Abstract
With cancer stem cells (CSCs) became the research hotspot, emerging studies attempt to reveal the functions of these special subsets in tumorigenesis. Although various approaches have been used in CSCs researches, only a few could really reflect or simulate the microenvironment in vivo. At present, CSCs theories are still difficult to apply for clinical remedy because CSCs subpopulations are always hard to identify and trace. Thus an ideal approach for clinicians and researchers is urgently needed. Circulating tumor cells (CTCs), as the method of noninvasive-liquid biopsy, could be detected in the peripheral blood (PB) from many tumors and even could be treated as procurators for CSCs deeper researches from patient-derived sample. However, CTCs, as a diagnostic marker, also raise much controversy over theirs clinical value. Mechanisms causing CTCs to shed from the tumor have not been fully characterized, thus it is unclear whether CTCs represent the entire makeup of cancer cells in the tumor or only a subset. The heterogeneity of CTCs also caused different clinical outcomes. To overcome these unsolved problems, recently, CTC researches are not just depend on enumerations, whereas those CTC subsets that could expand in vitro may play a pivotal role in the metastatic cascade. Here, we retrospect the CTC developmental history and discourse upon the enrichment of viable CTCs in functional assays, probe the further avenue at the crossroad.
Similar content being viewed by others
Background
For decades, tumor formation and development has been regarded as a mysterious issue, compelling scientists to seek the mechanism of origin. Much evidence hinted that some small subpopulations of tumorigenic cells were the causation of tumor recurrence and metastasis, but it may be difficult to draw definitive conception because of the lack of rigorous model and effective methods to identify these special subpopulations. Since 1960, when the Philadelphia (Ph) chromosome and its unique association with chronic myeloid leukaemia (CML) were discovered [1], evidence has been found that the appearance of clonal chromosomal aberrations caused abnormal cell proliferation in bone marrow. These pathological cells could be the culprit of tumorigenesis. Further research also found that these cells (Ph+) were always detected in circulation [2]. From then, cells with special markers had been noticed by researchers. The concept of cancer stem cell (CSCs) began to appear in the mid-1990s by isolating rare cells in the blood of patient with leukemia, these cells were capable to grow into a new leukemia when injected into mice [3]. The early discoveries contributed CSCs to become the hotspot and thus diverse CSC models were emerged subsequently. Many studies provided proof for the CSC hypotheses and managed to address and deduct the process of tumor initiation and development. Unfortunately, these relative hypotheses had not got the final conclusion [4,5,6,7], none could perfectly illustrate the details of every step in tumorigenesis and its relapse. It is still unknown about which CSCs paradigm is really suitable for modern clinical therapy. And now to solve these unsettled arguments, more researchers expect to focus on a single-cell level, which could have more convincing to reveal mechanisms of CSC. Therefore, the development of single-cell diagnostic methods is flourishing these years. Circulating tumor cells (CTCs) in the peripheral blood (PB) from different types of tumors are increasingly detected by various methods. However, the mechanisms causing CTCs to shed from the tumor have not been fully characterized, thus it is unclear whether CTCs represent the entire makeup of cancer cells in the tumor or only a subpopulation [8]. Nevertheless those CTC subsets, with CSCs feature, could expand in vitro may play a pivotal role in the metastatic cascade.
The viable CTCs with CSCs feature for functional analysis
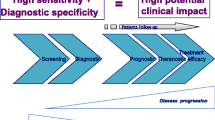
Since the CellSearch system was designed to detect detached tumor cells in PB, CTCs enumeration was thought to be an important method in the clinic relevance [9]. However, there were some limitations for CTC applications. The one was that the released CTCs number in different tumor types were quite disparity [10]. For example, inflammatory breast cancer (IBC) is characterized by high vascularity and increased microvessel density which may increase the chance for the CTCs release [11]. The higher incidence of CTC has also existed in SCLC patients with COPD, the inflammatory conditions and accumulation of airway macrophages which construct particular niches and enhance the invasive ability of CTCs to degrade the extracellular matrix (ECM) in early stage [10, 12] than other cancer types.. Apparently, a threshold of 3–5 CTCs/7.5 ml blood has been defined by the CellSearch system for prognostic stratification [10], which seems not compatible with all cancer patients. Other limitations were that enriched CTCs could not accurately cover the whole population and not all CTCs detected are clinically relevant [13]. Many isolated methods for CTCs relied on either defined surface marker or differences in the size of individual cell populations [13,14,15,16]. But CTCs are not a homogeneous group that can be captured by a set of identical markers or the same physicochemical properties. A few CTCs could remain the vitality in a very hostile environment during circulation [13, 14, 17, 18] by fusing with bone marrow-derived cells or altering the phenotype that could protect and hide them from the immune system attack. The methods based on the CD45 marker- even be considered that only express in mature mononuclear blood cells, was found that could even be appeared in CTCs by adhering to platelets or recruiting macrophages [10, 19]. And assumed epithelial markers, such as EpCAM, could also miss the CTCs subsets with low or absent expression [20] and inevitably cause decreased detection of CTCs that had undergone epithelial-mesenchymal transition (EMT), an important alteration involved in metastasis [13]. Contrary to transient disseminated tumor cells (DTC), these altered CTCs may be the key subsets which could manifest CSC features and significantly correlated to treatment response [15, 21,22,23]. In order to overcome these limitations, some specific markers that have a high specificity were used to define certain tumor types, such as mammaglobin for breast cancer and prostate-specific antigen (PSA) for prostate cancer. Nevertheless, these markers could be also downregulated during dedifferentiation of tumor cells [24] or absent in some particular CTCs due to the heterogeneity and plasticity [15]. These dynamic changes hindered CTC as a biomarker for clinical applications. For extending the understanding of relevant CTCs involved in metastasis, fortunately, the molecular technologies had integrated into CTCs identification by single-cell analyses such as RNA or exon sequencing [25, 26], which could be used to perform quantitative gene expression profiling for special CTCs and potentially guide patient management [26]. However, although studies of molecular characterization did identify different CTC subpopulations within a single blood sample, they had not addressed the biology of CTCs due to the scarcity of CTCs in the PB [25, 27]. To solve this issue, techniques on CTCs expansion both in vitro and in vivo had appeared (Fig. 1). The “viable CTCs”, which were enriched and isolated by label-free methods based on biophysical rather than biochemical properties, became the important role in experimental functional assays. One study reported that isolated human CTCs from murine blood showed an enhanced aggressive phenotype under hypoxic environment in vitro and in vivo [28]. The produced viable CTCs from xenografts in mouse manifested more biologic activity for functional researches. Other study also defined that qualified enrichment of viable CTCs must include some important parameters, such as capture efficiency, enrichment rate and even cell viability [29]. Recently, several groups have achieved a huge harvest in the expansion of CTCs from cancer patients. Two papers reported patient-derived CTCs culture for 6 months [30] and 1 year [31] respectively. Sufficient viable CTCs as a procurator for CSCs functional analyses could provide more biological information. But the next challenging obstacles had also existed. Many researchers concerned issues that were the efficient establishment of human-CTC cultures and the value for clinical applications. Recent years, the study reported that CTCs with CSCs phenotype derived from colorectal cancer patients could be designed to test drug sensitivity and integrate a personalized approach to clinical utility [25]. And then, much more CTC-platform provided the practicability on separation of viable CTCs by subsequent short-term growth in culture [27, 32,33,34] for functional test of CTC lines. Success in culturing human CTCs would overcome the difficulty of characterizing these rare cells and could extend new potential therapeutic strategy (Fig. 2).
CTC researches undergone the three stages CTC enumerations include various subsets such as dormant cells, apoptotic cells, and even normal hemopoietic stem cells, only depend on enumeration is not suitable for clinical evaluation. Whereas, further the studies in molecular characterization by RNA or exon sequencing could explain CTC heterogeneity, expanded CTCs could be the special subsets not only for deeper molecular-level researches but for functional analyses and guide the clinical therapy
The most influential events contributing to the causal relationship between CSCs and CTCs The fonts in black indicate events related to CSCs [1, 4, 32, 56, 57, 62,63,64,65,66,67,68]. The fonts in blue indicate events related to CTCs [9, 26, 28,29,30,31,32,33,34, 48, 54, 69,70,71,72,73,74,75,76,77,78]. And recent years, many evidence showed the inextricable connection between CSCs and CTCs, the expanded CTCs subsets are always used as a tool to reflect intrinsic characteristic of CSCs [25, 77, 78]. The abbreviations in Fig. 2: stem cells (SCs); mesenchymal stem cells (MSCs); serum-free medium (SFM); disseminated tumor cells (DTC); CTC-derived xenografts (CDX) [35,36,37,38,39, 43,44,45,46,47,48, 51, 52, 54]
Functional analysis of CTCs revealed modern individual treatment
Traditional CSC models suggested that there were intratumor heterogeneity in the primary site due to some special tumor cells get gene mutations which were able to become CSCs subpopulations and resulted in the tumor recurrence, metastasis or chemical drugs resistant. Current opinion even believed that these CSCs subpopulations were not immutable [16, 35,36,37,38]. Theoretically, under different environmental stress, CSCs and non-CSCs subpopulations were in a dynamic conversion [16]. Owing to the challenge of identify CSCs subpopulations, CTCs as a “monitoring method” were often used to study on the heterogeneity of CSCs in patient-derived samples in real-time. Some researchers had found CTCs and parental cells or primary tumor cells [28, 39] with some similarity such as hypoxia response both enhanced aggressive phenotype [23, 28] and others had found some differences in mutant gene [40] which could lead CTCs to acquire more aggressive behaviors. These researches showed that CTCs not only acted as an intermediate, they also as the potential precursor cells of metastasis [41] during the movement of tumor cells from the primary site to a distant location and the establishment of a new cancer growth. Different environmental stresses lead to different fates of CTCs. Some special CTCs could survive by some phenotypic and functional alteration to resistant environmental stress [42]. More aggressive CTCs could become potentially tumor-initiating cells, but they were unique and heterogeneous cell populations by their relation to a series of biological processes, such as EMT or mesenchymal-epithelial transition (MET), differed from the CSC-like cells in primary site as many researches previous described [35, 43,44,45]. These potentially tumor-initiating cells may not only infiltrate into distant sites, and also recruit some immunosuppressive cells, particularly tumor-associated macrophages (TAMs) to create a defensive shield and build the secondary niches [10, 12, 19]. The different cellular and intracellular interactions could cause totally different antitumor immune responses and metastatic prognoses [42, 45]. It could partially explain the source of heterogeneity of tumor metastasis and development in clinic. Recently a study investigated the different regrowth of the same CSCs population in primary and metastatic sites from a mouse model of colorectal cancer [46]. The authors found that the specific stem-cell subpopulation were eliminated by Lgr5+ target therapy in both primary and metastatic sites, but when the drugs treatment ceased, the two sites had different outcomes. In primary site, the tumor increased in the size and the specific stem-cell population reappeared, but in the metastatic site, there was no relapsed [46]. It could be explained that these CTCs, which had migrated through the bloodstream, could more contribute to act as the role of tumor-initiating cells and to drive metastasis formation, rather than act as the role of CSCs, which must have more self-renewal ability to maintain metastasis growth [46]. Conversely in primary site, the other cells may have the reversible ability and fulfilled stem-cell functions to fuel tumor regrowth [46, 47]. Thus, the target therapy might be more effective on metastatic site than primary site. Besides the difference between CTCs and primary tumor cells, the heterogeneity also exists in different CTC subpopulations. Malara N et al. even showed different biological behaviors in two expanded CTCs (eCTCs) subpopulations derived from patients with colon cancer. The eCTCs subpopulation expressed CXCR4+CK20+ were not tumorigenic but able to disseminate, and the other subpopulation expressed CD45−CD133+ were more tumorigenic. Patients with different prevalence of CTCs had different clinical outcomes [48]. Thus, on these basis of the CTCs heterogeneous composition, many researchers now do believe that traditional clinical treatment strategies might not be useful to patients with metastasis, because these treatment strategies often based on the pathological and molecular characterization of the primary tumor [49]. As current functional researches showed, CTCs should provide more useful resources for the mechanisms of metastasis formation [41]. The detailing of sufficient eCTCs in distinct subsets, by qualitative and quantitative measurement, might be useful to better define a personalized metastatic risk score [48] and lead to a better way in identification and isolation of metastasis-initiator cells for further clinical individual treatment decision regarding drug resistance [27] or prognosis [17].
The expanded methods of CTCs for clinical individual application
CSCs are known to be highly chemo-resistant [50] and more tumorigenic capacity under special microenvironment such as hypoxia-inducible condition [51]. They are always the key subsets that cause the treatment failed in whole tumor disease. Many researchers attempted to use the viable eCTCs to extend the knowledge of CSCs and figure out the process of metastasis formation. The methods that could get the more qualified eCTCs for reliable study are very crucial. CTC- derived xenograft (CDX) models is one of the expanded methods in vivo. Ameri, K et al. [28] using CDX to build a murine- derived CTCs model, showed that CTCs had an enhanced aggressive phenotype under chronic hypoxia. Their results revealed the micro-environmental stress could select for cells with phenotypes alterations and contributes to increased metastases. Successful CDX models could not only better mimic biological environment, it also recapitulate each individual patient’s cancer pathology and yield results more predictive of subsequent activity in patients [52]. However, using human cell line to generate murine-derived CTCs had its inevitable defects, because these CTCs from immunodeficient mice were not perfectly adequate for human [52]. For example, taken CTCs from cardiac puncture rather than from venous sampling, the most important differences are: i 2-7 ml blood is the minimal volume to human but is lethal to mouse, thus the enriched CTCs numbers are not on the same scale, CTCs in equal volume from mouse must be significantly higher than human. ii The sites that CTCs were directly punctured from heart means cardiogenic derived circulation in mouse, differed from peripheral venous and arterial circulation [53] in patients. And after that, although various studies reported that xenografts of CTCs were successful in many solid tumors, it should be also noted that many CDXs could be only obtained from advanced stage patients with high CTC counts, and even these xenotransplantation in vivo must take a long time [52].
The extended methods of CTCs in vitro were also reported. Many researchers suggested that the short term-eCTCs could distinguish from healthy or inflammation-derived cells that were isolated and unable to survive and expand [27, 48]. However, the maintenance of CTC culture in vitro from human blood samples is a complicated task, because many CTCs have limited proliferation ability and senesced after a few cell divisions in many cultural conditions such as adherent monolayer culture [30]. Lack of efficient conditions for eCTCs in vitro had become a bottleneck in clinic applications. Nevertheless, one study reported a microfluidic technology, human-CTC culture after enrichment by CTCiChip [54] showed the practicability of ex-vivo short term eCTCs in clinical trials. CTCs could be isolated and expanded from blood samples of early stage lung cancer patients, including patients with stage I [54]. In order to facilitate CTC expansion, the authors used a 3D co-culture condition, they introduced tumor associated fibroblasts to construct a tumor microenvironment [54]. Therefore, their expanded approach had high success rates to further characterize the biology of CTCs. And the long-term CTC cultures in vitro were reported by Min Y et al. They established oligoclonal CTC cultures sustained for > 6 months. CTCs were isolated from six of 36 patients with metastatic luminal subtype breast cancers [30]. In their serum free culture condition, the isolated CTCs could be maintained as suspended status and could form multi-cellular clusters, which were also named spheroids [55]. The eCTCs as non-adherent spheres may properly reflect intrinsic properties of CSCs that remain viable in the bloodstream after loss of attachment to basement membrane [30]. Spheroid culture of CTCs as a representative in vitro could reflect CTC cluster formation and growth in vivo [55]. Similar report was published by Cayrefourcq L et al. - the first CTC-derived permanent cell line isolated from the blood of a colon cancer patient, these CTCs had been cultured for more than 1 year [31]. It is a wealth of current functional researches on the biology of CTCs and raise the new perspective for drug testing in vitro and in vivo. But these long-term culture must also require high CTC counts from the advanced stage patients and were low success rate. Notably, there were another phenomenon might explain the low success rate. In Fan X et al.’s paper, the authors studied on 2 common prostate cancer cell lines named LNCaP and PC3 as research tools. Their results showed that PC3 could be formed spheres in suspension culture but LNCaP were failed [56] in the same condition. This suggested that different tumor cell lines, due to their different growth biology, could not either survive or expand in same culture mediums and environments in vitro. Thus, to better understanding CTCs biology from different origins, researchers must consider the merits and drawbacks in different culture conditions and approaches for clinical individual therapy (Table 1).
Optimize the current approaches of CTCs culture
Before strategies of CTC culture apply for clinical management, some problems should be concerned to address properly. The further characterization of the expanded CTC-derived cell lines must be required to define clearly, such as CTCs proliferated as tumor spheres always cultured in serum-free medium which were far from the conditions in vivo. How they differed from cells cultured from primary tumor biopsies or directly implanted into mouse models are concerned issues [30]. The other key technical problems are how to maintain CTCs phenotype and composition of population stable in culture. Some reports even hint that normal human mesenchymal stem cells (hMSC) are prone to genomic change and subsequent malignant transformation in long term culture [57]. Thus, CTC culture may be also meet the same situation that caused the genomic instability under various environmental stresses, especially long-term culture. 3D biomaterial for co-culture is an ideal way to solve this problem, which could maximize to mimic tumor physical and biochemical microenvironment by adding the different culture ingredients, i.e. growth factors, hormones, serums, matrix components, and growth factors. It also could facilitate CTC expansion [54]. Thus, 3D biomaterial could be considered to integrate the different culture methods for more realistic drug responses [58]. Furthermore, define and modify culture media supplements properly for different tumor cell lines are much important for CTC culture (Table 2).
Conclusions
Many studies have thought CTCs as a noninvasive method could provide a new perspective [59], but only enumeration is not sufficient [15, 60], it may only reflect relative tumor burden or leakiness of tumor-associated vasculature [40]. The quantification of CTCs with their viability are of high value for clinical evaluation, these CTCs with potential CSCs feature generally represent the tumor metastases and could be as procurators to facilitate real-time monitoring during systemic therapies by sequential peripheral blood sampling. But researchers must also keep an eye on those dormant CTCs in PB. A few of them may become the precursors of metastases in distant sites which offer appropriate conditions for them [61]. Thus, the optimal culture conditions for CTC expansion will need to be also considered for these special CTCs subsets. By utilizing different 3D biomaterials to improve culture microenvironment are the better options, it could screen out the more pertinent CTCs subsets and acquire more realistic information for strategy of personal therapy.
Abbreviations
- CDX:
-
CTC-derived xenografts
- CSCs:
-
Cancer stem cells
- CTCs:
-
Circulating tumor cells
- DTC:
-
Disseminated tumor cells
- ECM:
-
Extracellular matrix
- eCTCs:
-
Expanded CTCs
- EMT:
-
Epithelial-Mesenchymal Transition
- IBC:
-
Inflammatory breast cancer
- MET:
-
Mesenchymal-Epithelial Transition
- MSCs:
-
Mesenchymal stem cells
- PB:
-
Peripheral blood
- Ph:
-
Philadelphia chromosome
- SCs:
-
Stem cells
- SFM:
-
Serum-free medium
- TAMs:
-
Tumor-associated macrophages
- TICs:
-
Tumor-initiation cells
References
Nowell P, Hungerford D. A minute chromosome in human chronic granulocytic leukemia. Science. 1960;132:1497.
Hea LR. Persistent mitosis of transfused homologous leukocytes in children receiving antileukemic therapy. Science. 1963;142:1305–11.
Lapidot T, Sirard C, Vormoor J, Murdoch BTH. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8.
Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133–43.
Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–9.
Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJ. The origin of the cancer stem cell:current controversies and new insights. Nat Rev Cancer. 2005;5:899–904.
Avgustinova A, Benitah SA. The epigenetics of tumour initiation: cancer stem cells and their chromatin. Curr Opin Genet Dev. 2016;36:8–15.
Qin Z, Ljubimov VA, Zhou C, Tong Y, Liang J. Cell-free circulating tumor DNA in cancer. Chinese journal of cancer. 2016;35:36.
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91.
Hamilton G, Rath B. Circulating tumor cell interactions with macrophages: implications for biology and treatment. Transl Lung Cancer Res. 2017;6(4):418–30.
Pierga JY, Bidard FC, Autret A, Petit T, Andre F, Dalenc F, Levy C, Ferrero JM, Romieu G, Bonneterre J, et al. Circulating tumour cells and pathological complete response: independent prognostic factors in inflammatory breast cancer in a pooled analysis of two multicentre phase II trials (BEVERLY-1 and -2) of neoadjuvant chemotherapy combined with bevacizumab. Ann Oncol. 2017;28(1):103–9.
Hamilton G, Rath B, Klameth L, Hochmair MJ. Small cell lung cancer: recruitment of macrophages by circulating tumor cells. Oncoimmunology. 2015;5(3):e1093277.
Leong SM, Tan KM, Chua HW, Tan D, Fareda D, Osmany S, Li MH, Tucker S, Koay ES. Sampling circulating tumor cells for clinical benefits: how frequent? J Hematol Oncol. 2015;8:75.
Pesta M, Kulda V, Narsanska A, Fichtl J, Topolcan O. May CTC technologies promote better cancer management? EPMA J. 2015;6:1.
Nel I, David P, Gerken GG, Schlaak JF, Hoffmann AC. Role of circulating tumor cells and cancer stem cells in hepatocellular carcinoma. Hepatol Int. 2014;8:321–9.
Yang F, Xu J, Tang L, Guan X. Breast cancer stem cell: the roles and therapeutic implications. Cell Mol Life Sci. 2017;74:951–66.
Kolostova K, Cegan M, Bobek V. Circulating tumour cells in patients with urothelial tumours: enrichment and in vitro culture. Canadian Urological Association journal. 2014;8:E715–20.
Meyer CP, Pantel K, Tennstedt P, Stroelin P, Schlomm T, Heinzer H, Riethdorf S, Steuber T. Limited prognostic value of preoperative circulating tumor cells for early biochemical recurrence in patients with localized prostate cancer. Urol Oncol. 2016;34:235 e11–6.
Liu Q, Liao Q, Zhao Y. Myeloid-derived suppressor cells (MDSC) facilitate distant metastasis of malignancies by shielding circulating tumor cells (CTC) from immune surveillance. Med Hypotheses. 2016;87:34–9.
Mu Z, Benali-Furet N, Uzan G, Znaty A, Ye Z, Paolillo C, Wang C, Austin L, Rossi G, Fortina P, et al. Detection and characterization of circulating tumor associated cells in metastatic breast cancer. Int J Mol Sci. 2016;17(10):1665.
Li M, Zhang B, Zhang Z, Liu X, Qi X, Zhao J, Jiang Y, Zhai H, Ji Y, Luo D. Stem cell-like circulating tumor cells indicate poor prognosis in gastric cancer. Biomed Res Int. 2014;2014:981261.
Bidard FC, Proudhon C, Pierga JY. Circulating tumor cells in breast cancer. Mol Oncol. 2016;10:418–30.
Noman MZ, Messai Y, Muret J, Hasmim M, Chouaib S. Crosstalk between CTC, immune system and hypoxic tumor microenvironment. Cancer microenvironment: official journal of the International Cancer Microenvironment Society. 2014;7:153–60.
Alix-Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14(9):623–31.
Grillet F, Bayet E, Villeronce O, Zappia L. Circulating tumour cells from patients with colorectal cancer have cancer stem cell hallmarks in ex vivo culture. Gut. 2016; https://doi.org/10.1136/gutjnl-2016-311447.
Sieuwerts AM, Kraan J, Bolt-de Vries J, van der Spoel P, Mostert B, Martens JW, Gratama JW, Sleijfer S, Foekens JA. Molecular characterization of circulating tumor cells in large quantities of contaminating leukocytes by a multiplex real-time PCR. Breast Cancer Res Treat. 2009;118:455–68.
Khoo BL, Lee SC, Kumar P, Tan TZ, Warkiani ME, Ow SG, Nandi S, Lim CT, JP T. Short-term expansion of breast circulating cancer cells predicts response to anti-cancer therapy. Oncotarget. 2015;6:15578–93.
Ameri K, Luong R, Zhang H, Powell AA, Montgomery KD, Espinosa I, Bouley DM, Harris AL, Jeffrey SS. Circulating tumour cells demonstrate an altered response to hypoxia and an aggressive phenotype. Br J Cancer. 2010;102:561–9.
Zhou MD, Hao S, Williams AJ, Harouaka RA, Schrand B, Rawal S, Ao Z, Brenneman R, Gilboa E, Lu B, et al. Separable bilayer microfiltration device for viable label-free enrichment of circulating tumour cells. Sci Rep. 2014;4:7392.
Yu M, Bardia A, Aceto N, Bersani F, Madden MW, Donaldson MC, Desai R, Zhu H, Comaills V, Zheng Z, et al. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science. 2014;345:216–20.
Cayrefourcq L, Mazard T, Joosse S, Solassol J, Ramos J, Assenat E, Schumacher U, Costes V, Maudelonde T, Pantel K, et al. Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res. 2015;75:892–901.
Kulasinghe A, Perry C, Warkiani ME, Blick T, Davies A. Short term ex-vivo expansion of circulating head and neck tumour cells. Oncotarget. 2016;7:60101–9.
Kolostova K, Matkowski R, Gurlich R, Grabowski K, Soter K, Lischke R, Schutzner J, Bobek V. Detection and cultivation of circulating tumor cells in gastric cancer. Cytotechnology. 2016;68:1095–102.
Laget S, Broncy L, Hormigos K, Dhingra DM, BenMohamed F, Capiod T, Osteras M, Farinelli L, Jackson S, Paterlini-Brechot P. Technical insights into highly sensitive isolation and molecular characterization of fixed and live circulating tumor cells for early detection of tumor invasion. PLoS One. 2017;12:e0169427.
Klevebring D, Rosin G, Ma R, Lindberg J, Czene K, Kere J, Fredriksson I, Bergh J, Hartman J. Sequencing of breast cancer stem cell populations indicates a dynamic conversion between differentiation states in vivo. Breast cancer research : BCR. 2014;16:R72.
Iliopoulos D, Hirsch HA, Wang G, Struhl K. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci U S A. 2011;108:1397–402.
Fessler E, Dijkgraaf FE, De Sousa EMF, Medema JP. Cancer stem cell dynamics in tumor progression and metastasis: is the microenvironment to blame? Cancer Lett. 2013;341:97–104.
Vermeulen L, de Sousa e Melo F, Richel DJ, Medema JP. The developing cancer stem-cell model: clinical challenges and opportunities. The Lancet Oncology. 2012;13:e83–9.
Gallaher J, Babu A, Plevritis S, Anderson AR. Bridging population and tissue scale tumor dynamics: a new paradigm for understanding differences in tumor growth and metastatic disease. Cancer Res. 2014;74:426–35.
Miyamoto DT, Lee RJ, Stott SL, Ting DT, Wittner BS, Ulman M, Smas ME, Lord JB, Brannigan BW, Trautwein J, et al. Androgen receptor signaling in circulating tumor cells as a marker of hormonally responsive prostate cancer. Cancer Discov. 2012;2:995–1003.
Wang H, Stoecklein NH, Lin PP, Gires O. Circulating and disseminated tumor cells: diagnostic tools and therapeutic targets in motion. Oncotarget. 2017;8:1884–912.
Jie XX, Zhang XY, CJ X. Epithelial-to-mesenchymal transition, circulating tumor cells and cancer metastasis: mechanisms and clinical applications. Oncotarget. 2017; https://doi.org/10.18632/oncotarget.18277.
Ravasio R, Ceccacci E, Minucci S. Self-renewal of tumor cells: epigenetic determinants of the cancer stem cell phenotype. Curr Opin Genet Dev. 2016;36:92–9.
Zhang JX, Chen ZH, Xu Y, Chen JW, Weng HW, Yun M, Zheng ZS, Chen C, Wu BL, Li EM, et al. Downregulation of MicroRNA-644a promotes esophageal Squamous cell carcinoma aggressiveness and stem cell-like phenotype via Dysregulation of PITX2. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23:298–310.
Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10(6):717–28.
de Sousa e Melo F, Kurtova AV, Harnoss JM, Kljavin N, Hoeck JD, Hung J, Anderson JE, Storm EE, Modrusan Z, Koeppen H, et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature. 2017;543(7647):676–80.
Shimokawa M, Ohta Y, Nishikori S, Matano M, Takano A, Fujii M, Date S, Sugimoto S, Kanai T, Sato T. Visualization and targeting of LGR5+ human colon cancer stem cells. Nature. 2017;545:187–92.
Malara N, Trunzo V, Foresta U, Amodio N, De Vitis S, Roveda L, Fava M, Coluccio M, Macri R, Di Vito A, et al. Ex-vivo characterization of circulating colon cancer cells distinguished in stem and differentiated subset provides useful biomarker for personalized metastatic risk assessment. J Transl Med. 2016;14:133.
Gkountela S, Szczerba B, Donato C, Aceto N. Recent advances in the biology of human circulating tumour cells and metastasis. ESMO Open. 2016;1:e000078.
Deshmukh A, Deshpande K, Arfuso F, Newsholme P, Dharmarajan A. Cancer stem cell metabolism: a potential target for cancer therapy. Mol Cancer. 2016;15:69.
Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–13.
Lai Y, Wei X, Lin S, Qin L, Cheng L, Li P. Current status and perspectives of patient-derived xenograft models in cancer research. J Hematol Oncol. 2017;10:106.
Jiao LR, Apostolopoulos C, Jacob J, Szydlo RNJ. Unique localization of circulating tumor cells in patients with hepatic metastases. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27:6160–5.
Zhang Z, Shiratsuchi H, Lin J, Chen G, Reddy RM, Azizi E, Fouladdel S, Chang AC, Lin L, Jiang H, et al. Expansion of CTCs from early stage lung cancer patients using a microfluidic co-culture model. Oncotarget. 2014;5:12383–97.
Braunholz D, Saki M, Niehr F, Öztürk M, Borràs Puértolas B, Konschak R, Budach V, Tinhofer I. Spheroid culture of head and neck cancer cells reveals an important role of EGFR Signalling in anchorage independent survival. PLoS One. 2016;11:e0163149.
Fan X, Liu S, Su F, Pan Q, Lin T. Effective enrichment of prostate cancer stem cells from spheres in a suspension culture system. Urol Oncol. 2012;30:314–8.
Rosland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R, Lonning PE, et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331–9.
Bielecka ZF, Maliszewska-Olejniczak K, Safir IJ, Szczylik C, Czarnecka AM. Three-dimensional cell culture model utilization in cancer stem cell research. Biol Rev Camb Philos Soc. 2017;92:1505–20.
Smerage JB, Barlow WE, Hortobagyi GN, Winer EP, Leyland-Jones B, Srkalovic G, Tejwani S, Schott AF, O'Rourke MA, Lew DL, et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:3483–9.
Lu SH, Tsai WS, Chang YH, Chou TY, Pang ST, Lin PH, Tsai CM, Chang YC. Identifying cancer origin using circulating tumor cells. Cancer biology & therapy. 2016;17:430–8.
Schindlbeck C, Andergassen U, Jueckstock J, Rack B, Janni W, Jeschke U. Disseminated and circulating tumor cells in bone marrow and blood of breast cancer patients: properties, enrichment, and potential targets. J Cancer Res Clin Oncol. 2016;142(9):1883–95.
Weiss S, Reynolds BA, Vescovi AL, Morshead C, Craig CG. Der Kooy Dv. Is there a neural stem cell in the mammalian forebrain? Trends Neurosci. 1996;19:387–93.
Vescovi AL, Reynolds BA, Fraser DD, S W. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron. 1993;11:951–66.
Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of Tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–11.
Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403.
Liao MJ, Zhang CC, Zhou B, Zimonjic DB, Mani SA, Kaba M, Gifford A, Reinhardt F, Popescu NC, Guo W, et al. Enrichment of a population of mammary gland cells that form mammospheres and have in vivo repopulating activity. Cancer Res. 2007;67:8131–8.
Wang W, Itaka K, Ohba S, Nishiyama N, Chung UI, Yamasaki Y, Kataoka K. 3D spheroid culture system on micropatterned substrates for improved differentiation efficiency of multipotent mesenchymal stem cells. Biomaterials. 2009;14:2705–15.
Han ME, Jeon TY, Hwang SH, Lee YS, Kim HJ, Shim HE, Yoon S, Baek SY, Kim BS, Kang CD, et al. Cancer spheres from gastric cancer patients provide an ideal model system for cancer stem cell research. Cellular and molecular life sciences : CMLS. 2011;68:3589–605.
Ashworth TR. A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Aus Medj. 1869;14:146–9.
Seal SH. Silicone flotation: a simple quantitative method for the isolation of free-floating cancer cells from the blood. Cancer biology & therapy. 1959;12:590–5.
Alix-Panabieres C, Bartkowiak K, Pantel K. Functional studies on circulating and disseminated tumor cells in carcinoma patients. Mol Oncol. 2016;10:443–9.
Racila E, Euhus D, Weiss AJ, Rao CJM. Detection and characterization of carcinoma cells in the blood. Proc Natl Acad Sci U S A. 1998;95:4589–94.
Pretlow TG, Schwartz S, Giaconia JM, Wright AL, Grimm HA, Edgehouse NL, Murphy JR, Markowitz SD, Jamison JM, Summers JL, et al. Prostate cancer and other Xenografts from cells in peripheral blood of patients. Cancer Res. 2000;60:4033–6.
Zheng S, Lin HK, Lu B, Williams A, Datar R, Cote RJ, Tai YC. 3D microfilter device for viable circulating tumor cell (CTC) enrichment from blood. Biomed Microdevices. 2011;13:203–13.
Deneve E, Riethdorf S, Ramos J, Nocca D, Coffy A, Daures JP, Maudelonde T, Fabre JM, Pantel K, Alix-Panabieres C. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. Clin Chem. 2013;59:1384–92.
Alix-Panabières C. EPISPOT assay: detection of viable DTCs/CTCs in solid tumor patients. Recent Results Cancer Res. 2012;195:69–76.
Zhang L, Ridgway LD, Wetzel MD, Ngo J, Yin W, Kumar D, Goodman JC, Groves MD, Marchetti D. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci Transl Med. 2013;5:180ra48.
Gao D, Vela I, Sboner A, Iaquinta PJ, Karthaus WR, Gopalan A, Dowling C, Wanjala JN, Undvall EA, Arora VK, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159:176–87.
Yang Z, Li C, Fan Z, Liu H, Zhang X, Cai Z, Xu L, Luo J, Huang Y, He L, et al. Single-cell sequencing reveals variants in ARID1A, GPRC5A and MLL2 driving self-renewal of human bladder cancer stem cells. Eur Urol. 2017;71:8–12.
Dosch JS, Ziemke EK, Shettigar A, Rehemtulla A, Sebolt-Leopold JS. Cancer stem cell marker phenotypes are reversible and functionally homogeneous in a preclinical model of pancreatic cancer. Cancer Res. 2015;75:4582–92.
Lichner Z, Saleh C, Subramaniam V, Seivwright A, Prud'homme GJ, Yousef GM. miR-17 inhibition enhances the formation of kidney cancer spheres with stem cell/ tumor initiating cell properties. Oncotarget. 2014;6:5567–81.
Nolte SM, Venugopal C, McFarlane N, Morozova O, Hallett RM, O'Farrell E, Manoranjan B, Murty NK, Klurfan P, Kachur E, et al. A cancer stem cell model for studying brain metastases from primary lung cancer. J Natl Cancer Inst. 2013;105:551–62.
Paranjape AN, Mandal T, Mukherjee G, Kumar MV, Sengupta K, Rangarajan A. Introduction of SV40ER and hTERT into mammospheres generates breast cancer cells with stem cell properties. Oncogene. 2012;31:1896–909.
Dey D, Saxena M, Paranjape AN, Krishnan V, Giraddi R, Kumar MV, Mukherjee G, Rangarajan A. Phenotypic and functional characterization of human mammary stem/progenitor cells in long term culture. PLoS One. 2009;4:e5329.
Lombardo Y, Filipovic A, Molyneux G, Periyasamy M. Nicastrin regulates breast cancer stem cell properties and tumor growth in vitro and in vivo. Proc Natl Acad Sci U S A. 2012;109:16558–63.
Hussein D, Punjaruk W, Storer LC, Shaw L, Othman R, Peet A, Miller S, Bandopadhyay G, Heath R, Kumari R, et al. Pediatric brain tumor cancer stem cells: cell cycle dynamics, DNA repair, and etoposide extrusion. Neuro-Oncology. 2011;13:70–83.
Wang K, Kievit FM, Erickson AE, Silber JR, Ellenbogen RG, Zhang M. Culture on 3D Chitosan-Hyaluronic acid scaffolds enhances stem cell marker expression and drug resistance in human Glioblastoma cancer stem cells. Advanced healthcare materials. 2016;5:3173–81.
Kolostova K, Matkowski R, Jędryka M, Soter K. The added value of circulating tumor cells examination in ovarian cancer staging. Am J Cancer Res. 2015;5:3363–75.
Kolostova K, Spicka J, Matkowski R, Bobek V. Isolation, primary culture, morphological and molecular characterization of circulating tumor cells in gynecological cancers. Am J Transl Res. 2015;7:1203–13.
Kolostova K, Pinkas M, Jakabova A, Pospisilova E, Svobodova P. Molecular characterization of circulating tumor cells in ovarian cancer. Am J Cancer Res. 2016;6:973–80.
Bobek V, Gurlich R, Eliasova P, Kolostova K. Circulating tumor cells in pancreatic cancer patients: enrichment and cultivation. World J Gastroenterol. 2014;20:17163–70.
Kolostova K, Zhang Y, Hoffman RM, Bobek V. In vitro culture and characterization of human lung cancer circulating tumor cells isolated by size exclusion from an orthotopic nude-mouse model expressing fluorescent protein. J Fluoresc. 2014;24:1531–6.
Acknowledgements
Not applicable
Funding
Not applicable
Availability of data and materials
Not applicable
Author information
Authors and Affiliations
Contributions
Luo YT wrote and edited the manuscript, Cheng J revised the manuscript and gave critical review of the manuscript, Feng X, He SJ and Wang YW contributed to language editing, Huang Q gave suggestions and gave revisions. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Luo, Y.T., Cheng, J., Feng, X. et al. The viable circulating tumor cells with cancer stem cells feature, where is the way out?. J Exp Clin Cancer Res 37, 38 (2018). https://doi.org/10.1186/s13046-018-0685-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13046-018-0685-7