biopsy. Future Oncol Lond Engl. 2015;11(11):1611–23.
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–25.
Passaro A, Jänne PA, Mok T, Peters S. Overcoming therapy resistance in EGFR-mutant lung cancer. Nat Cancer. 2021;2(4):377–91.
Gristina V, Barraco N, La Mantia M, Castellana L, Insalaco L, Bono M, et al. Clinical potential of circulating cell-free DNA (cfDNA) for longitudinally monitoring clinical outcomes in the first-line setting of non-small-cell lung cancer (NSCLC): a real-world prospective study. Cancers. 2022;14(23):6013.
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–40.
Wu W, Haderk F, Bivona TG. Non-canonical thinking for targeting ALK-Fusion onco-proteins in lung cancer. Cancers. 2017;9(12):164.
Shaw AT, Solomon BJ, Besse B, Bauer TM, Lin CC, Soo RA, et al. ALK resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2019;37(16):1370–9.
Gristina V, La Mantia M, Iacono F, Galvano A, Russo A, Bazan V. The emerging therapeutic landscape of ALK inhibitors in non-small cell lung cancer. Pharm Basel Switz. 2020;13(12):474.
Li W, Liu JB, Hou LK, Yu F, Zhang J, Wu W, et al. Liquid biopsy in lung cancer: significance in diagnostics, prediction, and treatment monitoring. Mol Cancer. 2022;21(1):25.
Chabon JJ, Simmons AD, Lovejoy AF, Esfahani MS, Newman AM, Haringsma HJ, et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat Commun. 2016;7:11815.
Patel A, Walters JN, Reuschel EL, Schultheis K, Parzych E, Gary EN, et al. Intradermal-delivered DNA vaccine induces durable immunity mediating a reduction in viral load in a rhesus macaque SARS-CoV-2 challenge model. Cell Rep Med. 2021;2(10):100420.
Wan JCM, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223–38.
Mosele F, Remon J, Mateo J, Westphalen CB, Barlesi F, Lolkema MP, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO precision medicine working group. Ann Oncol Off J Eur Soc Med Oncol. 2020;31(11):1491–505.
Page RD, Drusbosky LM, Dada H, Raymond VM, Daniel DB, Divers SG, et al. Clinical outcomes for plasma-based comprehensive genomic profiling versus standard-of-care tissue testing in advanced non-small cell lung cancer. Clin Lung Cancer. 2022;23(1):72–81.
Hann HW, Jain S, Park G, Steffen JD, Song W, Su YH. Detection of urine DNA markers for monitoring recurrent hepatocellular carcinoma. Hepatoma Res. 2017;3:105–11.
Mok T, Camidge DR, Gadgeel SM, Rosell R, Dziadziuszko R, Kim DW, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol Off J Eur Soc Med Oncol. 2020;31(8):1056–64.
Scherer F. Capturing Tumor Heterogeneity and Clonal Evolution by Circulating Tumor DNA Profiling. Recent Results Cancer Res Fortschritte Krebsforsch Progres Dans Rech Sur Cancer. 2020;215:213–30.
Mack PC, Miao J, Redman MW, Moon J, Goldberg SB, Herbst RS, et al. Circulating tumor DNA kinetics predict progression-free and overall survival in EGFR TKI-treated patients with EGFR-mutant NSCLC (SWOG S1403). Clin Cancer Res Off J Am Assoc Cancer Res. 2022;28(17):3752–60.
Iwata H, Im SA, Masuda N, Im YH, Inoue K, Rai Y, et al. PALOMA-3: phase III trial of fulvestrant with or without palbociclib in premenopausal and postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer that progressed on prior endocrine therapy-safety and efficacy in asian patients. J Glob Oncol. 2017;3(4):289–303.
O’Leary B, Cutts RJ, Liu Y, Hrebien S, Huang X, Fenwick K, et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov. 2018;8(11):1390–403.
Turner NC, Kingston B, Kilburn LS, Kernaghan S, Wardley AM, Macpherson IR, et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): a multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 2020;21(10):1296–308.
Kingston B, Cutts RJ, Bye H, Beaney M, Walsh-Crestani G, Hrebien S, et al. Genomic profile of advanced breast cancer in circulating tumour DNA. Nat Commun. 2021;12(1):2423.
Hrebien S, Citi V, Garcia-Murillas I, Cutts R, Fenwick K, Kozarewa I, et al. Early ctDNA dynamics as a surrogate for progression-free survival in advanced breast cancer in the BEECH trial. Ann Oncol Off J Eur Soc Med Oncol. 2019;30(6):945–52.
Galvano A, Castellana L, Gristina V, La Mantia M, Insalaco L, Barraco N, et al. The diagnostic accuracy of PIK3CA mutations by circulating tumor DNA in breast cancer: an individual patient data meta-analysis. Ther Adv Med Oncol. 2022;14:17588359221110162.
Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5):e192535.
Passiglia F, Galvano A, Gristina V, Barraco N, Castiglia M, Perez A, et al. Is there any place for PD-1/CTLA-4 inhibitors combination in the first-line treatment of advanced NSCLC?-a trial-level meta-analysis in PD-L1 selected subgroups. Transl Lung Cancer Res. 2021;10(7):3106–19.
Sivapalan L, Murray JC, Canzoniero JV, Landon B, Jackson J, Scott S, et al. Liquid biopsy approaches to capture tumor evolution and clinical outcomes during cancer immunotherapy. J Immunother Cancer. 2023;11(1):e005924.
Incorvaia L, Fanale D, Badalamenti G, Barraco N, Bono M, Corsini LR, et al. Programmed Death Ligand 1 (PD-L1) as a Predictive Biomarker for Pembrolizumab Therapy in Patients with Advanced Non-Small-Cell Lung Cancer (NSCLC). Adv Ther. 2019;36(10):2600–17.
Gristina V, Galvano A, Castellana L, Insalaco L, Cusenza S, Graceffa G, et al. Is there any room for PD-1 inhibitors in combination with platinum-based chemotherapy as frontline treatment of extensive-stage small cell lung cancer? A systematic review and meta-analysis with indirect comparisons among subgroups and landmark survival analyses. Ther Adv Med Oncol. 2021;13:17588359211018018.
Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med. 2017;377(25):2500–1.
Pepe F, Pisapia P, Gristina V, Rocco D, Micheli M, Micheli P, et al. Tumor mutational burden on cytological samples: a pilot study. Cancer Cytopathol. 2021;129(6):460–7.
Hofman P, Heeke S, Alix-Panabières C, Pantel K. Liquid biopsy in the era of immuno-oncology: is it ready for prime-time use for cancer patients? Ann Oncol Off J Eur Soc Med Oncol. 2019;30(9):1448–59.
Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371(6529):595–602.
Peters S, Dziadziuszko R, Morabito A, Felip E, Gadgeel SM, Cheema P, et al. Atezolizumab versus chemotherapy in advanced or metastatic NSCLC with high blood-based tumor mutational burden: primary analysis of BFAST cohort C randomized phase 3 trial. Nat Med. 2022;28(9):1831–9.
He J, Kalinava N, Doshi P, Ma J, Pavlick DC, Albacker LA, Tukachinsky H, Fusaro G, Oxnard GR, Green G, Fabrizio D. Evaluation of tissue-and plasma-derived tumor mutational burden and genomic alterations of interest from the CheckMate 848 clinical trial. Cancer Res. 2022;82(12_Supplement):2139-39. https://doi.org/10.1158/1538-7445.AM2022-2139.
Schenker M, Burotto M, Richardet M, Ciuleanu T, Goncalves A, Steeghs N, Schoffski P, Ascierto PA, Maio M, Lugowska I, Lupinacci L. CheckMate 848: a randomized, open-label, phase 2 study of nivolumab in combination with ipilimumab or nivolumab monotherapy in patients with advanced or metastatic solid tumors of high tumor mutational burden. In: Cancer Research 2022 Jun 15 (Vol. 82, No. 12). 615 Chestnut St, 17th Floor, Philadelphia, PA 19106-4404 USA: Amer Assoc Cancer Research; 2022. https://doi.org/10.1158/1538-7445.AM2022-CT022.
de Castro G, Rizvi NA, Schmid P, Syrigos K, Martin C, Yamamoto N, et al. NEPTUNE: phase 3 study of first-line durvalumab plus tremelimumab in patients with metastatic NSCLC. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2023;18(1):106–19.
Si H, Kuziora M, Quinn KJ, Helman E, Ye J, Liu F, et al. A Blood-based Assay for Assessment of Tumor Mutational Burden in First-line Metastatic NSCLC Treatment: Results from the MYSTIC Study. Clin Cancer Res Off J Am Assoc Cancer Res. 2021;27(6):1631–40.
Wang X, Li M. Correlate tumor mutation burden with immune signatures in human cancers. BMC Immunol. 2019;20:1–3. https://doi.org/10.1186/s12865-018-0285-5.
Khagi Y, Goodman AM, Daniels GA, Patel SP, Sacco AG, Randall JM, Bazhenova LA, Kurzrock R. Hypermutated circulating tumor DNA: correlation with response to checkpoint inhibitor–based immunotherapy. Clin Cancer Res. 2017;23(19):5729–36. https://doi.org/10.1158/1078-0432.CCR-17-1439.
Georgiadis A, Durham JN, Keefer LA, Bartlett BR, Zielonka M, Murphy D, et al. Noninvasive detection of microsatellite instability and high tumor mutation burden in cancer patients treated with PD-1 blockade. Clin Cancer Res Off J Am Assoc Cancer Res. 2019;25(23):7024–34.
Bratman SV, Yang SC, Iafolla MA, Liu Z, Hansen AR, Bedard PL, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer. 2020;1(9):873–81. https://doi.org/10.1038/s43018-020-0096-5.
Váraljai R, Wistuba-Hamprecht K, Seremet T, Diaz JMS, Nsengimana J, Sucker A, et al. Application of circulating cell-free tumor DNA Profiles for therapeutic monitoring and outcome prediction in genetically heterogeneous metastatic melanoma. JCO Precis Oncol. 2019;3:1–10.
Guibert N, Jones G, Beeler JF, Plagnol V, Morris C, Mourlanette J, et al. Targeted sequencing of plasma cell-free DNA to predict response to PD1 inhibitors in advanced non-small cell lung cancer. Lung Cancer Amst Neth. 2019;137:1–6.
Goldberg SB, Narayan A, Kole AJ, Decker RH, Teysir J, Carriero NJ, et al. Early Assessment of Lung Cancer Immunotherapy Response via Circulating Tumor DNA. Clin Cancer Res Off J Am Assoc Cancer Res. 2018;24(8):1872–80.
Kim WT, Kim YH, Jeong P, Seo SP, Kang HW, Kim YJ, et al. Urinary cell-free nucleic acid IQGAP3: a new non-invasive diagnostic marker for bladder cancer. Oncotarget. 2018;9(18):14354–65.
Ilié M, Szafer-Glusman E, Hofman V, Chamorey E, Lalvée S, Selva E, et al. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann Oncol Off J Eur Soc Med Oncol. 2018;29(1):193–9.
Guibert N, Delaunay M, Lusque A, Boubekeur N, Rouquette I, Clermont E, et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer Amst Neth. 2018;120:108–12.
Nicolazzo C, Raimondi C, Mancini M, Caponnetto S, Gradilone A, Gandini O, et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci Rep. 2016;6:31726.
Hogan SA, Courtier A, Cheng PF, Jaberg-Bentele NF, Goldinger SM, Manuel M, et al. Peripheral blood TCR repertoire profiling may facilitate patient stratification for immunotherapy against melanoma. Cancer Immunol Res. 2019;7(1):77–85.
Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S, et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med. 2016;22(4):433–8.
Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545(7652):60–5.
Bian B, Fanale D, Dusetti N, Roque J, Pastor S, Chretien AS, et al. Prognostic significance of circulating PD-1, PD-L1, pan-BTN3As, BTN3A1 and BTLA in patients with pancreatic adenocarcinoma. Oncoimmunology. 2019;8(4):e1561120.
Incorvaia L, Badalamenti G, Rinaldi G, Iovanna JL, Olive D, Swayden M, et al. Can the plasma PD-1 levels predict the presence and efficiency of tumor-infiltrating lymphocytes in patients with metastatic melanoma? Ther Adv Med Oncol. 2019;11:1758835919848872.
Fanale D, Incorvaia L, Badalamenti G, De Luca I, Algeri L, Bonasera A, et al. Prognostic role of plasma PD-1, PD-L1, pan-BTN3As and BTN3A1 in patients affected by metastatic gastrointestinal stromal tumors: can immune checkpoints act as a sentinel for short-term survival? Cancers. 2021;13(9):2118.
Incorvaia L, Fanale D, Badalamenti G, Porta C, Olive D, De Luca I, et al. Baseline plasma levels of soluble PD-1, PD-L1, and BTN3A1 predict response to nivolumab treatment in patients with metastatic renal cell carcinoma: a step toward a biomarker for therapeutic decisions. Oncoimmunology. 2020;9(1):1832348.
Incorvaia L, Fanale D, Badalamenti G, Brando C, Bono M, De Luca I, et al. A «Lymphocyte MicroRNA Signature» as predictive biomarker of immunotherapy response and plasma PD-1/PD-L1 expression levels in patients with metastatic renal cell carcinoma: pointing towards epigenetic reprogramming. Cancers. 2020;12(11):3396.
Incorvaia L, Rinaldi G, Badalamenti G, Cucinella A, Brando C, Madonia G, et al. Prognostic role of soluble PD-1 and BTN2A1 in overweight melanoma patients treated with nivolumab or pembrolizumab: finding the missing links in the symbiotic immune-metabolic interplay. Ther Adv Med Oncol. 2023;15:17588359231151844.
Galvano A, Gristina V, Malapelle U, Pisapia P, Pepe F, Barraco N, et al. The prognostic impact of tumor mutational burden (TMB) in the first-line management of advanced non-oncogene addicted non-small-cell lung cancer (NSCLC): a systematic review and meta-analysis of randomized controlled trials. ESMO Open. 2021;6(3):100124.
Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973–85.
Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–58.
He Y, Zhang X, Zhu M, He W, Hua H, Ye F, et al. Soluble PD-L1: a potential dynamic predictive biomarker for immunotherapy in patients with proficient mismatch repair colorectal cancer. J Transl Med. 2023;21(1):25.
Mathew M, Zade M, Mezghani N, Patel R, Wang Y, Momen-Heravi F. Extracellular Vesicles as Biomarkers in Cancer Immunotherapy. Cancers. 2020;12(10):2825.
Lane RE, Korbie D, Hill MM, Trau M. Extracellular vesicles as circulating cancer biomarkers: opportunities and challenges. Clin Transl Med. 2018;7(1):14.
Liu SY, Liao Y, Hosseinifard H, Imani S, Wen QL. Diagnostic role of extracellular vesicles in cancer: a comprehensive systematic review and meta-analysis. Front Cell Dev Biol. 2021;9:705791.
Cammarata G, Barraco N, Giusti I, Gristina V, Dolo V, Taverna S. Extracellular vesicles-ceRNAs as ovarian cancer biomarkers: looking into circRNA-miRNA-mRNA code. Cancers. 2022;14(14):3404.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977.
Majood M, Rawat S, Mohanty S. Delineating the role of extracellular vesicles in cancer metastasis: a comprehensive review. Front Immunol. 2022;13:966661.
Soekmadji C, Li B, Huang Y, Wang H, An T, Liu C, et al. The future of extracellular vesicles as theranostics - an ISEV meeting report. J Extracell Vesicle. 2020;9(1):1809766.
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750.
Kang T, Atukorala I, Mathivanan S. Biogenesis of extracellular vesicles. Subcell Biochem. 2021;97:19–43.
Palviainen M, Saraswat M, Varga Z, Kitka D, Neuvonen M, Puhka M, et al. Extracellular vesicles from human plasma and serum are carriers of extravesicular cargo-Implications for biomarker discovery. PLoS ONE. 2020;15(8):e0236439.
Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2.
Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269–88.
Amintas S, Vendrely V, Dupin C, Buscail L, Laurent C, Bournet B, et al. Next-generation cancer biomarkers: extracellular vesicle DNA as a circulating surrogate of tumor DNA. Front Cell Dev Biol. 2020;8:622048.
Cai X, Janku F, Zhan Q, Fan JB. Accessing genetic information with liquid biopsies. Trends Genet TIG. 2015;31(10):564–75.
Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–35.
Leetanaporn K, Hanprasertpong J, Navakanitworakul R. Molecular insights and clinical impacts of extracellular vesicles in cancer. Oncol Rev. 2021;15(2):542.
Hosseini K, Ranjbar M, Pirpour Tazehkand A, Asgharian P, Montazersaheb S, Tarhriz V, et al. Evaluation of exosomal non-coding RNAs in cancer using high-throughput sequencing. J Transl Med. 2022;20(1):30.
de Miguel-Perez D, Russo A, Arrieta O, Ak M, Barron F, Gunasekaran M, et al. Extracellular vesicle PD-L1 dynamics predict durable response to immune-checkpoint inhibitors and survival in patients with non-small cell lung cancer. J Exp Clin Cancer Res CR. 2022;41(1):186.
Svedman FC, Lohcharoenkal W, Bottai M, Brage SE, Sonkoly E, Hansson J, et al. Extracellular microvesicle microRNAs as predictive biomarkers for targeted therapy in metastastic cutaneous malignant melanoma. PLoS ONE. 2018;13(11):e0206942.
Corcoran C, Rani S, O’Driscoll L. miR-34a is an intracellular and exosomal predictive biomarker for response to docetaxel with clinical relevance to prostate cancer progression. Prostate. 2014;74(13):1320–34.
Vinik Y, Ortega FG, Mills GB, Lu Y, Jurkowicz M, Halperin S, et al. Proteomic analysis of circulating extracellular vesicles identifies potential markers of breast cancer progression, recurrence, and response. Sci Adv. 2020;6(40):eaba5714.
Bernard V, Kim DU, San Lucas FA, Castillo J, Allenson K, Mulu FC, et al. Circulating nucleic acids are associated with outcomes of patients with pancreatic cancer. Gastroenterology. 2019;156(1):108-118.e4.
Castellanos-Rizaldos E, Grimm DG, Tadigotla V, Hurley J, Healy J, Neal PL, et al. Exosome-based detection of EGFR T790M in plasma from non-small cell lung cancer patients. Clin Cancer Res Off J Am Assoc Cancer Res. 2018;24(12):2944–50.
Ma C, Jiang F, Ma Y, Wang J, Li H, Zhang J. Isolation and detection technologies of extracellular vesicles and application on cancer diagnostic. Dose-Response Publ Int Hormesis Soc. 2019;17(4):1559325819891004.
Heitzer E, Haque IS, Roberts CES, Speicher MR. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet. 2019;20(2):71–88.
Tivey A, Church M, Rothwell D, Dive C, Cook N. Circulating tumour DNA - looking beyond the blood. Nat Rev Clin Oncol. 2022;19(9):600–12.
Oshi M, Murthy V, Takahashi H, Huyser M, Okano M, Tokumaru Y, et al. Urine as a source of liquid biopsy for cancer. Cancers. 2021;13(11):2652.
Green EA, Li R, Albiges L, Choueiri TK, Freedman M, Pal S, et al. Clinical utility of cell-free and circulating tumor DNA in kidney and bladder cancer: a critical review of current literature. Eur Urol Oncol. 2021;4(6):893–903.
Wang X, Meng Q, Wang C, Li F, Zhu Z, Liu S, et al. Investigation of transrenal KRAS mutation in late stage NSCLC patients correlates to disease progression. Biomark Biochem Indic Expo Response Susceptibility Chem. 2017;22(7):654–60.
Xie F, Li P, Gong J, Tan H, Ma J. Urinary cell-free DNA as a prognostic marker for KRAS-positive advanced-stage NSCLC. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex. 2018;20(5):591–8.
Wang Z, Wang X, Zhang D, Yu Y, Cai L, Zhang C. Long non-coding RNA urothelial carcinoma-associated 1 as a tumor biomarker for the diagnosis of urinary bladder cancer. Tumour Biol J Int Soc Oncodevelopmental Biol Med. 2017;39(6):1010428317709990.
Yu H, Han L, Yuan J, Sun Y. Circulating tumor cell free DNA from plasma and urine in the clinical management of colorectal cancer. Cancer Biomark Sect Dis Markers. 2020;27(1):29–37.
Tian F, Liao Y, Zhang Y. Variations in transrenal DNA and comparison with plasma DNA as a diagnostic marker for colorectal cancer. Int J Biol Markers. 2017;32(4):e434-40.
Zuo Z, Tang J, Cai X, Ke F, Shi Z. Probing of breast cancer using a combination of plasma and urinary circulating cell-free DNA. Biosci Rep. 2020;40(11):BSR20194306.
Zhang R, Zang J, Xie F, Zhang Y, Wang Y, Jing Y, et al. Urinary molecular pathology for patients with newly diagnosed urothelial bladder cancer. J Urol. 2021;206(4):873–84.
Oto J, Santillana N, Solmoirago MJ, Pérez AJ, Sánchez GJV, Plana E, et al. Mp21-11 diagnostic and prognostic value of urine circulating cell-free dna in renal cell carcinoma. J Urol. 2019;201(Supplement 4):e296–e296.
Springer SU, Chen CH, Rodriguez Pena MDC, Li L, Douville C, Wang Y, et al. Non-invasive detection of urothelial cancer through the analysis of driver gene mutations and aneuploidy. eLife. 2018;7:e32143.
Dudley JC, Schroers-Martin J, Lazzareschi DV, Shi WY, Chen SB, Esfahani MS, et al. Detection and surveillance of bladder cancer using urine tumor DNA. Cancer Discov. 2019;9(4):500–9.
Nuzzo PV, Berchuck JE, Korthauer K, Spisak S, Nassar AH, Abou Alaiwi S, et al. Detection of renal cell carcinoma using plasma and urine cell-free DNA methylomes. Nat Med. 2020;26(7):1041–3.
Casadio V, Calistri D, Salvi S, Gunelli R, Carretta E, Amadori D, et al. Urine cell-free DNA integrity as a marker for early prostate cancer diagnosis: a pilot study. BioMed Res Int. 2013;2013:270457.
McKiernan J, Donovan MJ, O’Neill V, Bentink S, Noerholm M, Belzer S, et al. A novel urine exosome gene expression assay to predict high-grade prostate cancer at initial biopsy. JAMA Oncol. 2016;2(7):882–9.
Sanguedolce F, Cormio A, Brunelli M, D’Amuri A, Carrieri G, Bufo P, et al. Urine TMPRSS2: ERG fusion transcript as a biomarker for prostate cancer: literature review. Clin Genitourin Cancer. 2016;14(2):117–21.
Groskopf J, Aubin SMJ, Deras IL, Blase A, Bodrug S, Clark C, et al. APTIMA PCA3 molecular urine test: development of a method to aid in the diagnosis of prostate cancer. Clin Chem. 2006;52(6):1089–95.
Whitman EJ, Groskopf J, Ali A, Chen Y, Blase A, Furusato B, et al. PCA3 score before radical prostatectomy predicts extracapsular extension and tumor volume. J Urol. 2008;180(5):1975–8 discussion 1978-1979.
Tutrone R, Donovan MJ, Torkler P, Tadigotla V, McLain T, Noerholm M, et al. Clinical utility of the exosome based ExoDx Prostate(IntelliScore) EPI test in men presenting for initial Biopsy with a PSA 2–10 ng/mL. Prostate Cancer Prostatic Dis. 2020;23(4):607–14.
Hendriks RJ, van der Leest MMG, Israël B, Hannink G, YantiSetiasti A, Cornel EB, et al. Clinical use of the SelectMDx urinary-biomarker test with or without mpMRI in prostate cancer diagnosis: a prospective, multicenter study in biopsy-naïve men. Prostate Cancer Prostatic Dis. 2021;24(4):1110–9.
Srivastava AK, Singh PK, Rath SK, Dalela D, Goel MM, Bhatt MLB. Appraisal of diagnostic ability of UCA1 as a biomarker of carcinoma of the urinary bladder. Tumour Biol J Int Soc Oncodevelopmental Biol Med. 2014;35(11):11435–42.
Berz D, Raymond VM, Garst JH, Erlander MG. Non-invasive urine testing of EGFR activating mutation and T790M resistance mutation in non-small cell lung cancer. Exp Hematol Oncol. 2015;5:24.
Chen W, Liao Y, Yang C, Fang Z, Liu B, Zheng X, et al. Potential use of transrenal DNA for non-invasive monitoring and prognosis of colorectal cancer. Biomark Biochem Indic Expo Response Susceptibility Chem. 2019;24(6):524–9.
Smith CG, Moser T, Mouliere F, Field-Rayner J, Eldridge M, Riediger AL, et al. Comprehensive characterization of cell-free tumor DNA in plasma and urine of patients with renal tumors. Genome Med. 2020;12(1):23.
Silva R, Moran B, Russell NM, Fahey C, Vlajnic T, Manecksha RP, et al. Evaluating liquid biopsies for methylomic profiling of prostate cancer. Epigenetics. 2020;15(6–7):715–27.
Augustus E, Van Casteren K, Sorber L, van Dam P, Roeyen G, Peeters M, et al. The art of obtaining a high yield of cell-free DNA from urine. PLoS ONE. 2020;15(4):e0231058.
Kim WT, Jeong P, Yan C, Kim YH, Lee IS, Kang HW, et al. UBE2C cell-free RNA in urine can discriminate between bladder cancer and hematuria. Oncotarget. 2016;7(36):58193–202.
Qu L, Ding J, Chen C, Wu ZJ, Liu B, Gao Y, et al. Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell. 2016;29(5):653–68.
Iliev R, Fedorko M, Machackova T, Mlcochova H, Svoboda M, Pacik D, et al. Expression levels of PIWI-interacting RNA, piR-823, are deregulated in tumor tissue, blood serum and urine of patients with renal cell carcinoma. Anticancer Res. 2016;36(12):6419–23.
Chen X, Chen RX, Wei WS, Li YH, Feng ZH, Tan L, et al. PRMT5 Circular RNA promotes metastasis of urothelial carcinoma of the bladder through sponging miR-30c to induce epithelial-mesenchymal transition. Clin Cancer Res Off J Am Assoc Cancer Res. 2018;24(24):6319–30.
Zimmermann BG, Park NJ, Wong DT. Genomic targets in saliva. Ann N Y Acad Sci. 2007;1098:184–91.
Kaczor-Urbanowicz KE, Wei F, Rao SL, Kim J, Shin H, Cheng J, et al. Clinical validity of saliva and novel technology for cancer detection. Biochim Biophys Acta Rev Cancer. 2019;1872(1):49–59.
Sethi S, Benninger MS, Lu M, Havard S, Worsham MJ. Noninvasive molecular detection of head and neck squamous cell carcinoma: an exploratory analysis. Diagn Mol Pathol Am J Surg Pathol Part B. 2009;18(2):81–7.
Ahn SM, Chan JYK, Zhang Z, Wang H, Khan Z, Bishop JA, et al. Saliva and plasma quantitative polymerase chain reaction-based detection and surveillance of human papillomavirus-related head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2014;140(9):846–54.
Hanna GJ, Lau CJ, Mahmood U, Supplee JG, Mogili AR, Haddad RI, et al. Salivary HPV DNA informs locoregional disease status in advanced HPV-associated oropharyngeal cancer. Oral Oncol. 2019;95:120–6.
Ding S, Song X, Geng X, Liu L, Ma H, Wang X, et al. Saliva-derived cfDNA is applicable for EGFR mutation detection but not for quantitation analysis in non-small cell lung cancer. Thorac Cancer. 2019;10(10):1973–83.
Wei F, Lin CC, Joon A, Feng Z, Troche G, Lira ME, et al. Noninvasive saliva-based EGFR gene mutation detection in patients with lung cancer. Am J Respir Crit Care Med. 2014;190(10):1117–26.
Pu D, Liang H, Wei F, Akin D, Feng Z, Yan Q, et al. Evaluation of a novel saliva-based epidermal growth factor receptor mutation detection for lung cancer: a pilot study. Thorac Cancer. 2016;7(4):428–36.
Li F, Wei F, Liao W, Huang WL, Lin C chung, Chia D, et al. EFIRM liquid biopsy (eLB): Detection of ultra-short circulating tumor DNA (usctDNA) in plasma and saliva of non-small cell lung cancer (NSCLC) patients. J Clin Oncol. 2018;36(15_suppl):e24062–e24062.
Li F, Wei F, Huang WL, Lin CC, Li L, Shen MM, et al. Ultra-short circulating tumor DNA (usctDNA) in plasma and saliva of non-small cell lung cancer (NSCLC) patients. Cancers. 2020;12(8):2041.
Li Y, St John MAR, Zhou X, Kim Y, Sinha U, Jordan RCK, et al. Salivary transcriptome diagnostics for oral cancer detection. Clin Cancer Res Off J Am Assoc Cancer Res. 2004;10(24):8442–50.
Elashoff D, Zhou H, Reiss J, Wang J, Xiao H, Henson B, et al. Prevalidation of salivary biomarkers for oral cancer detection. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2012;21(4):664–72.
Bu J, Bu X, Liu B, Chen F, Chen P. Increased Expression of Tissue/Salivary Transgelin mRNA Predicts Poor Prognosis in Patients with Oral Squamous Cell Carcinoma (OSCC). Med Sci Monit Int Med J Exp Clin Res. 2015;21:2275–81.
Chai RC, Lim Y, Frazer IH, Wan Y, Perry C, Jones L, et al. A pilot study to compare the detection of HPV-16 biomarkers in salivary oral rinses with tumour p16(INK4a) expression in head and neck squamous cell carcinoma patients. BMC Cancer. 2016;16:178.
Han B, Chao J, Yao H. Circular RNA and its mechanisms in disease: from the bench to the clinic. Pharmacol Ther. 2018;187:31–44.
Zahran F, Ghalwash D, Shaker O, Al-Johani K, Scully C. Salivary microRNAs in oral cancer. Oral Dis. 2015;21(6):739–47.
Wu L, Zheng K, Yan C, Pan X, Liu Y, Liu J, et al. Genome-wide study of salivary microRNAs as potential noninvasive biomarkers for detection of nasopharyngeal carcinoma. BMC Cancer. 2019;19(1):843.
Uma Maheswari TN, Nivedhitha MS, Ramani P. Expression profile of salivary micro RNA-21 and 31 in oral potentially malignant disorders. Braz Oral Res. 2020;34:e002.
Ahmad P, Sana J, Slavik M, Gurin D, Radova L, Gablo NA, et al. MicroRNA-15b-5p predicts locoregional relapse in head and neck carcinoma patients treated with intensity-modulated radiotherapy. Cancer Genomics Proteomics. 2019;16(2):139–46.
Tang H, Wu Z, Zhang J, Su B. Salivary lncRNA as a potential marker for oral squamous cell carcinoma diagnosis. Mol Med Rep. 2013;7(3):761–6.
Bahn JH, Zhang Q, Li F, Chan TM, Lin X, Kim Y, et al. The landscape of microRNA, Piwi-interacting RNA, and circular RNA in human saliva. Clin Chem. 2015;61(1):221–30.
Zhao SY, Wang J, Ouyang SB, Huang ZK, Liao L. Salivary Circular RNAs Hsa_Circ_0001874 and Hsa_Circ_0001971 as Novel Biomarkers for the Diagnosis of Oral Squamous Cell Carcinoma. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2018;47(6):2511–21.
Li K, Lin Y, Luo Y, Xiong X, Wang L, Durante K, et al. A signature of saliva-derived exosomal small RNAs as predicting biomarker for esophageal carcinoma: a multicenter prospective study. Mol Cancer. 2022;21(1):21.
Patel A, Patel S, Patel P, Tanavde V. Saliva based liquid biopsies in head and neck cancer: how far are we from the clinic? Front Oncol. 2022;12:828434.
Greither T, Vorwerk F, Kappler M, Bache M, Taubert H, Kuhnt T, et al. Salivary miR-93 and miR-200a as post-radiotherapy biomarkers in head and neck squamous cell carcinoma. Oncol Rep. 2017;38(2):1268–75.
Yuvaraj M, Udayakumar K, Jayanth V, Prakasa Rao A, Bharanidharan G, Koteeswaran D, et al. Fluorescence spectroscopic characterization of salivary metabolites of oral cancer patients. J Photochem Photobiol B. 2014;130:153–60.
Deo PN, Deshmukh R. Oral microbiome and oral cancer - The probable nexus. J Oral Maxillofac Pathol JOMFP. 2020;24(2):361–7.
Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME, Colorectal Cancer Study Group. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med. 2004;351(26):2704–14.
Prince M, Lester L, Chiniwala R, Berger B. Multitarget stool DNA tests increases colorectal cancer screening among previously noncompliant medicare patients. World J Gastroenterol. 2017;23(3):464–71.
Redwood DG, Asay ED, Blake ID, Sacco PE, Christensen CM, Sacco FD, et al. Stool DNA testing for screening detection of colorectal neoplasia in Alaska native people. Mayo Clin Proc. 2016;91(1):61–70.
Naber SK, Knudsen AB, Zauber AG, Rutter CM, Fischer SE, Pabiniak CJ, et al. Cost-effectiveness of a multitarget stool DNA test for colorectal cancer screening of Medicare beneficiaries. PLoS ONE. 2019;14(9):e0220234.
Caldas C, Hahn SA, Hruban RH, Redston MS, Yeo CJ, Kern SE. Detection of K-ras mutations in the stool of patients with pancreatic adenocarcinoma and pancreatic ductal hyperplasia. Cancer Res. 1994;54(13):3568–73.
Youssef O, Sarhadi V, Ehsan H, Böhling T, Carpelan-Holmström M, Koskensalo S, et al. Gene mutations in stool from gastric and colorectal neoplasia patients by next-generation sequencing. World J Gastroenterol. 2017;23(47):8291–9.
Allen-Vercoe E, Coburn B. A microbiota-derived metabolite augments cancer immunotherapy responses in mice. Cancer Cell. 2020;38(4):452–3.
Xu X, Lv J, Guo F, Li J, Jia Y, Jiang D, et al. Gut microbiome influences the efficacy of PD-1 antibody immunotherapy on MSS-type colorectal cancer via metabolic pathway. Front Microbiol. 2020;11:814.
Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371(6529):602–9.
Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–9.
Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–84.
Wu CW, Ng SSM, Dong YJ, Ng SC, Leung WW, Lee CW, et al. Detection of miR-92a and miR-21 in stool samples as potential screening biomarkers for colorectal cancer and polyps. Gut. 2012;61(5):739–45.
Raut JR, Schöttker B, Holleczek B, Guo F, Bhardwaj M, Miah K, et al. A microRNA panel compared to environmental and polygenic scores for colorectal cancer risk prediction. Nat Commun. 2021;12(1):4811.
Liu H, Gong W, Lou J, Ju H, Yin X, Liu Y, et al. MicroRNA-21 and microRNA-146a identification in stool and its clinical significance in colorectal neoplasms. 2016;9:16441–9
Bastaminejad S, Taherikalani M, Ghanbari R, Akbari A, Shabab N, Saidijam M. Investigation of MicroRNA-21 expression levels in serum and stool as a potential non-invasive biomarker for diagnosis of colorectal cancer. Iran Biomed J. 2017;21(2):106–13.
Phua LC, Chue XP, Koh PK, Cheah PY, Chan ECY, Ho HK. Global fecal microRNA profiling in the identification of biomarkers for colorectal cancer screening among Asians. Oncol Rep. 2014;32(1):97–104.
Duran-Sanchon S, Moreno L, Gómez-Matas J, Augé JM, Serra-Burriel M, Cuatrecasas M, et al. Fecal MicroRNA-based algorithm increases effectiveness of fecal immunochemical test-based screening for colorectal cancer. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2021;19(2):323-330.e1.
Duran-Sanchon S, Moreno L, Augé JM, Serra-Burriel M, Cuatrecasas M, Moreira L, et al. Identification and validation of MicroRNA profiles in fecal samples for detection of colorectal cancer. Gastroenterology. 2020;158(4):947-957.e4.
Gharib E, Nazemalhosseini-Mojarad E, Baghdar K, Nayeri Z, Sadeghi H, Rezasoltani S, et al. Identification of a stool long non-coding RNAs panel as a potential biomarker for early detection of colorectal cancer. J Clin Lab Anal. 2021;35(2):e23601.
Zhao Z, Zhu A, Bhardwaj M, Schrotz-King P, Brenner H. Fecal microRNAs, fecal microRNA Panels, or combinations of fecal microRNAs with fecal hemoglobin for early detection of colorectal cancer and its precursors: a systematic review. Cancers. 2021;14(1):65.
Klaassen CHW, Jeunink MAF, Prinsen CFM, Ruers TJM, Tan ACITL, Strobbe LJA, et al. Quantification of human DNA in feces as a diagnostic test for the presence of colorectal cancer. Clin Chem. 2003;49(7):1185–7.
van Dam L, Korfage IJ, Kuipers EJ, Hol L, van Roon AHC, Reijerink JCIY, et al. What influences the decision to participate in colorectal cancer screening with faecal occult blood testing and sigmoidoscopy? Eur J Cancer Oxf Engl 1990. 2013;49(10):2321–30.
Osborne JM, Flight I, Wilson CJ, Chen G, Ratcliffe J, Young GP. The impact of sample type and procedural attributes on relative acceptability of different colorectal cancer screening regimens. Patient Prefer Adherence. 2018;12:1825–36.
Funding
Other than regular employment, the authors received no financial support for the research, authorship and/or publication of this article.
Author information
Authors and Affiliations
Contributions
DS was responsible to coordinate the concept, design, and drafting of manuscript and has the first authorship. AB and SS were responsible for performing literature search, extracting data and contributed to the drafting of manuscript. AG, LI, VG and AR were responsible for performing literature search, extracting data and contributed to the drafting of manuscript. MI, SF, SS and FP were responsible for performing literature search, extracting data and contributed to the drafting of manuscript. CT and GP were responsible for performing literature search, extracting data and contributed to the drafting of manuscript. EP and EDF were responsible for performing literature search, extracting data and contributed to the drafting of manuscript. FF and GPS supervised the elaboration of the design and drafting of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
GP reports research funding (to Institution) from Astrazeneca, Exact Sciences, Diapath; personal honoraria as invited speaker from Amgen, AstraZeneca, Bio-Optica, Boehringer-Ingelheim; Diatech Pharmacogenetics, Exact Sciences, GlaxoSmithKline, Incyte, Janssen-Cilag; Lilly, Novartis, Roche, Merck Serono, Veracyte; participation in advisory board for Amgen, Astrazeneca, Novartis, Exact Sciences, Roche.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Santini, D., Botticelli, A., Galvano, A. et al. Network approach in liquidomics landscape. J Exp Clin Cancer Res 42, 193 (2023). https://doi.org/10.1186/s13046-023-02743-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13046-023-02743-9