Abstract
Background
Alzheimer’s disease (AD) is the leading cause of dementia, clinically characterized by memory deficits and progressive cognitive decline. Despite decades of research effective therapies are lacking, and a large part of the genetic heritability remains unidentified. ABCA7 and ABCA1, members of the ATP-binding cassette subfamily A (ABCA), were identified as AD risk genes in genome-wide association studies. Nevertheless, genetic and/or functional studies propose a link between AD and two other members of the ABCA subclass, i.e., ABCA2 and ABCA5.
Main body
Changes in expression or dysfunction of these transporters were found to increase amyloid β levels. This might be related to the common role of ABCA transporters in cellular cholesterol homeostasis, for which a prominent role in AD development has been suggested. In this review, we provide a comprehensive overview and discussion on the contribution of the ABCA subfamily to the etiopathogenesis of AD.
Conclusions
A better understanding of the function and identification of disease-associated genetic variants in ABCA transporters can contribute to the development of novel therapeutic strategies for AD.
Similar content being viewed by others
Background
Alzheimer’s disease (AD) is the most common cause of dementia and represents the sixth-leading cause of death in the United States [1]. Age is the most important known risk factor for AD, with the risk approximately doubling every five years after the age of 65 [2]. While most patients have an onset age above 65 years (late-onset AD or LOAD), in 10% of the patients the disease manifests before the age of 65 years, known as early-onset AD (EOAD) [3]. EOAD is almost entirely genetically determined, with a heritability between 92 and 100% [4]. In the early 1990’s, genetic studies in large pedigrees with autosomal dominant inheritance patterns of AD, led to the discovery of pathogenic mutations in three genes: amyloid precursor protein (APP), presenilin-1 (PSEN1) and presenilin-2 (PSEN2) [5,6,7]. The discovery of these genes contributed significantly to the understanding of the disease and shaped the amyloid β (Aβ) cascade hypothesis. Neurotoxic Aβ peptides are the major component of senile plaques, an important pathological hallmark of AD, and are generated through the successive proteolytic cleavage of APP by β- and γ-secretases [8]. PSEN1 and PSEN2 represent catalytic subunits of γ-secretase [9]. Mutations in APP and the presenilins explain around 10% of the EOAD cases, leaving the majority of the patients genetically unexplained [4]. LOAD is a more complex disorder, with an estimated heritability of 58% to 79% [10]. The ε4 allele of the apolipoprotein E (APOE) gene is recognized as a strong risk factor for LOAD [11, 12]. In heterozygous carriers, the risk for developing AD is 3 to 4 times higher compared to APOE ε4 noncarriers and increases 9 to 15 times in homozygous APOE ε4 carriers [13]. Genome-wide association studies (GWAS) in large LOAD patient and control cohorts led to the identification of common variants associated with AD in numerous genomic risk loci, including two members of the ATP-binding cassette subfamily A (ABCA), ABCA7 and ABCA1 [14, 15].
Main text
ABC transporters and the A-subfamily
The ATP-binding cassette (ABC) transporter family is a superfamily of highly conserved integral membrane proteins responsible for the transport of various substrates across cellular membranes. Based on amino acid sequence similarity and phylogeny, seven subfamilies from ABCA to ABCG are defined, which classify all 48 functional human ABC transporters [16]. ABC transporters share a characteristic architecture, consisting of four core domains: two nucleotide binding domains (NBD) and two transmembrane domains (TMD) (Fig. 1). The NBDs provide the energy for substrate transport by ATP-binding and ATP-hydrolysis and contain three highly conserved motifs: Walker A and B motifs and a signature (C) motif. The TMDs typically contain six membrane-spanning α-helices and provide a pathway across the membrane for substrate transport [17]. These domains also harbor ligand-binding sites that determine the substrate specificity [18].
The ABCA subfamily comprises 12 functional transporters, ABCA1 to ABCA13, with ABCA11 representing a transcribed pseudogene. The A-subfamily is characterized by two large extracellular loops between the first and second helix of each transmembrane domain, which can function as ligand binding sites (Fig. 1) [19]. Several members have been identified as lipid transporters in different body locations [20]. The subfamily can be divided in two subgroups, based on phylogenetic analysis and chromosomal location [17]. The first subgroup of five genes (ABCA5-6 and ABCA8-10) is organized in a head-to-tail cluster on chromosome 17q24, while the second group of seven genes (ABCA1-4, ABCA7 and ABCA12-13) is dispersed on six chromosomes [17].
Over the past years, A-subclass ABC proteins have gained a lot of attention due to their implication in human diseases. To date, mutations in five ABCA genes are causatively linked to monogenic recessive disorders: ABCA1 (Tangier disease), ABCA3 (neonatal surfactant deficiency), ABCA4 (Stargardt disease), ABCA12 (harlequin ichthyosis) and most recently ABCA5 was linked to congenital generalized hypertrichosis terminalis [21,22,23,24,25]. Additionally, rare coding variants in ABCA13, increase the susceptibility to schizophrenia and bipolar disorder [26, 27]. Moreover, GWAS recognized ABCA7 and more recently ABCA1 as risk genes for LOAD [15, 28]. Post-GWAS genetic studies identified common and rare ABCA7 variants that influence AD risk, establishing ABCA7 as an important AD risk gene. Although the underlying mechanism linking ABCA7 risk variants to AD pathogenesis is poorly understood, ABCA7 is functionally involved in several molecular processes linked to AD etiology.
Besides ABCA1 and ABCA7, two additional ABCA members, ABCA2 and ABCA5, have been genetically and/or functionally linked to AD, supporting a broader function of this protein subfamily to the etiopathogenesis of AD (Fig. 1). Interestingly, these ABCA transporters are all implicated in cholesterol homeostasis, a pathway for which an important role in AD has been suggested, as highlighted below. In this review, we will first discuss the link between cholesterol metabolism and AD, before reviewing the genetic and functional evidence linking ABCA1, ABCA2, ABCA5 and ABCA7 to AD.
The link between cholesterol homeostasis and AD
Cholesterol is a key component of mammalian cell membranes and it is involved in a large number of cellular processes [29]. Membrane cholesterol regulates membrane fluidity, rigidity and permeability by interacting with surrounding bilayer lipids and regulates signal transduction by interacting with transmembrane proteins [29]. The brain contains the highest cholesterol levels in the body, and tight regulation of its synthesis, storage, transport and removal is essential for neuronal functioning [30]. Brain cholesterol mainly originates from de novo synthesis, since systemic lipoprotein uptake is prevented by the blood–brain barrier (BBB) [31]. In the adult brain, cholesterol synthesis is mostly dedicated to astrocytes, which then redistribute cholesterol to neurons, a process mediated by ABCA1 [32]. ABCA1 exports excess cellular cholesterol and phospholipids to apolipoproteins [33]. While apolipoprotein A1 (ApoA1) is the major component of high-density lipoprotein (HDL) particles in the plasma, shuttling cholesterol to the liver for excretion, ApoE is the main cholesterol transporter in the central nervous system and is predominantly produced by astrocytes [33]. In the brain, the HDL-like ApoE-cholesterol-phospholipid complexes can be internalized by neurons, by binding to cell surface receptors, such as the low-density lipoprotein (LDL) receptor [34]. Excess cholesterol can be excreted by conversion to 24-S-hydroxycholesterol, which can readily pass the BBB to be further metabolized by the liver or can be esterified and stored intracellularly as lipid droplets [35, 36]. In addition, it is hypothesized that brain cholesterol is eliminated through the BBB by efflux transporters, such as ABC transporters [37].
β- and γ-secretases mainly operate in cholesterol-enriched membrane microdomains termed lipid rafts, while α-secretase mainly localizes to non-raft regions. High plasma membrane cholesterol levels facilitate the colocalization of APP with β- and γ-secretases, promoting amyloidogenic APP processing and therefore Aβ production [38]. In line, cholesterol depletion promotes the nonamyloidogenic α-secretase cleavage of APP, leading to a reduced Aβ production [39]. Despite the separation of brain and peripheral cholesterol pools, epidemiological studies identified a link between high serum cholesterol levels and AD risk [40]. In parallel, the use of cholesterol-lowering agents, i.e. statins, is associated with lower AD risk [41]. The flux of plasma oxysterols towards the central nervous system following hypercholesterolemia, together with disruption of the BBB might explain the link between serum cholesterol and AD [42].
A first genetic link between AD and lipid metabolism was established when the ε4 allele of APOE was identified as a major genetic risk factor for AD and cerebral amyloid angiopathy (CAA) [43, 44]. ApoE ε4 is suggested to increase Aβ aggregation and decrease Aβ clearance [45, 46]. Indeed, ApoE colocalizes with senile plaques, neurofibrillary tangles, and vascular amyloid [12], and was found to bind Aβ, although the ApoE ε4 isoform shows a decreased Aβ binding affinity [47, 48]. In addition, an isoform-dependent difference in cellular cholesterol efflux is observed, with ApoE ε4 showing the least efflux capacity [49]. Decades after the identification of APOE ε4 as a strong AD risk factor, GWAS in LOAD cohorts identified a high number of risk genes that are implicated in lipid metabolism, including two genes of the ABCA subfamily: ABCA1 and ABCA7 (Fig. 1) [14, 50, 51].
ABCA1
In the periphery, ABCA1 promotes the release of cellular cholesterol and phospholipids to lipid-poor apolipoproteins, mainly ApoA1, to generate HDL [52]. Since cholesterol is mainly catabolized in the liver, efflux of excessive cellular cholesterol by ABCA1 to ApoA1 plays a key role in the reverse cholesterol transport pathway in order to deliver HDL to the liver for excretion [53]. The identification of ABCA1 loss of function mutations in patients with HDL-deficiency syndromes, including Tangier disease, confirmed the role of ABCA1 in cellular cholesterol homeostasis [54]. Tangier disease is a recessive disorder characterized by extremely low plasma HDL and ApoA1 levels, intracellular cholesterol depositions, premature atherosclerosis and peripheral neuropathy [54]. The role of ABCA1 in the periphery has been extensively studied. Nevertheless, ABCA1 is highly expressed in the human brain, with the highest expression in neurons and microglia [55]. Studies in mice showed that in the central nervous system, ABCA1 is directly involved in brain cholesterol homeostasis by exporting cholesterol through the BBB [56]. In addition, loss of Abca1 results in a major decrease in ApoE protein levels and ApoE lipidation, as well as an impaired hippocampal neurite morphology in mice, suggesting a role for ABCA1 in AD [33, 57]. Lipidation of ApoE is required for its functioning, including the ability to bind Aβ [48], and a lower lipidation status has been observed in ApoE ε4 compared to ApoE ε3 produced by human iPSC-derived astrocytes [58]. Furthermore, Abca1 deficiency increases Aβ deposition as well as CAA in two AD mouse models [59, 60], and is linked to cognitive deficits in mice [57, 61]. Fitz et al. demonstrate that Abca1 deficiency in an AD mouse model negatively impacts amyloid deposition, Aβ clearance and memory in mice expressing human APOE ε4 but not APOE ε3, suggesting an interaction between ABCA1 and other genetic risk factors [62]. In parallel, overexpression of Abca1 in an AD mouse model reduced fibrillogenesis and deposition of Aβ in the brain, possibly related to the increased lipidation of ApoE [63]. Selective stimulation of Abca1 with an ABCA1 agonist in mice expressing human APOE ε4, increased lipidation of ApoE ε4 and ameliorated ApoE ε4-driven cognitive impairments and brain pathology, rendering it to a similar level as the mice expressing ApoE ε3 [64]. In addition, ABCA1 membrane expression in mice primary astrocytes is diminished in cells expressing human ApoE ε4 compared to ApoE ε3 expressing cells due to a reduced ABCA1 recycling [65]. In parallel, a reduction in ABCA1 protein levels is observed in human astrocytes expressing ApoE ε4, possibly contributing to the ineffective cholesterol efflux in ApoE ε4 cells [66]. Upregulation of ABCA1 and the subsequent increase in APOE lipidation might present a potential therapeutic strategy to ameliorate AD-pathology driven by APOE ε4.
ABCA1 is transcriptionally regulated by oxysterol-activated liver X receptors (LXRs), nuclear receptors which bind to DNA sequences of their target genes as heterodimers with retinoid X receptors (RXRs) to activate transcription [67]. Numerous studies have pursued the use of LXR or RXR agonists to reduce AD-related brain pathology and cognitive impairment, as recently reviewed by Fitz and colleagues [68]. Following LXR activation, a decrease in amyloidogenic APP processing and Aβ secretion has been demonstrated in vitro and in AD mouse models, and improvement of cognitive deficits has been observed in AD mice [69, 70]. These changes were associated with an increased ABCA1 expression and propose the induction of functional ABCA1 as a promising therapeutic option for AD [69, 70].
In vitro experiments with skin fibroblasts derived from two Tangier disease patients carrying homozygous ABCA1 premature termination codon (PTC) or missense mutations leading to a loss of functional protein show an increased production of Aβ compared to control cells [71]. Interestingly, upregulation of ABCA1 expression via a synthetic LXR ligand led to a further Aβ increase in cells carrying a missense mutation (N935S) and stayed the same in cells carrying a nonsense mutation, signaling that functional and full-length ABCA1 is required to benefit from the effect of LXR/RXR agonists on Aβ secretion [71]. This is in line with the clinical phenotype of the N935S patient, who had extremely low HDL levels and developed severe dementia and amyloid depositions by the age of 60 [71]. Another case with a relevant link with AD is a patient carrying a compound heterozygous mutation (D1099Y and F2009S) in ABCA1, who presented with low HDL but no cardiovascular disease, and later developed and died of CAA [72].
The ABCA1 gene is located near a linkage peak on chromosome 9, previously identified through genome-wide AD linkage studies, and is a good candidate gene given its function in cholesterol homeostasis [73, 74]. Since the early 2000’s, 20 studies exploring the association of ABCA1 common single nucleotide polymorphisms (SNPs) with AD have been published, reporting conflicting results (PubMed, accessed 20 September 2021). An established ABCA1 loss-of-function mutation involved in familial HDL-deficiency, N1800H, is associated with low ApoE plasma levels and a higher risk for AD and cerebrovascular disease [75]. In a family with 4 AD patients, co-segregation of a missense variant (rs137854495; p.A937V) with AD was reported [76]. This variant was previously identified in Tangier disease patients as part of a compound heterozygous mutation [25]. The mutation resides in the Walker A motif of the first NBD and abolishes cholesterol efflux [77]. Interestingly, the same conserved Alanine to Valine substitution in ABCA7 (p.A845V) was identified in a patient with AD (Fig. 2). Subcellular localization studies found that this variant leads to a loss of functional ABCA7 by means of mislocalization from the plasma membrane to the ER [78]. Finally, the largest AD GWAS/GWAX to date, i.e., including AD-by-proxy cases based on parental history of AD, recently identified ABCA1 as a candidate AD gene [14].
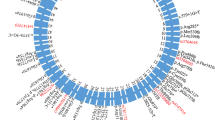
Topological model of ABCA7. Pathogenic ABCA7 missense mutations leading to mislocalization and subsequent loss of functional protein as well as ABCA7 missense mutations corresponding to pathogenic mutations in ABCA transporters implicated in human disease are shown. The ABCA7 sequence was aligned with sequences of ABCA1, 3, 4, 5 and 12. Pathogenic missense mutations in these five genes were downloaded from the ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar/). ABCA7 missense mutations, previously reported by Le Guennec et al., Sassi et al., Bellenguez et al., De Roeck et al., and Bossaerts et al., that correspond to pathogenic missense mutations in ABCA1, 3, 4, 5 and 12 are shown in the figure [78,79,80,81,82]. Variants marked with a ‘*’ were identified in control individual(s) only. Protein domain and motif information was based on alignment with ABCA1 [83, 84]. ABCA7 missense variants are shown in red. Corresponding pathogenic ABCA1, ABCA4 and ABCA12 mutations are shown in black, blue and purple respectively
ABCA2
The ABCA2 gene is located close to ABCA1 on chromosome 9q and encodes a 2436 amino acid polypeptide [85, 86]. ABCA2 mRNA is predominantly expressed in human brain compared to other organs, where it is localized mainly in oligodendrocytes [55, 87]. ABCA2 mRNA expression in macrophages is upregulated in response to cholesterol influx, classifying ABCA2 as a sterol-responsive gene [85]. Given its high expression in the brain, a plausible role for ABCA2 in brain lipid homeostasis is hypothesized [88]. Subcellular localization studies in HEK293 cells overexpressing human ABCA2 show high ABCA2 expression in late endosomes/lysosomes, proposing an intracellular lipid trafficking role, rather than transport across the plasma membrane like ABCA1 and ABCA7 [87]. Human ABCA2 expression in Chinese hamster ovary cells leads to the sequestering of LDL-free cholesterol in the lysosome and blocks its delivery to the endoplasmic reticulum (ER) for esterification, mimicking sterol-deprived conditions, and confirming a role in intracellular cholesterol trafficking [89]. Expression of human ABCA2 in HEK293 cells did not significantly alter cholesterol efflux to ApoA1 or ApoE, which again might reflect the endolysosomal location of ABCA2 [90]. However, later research found a decrease in total and membrane cholesterol levels as well as a reduced cholesterol efflux to ApoE ε3 acceptors in mouse neuroblastoma cells expressing human ABCA2, without perturbing lipid rafts [91]. In addition, ABCA2 regulates cholesterol levels by decreasing LDL receptor mRNA and protein expression [91]. The ABCA2 protein expression dynamics in developing rat brain oligodendrocytes coincide with the myelination process, proposing a role for ABCA2 in myelin formation [92, 93]. In fact, Abca2 knockout mice show abnormal myelin sheet ultrastructure and present with prominent tremor, reduced body weight and hyperactivity, of which the latter two were more prominent in female mice [94]. A second study also observed a tremor in Abca2 knockout mice, and identified alterations in brain sphingolipid levels, but could not confirm abnormal myelin structure [95].
Differential gene expression analysis of HEK293 cells overexpressing human ABCA2 identified several genes involved in the pathogenesis of AD [96]. Increased expression of human ABCA2 is associated with an increase in APP synthesis and amyloidogenic processing via β-secretase [96, 97]. In parallel, in vitro and in vivo depletion of ABCA2 is associated with a decrease in Aβ production, due to a decreased γ-secretase cleavage of APP [98]. Confocal microscopy identified colocalization of ABCA2 with both Aβ and APP in intracellular vesicles in human neuroblastoma cells [96]. Of note, the endosomal/lysosomal pathway, showing high levels of ABCA2 expression, is the major site for Aβ generation [99]. ABCA2 mRNA expression is also significantly increased in brain tissue of the prefrontal cortex and blood of AD patients compared to control individuals [100]. These data suggest a link between elevated ABCA2 expression and AD, and studies clarifying the exact function of the transporter might shed more light on the use of ABCA2 downregulation as a potential therapeutic option for AD.
Methylation of ABCA2 CpG site cg03349123 is negatively associated with AD risk [100]. Macé and colleagues reported a significant association of a synonymous SNP (rs908832) within exon 14, with EOAD in a French case–control cohort [101]. This association was later confirmed in a Swiss cohort, while in a Greek cohort, the minor allele was significantly more frequent in the control group [102]. Furthermore, rs908832 was monomorphic in a Japanese cohort, suggesting an ethnicity-dependent association with AD [102]. In a Caucasian-American cohort, no significant association was observed either with EOAD or LOAD [103]. Meta-analysis comprising these studies found a strong association of rs908832 with AD (OR = 1.55, 95% CI = 1.12–2.16, P = 0.009) [104]. However, rs908832 is not associated with serum cholesterol profiles, and further research is needed to clarify the underlying molecular mechanism this SNP [102].
ABCA5
ABCA5 is part of the ABCA gene cluster on chromosome 17q24 [105]. Macrophages from Abca5 knockout mice show a decreased cholesterol efflux to HDL, identifying Abca5 as a sterol-responsive gene [106]. A compensatory role for ABCA5 was identified in macrophages after ABCA1 downregulation under hyper-cholesterol conditions [107]. High ABCA5 mRNA expression is found in the human and mouse brain, where it is predominantly present in neurons [106, 108]. Subcellularly, murine Abca5 localizes in lysosomes and late endosomes as well as at the plasma membrane [107, 109]. Abca5 knockout mice present with trembling and lysosomal disease-like symptoms such as heart abnormalities, although no abnormalities were detected in brain [109]. ABCA5 is highly expressed in human skin and hair follicles and bi-allelic loss-of-function mutations in ABCA5 are linked to excessive hair overgrowth (hypertrichosis) [22]. These mutations lead to a disturbed lysosomal function, resulting in the accumulation of autophagosomes and of free cholesterol in endolysosomes in patient hair follicles [22]. A first link with neurodegenerative diseases was established when four SNPs in ABCA5 were associated with a reduced risk for Parkinson’s disease (PD) in GWAS [110]. In fact, ABCA5 mRNA expression is significantly increased in PD patient brains [108]. Similar to ABCA1, ABCA5 mRNA expression is significantly elevated in the hippocampus of AD patient brains [106, 111]. Increased expression of human ABCA5 in vitro significantly reduces Aβ load, mediated by changes in the processing of APP, suggesting a potential protective role for ABCA5 in AD [106].
ABCA7
Lipid metabolism
Like ABCA1, ABCA7 transfected in vitro mediates plasma HDL formation by releasing cholesterol and phospholipids to ApoA1, the major apolipoprotein in the blood. However, the relative release of cholesterol to phospholipids is much lower than for ABCA1 [112,113,114,115]. Recent research showed an increase in ATPase activity of purified ABCA7 in the presence of ApoA1 and ApoE, suggesting a direct interaction between ABCA7 and apolipoproteins [83]. Interestingly, an isoform-dependent stimulation was seen for ApoE, with both ApoE ε4 and ApoE ε2 resulting in a weaker ATPase stimulation compared to ApoE ε3, despite their opposite effects on AD risk, suggesting distinct binding efficiencies between ABCA7 and ApoE isoforms [83]. Additionally, Abca7 knockout mice showed lower serum and HDL cholesterol levels than wild type mice but only in females, despite the fact that ApoA1-stimulated lipid efflux from macrophages did not differ between wild type and Abca7 knockout mice [116]. Likewise, suppression of endogenous Abca7 mRNA by siRNA in mouse fibroblasts did not influence ApoA1-mediated cellular lipid release [117]. In contrast to ABCA1, ABCA7 gene and protein expression is downregulated in BALB/3T3 cells by increased cellular cholesterol and upregulated by cholesterol depletion via the sterol regulatory element-binding protein 2 (SREBP2) pathway [118]. Despite the high homology between ABCA1 and ABCA7, the latter does not compensate for the loss of ABCA1 in Tangier disease patients, further questioning the exact role of ABCA7 in cellular cholesterol release [119]. Moreover, thymocytes and antigen-presenting cells from Abca7 knockout mice show a reduced trafficking of CD1d, a glycoprotein that presents lipid antigens to natural-killer T (NKT) cells, to the plasma membrane as well as a reduction in lipid rafts, leading to a decreased CD1d content within lipid rafts and subsequently a reduction in NKT cell activation [120]. The role of ABCA1 in NKT cell development has not been studied yet.
Phagocytosis
Besides its role in lipid regulation, ABCA7 is functionally linked with phagocytosis. This hypothesis is based on the sequence similarity of ABCA1 and ABCA7 with ced-7, a C. elegans gene involved in the engulfment of cell corpses during programmed cell death [121, 122]. Indeed, Iwamoto and colleagues found that suppression of Abca7 with siRNA in mouse fibroblasts results in impaired phagocytic activity, while lipid release is not influenced [118]. Both in vitro and in vivo studies show abolished phagocytosis in Abca7 deficient mouse macrophages, while phagocytosis was not altered in ABCA1 deficient cells [117, 122]. Decreasing cellular lipid content using statins was found to enhance phagocytosis via upregulation of ABCA7 in vitro and in mice, indicating a direct link between cholesterol homeostasis and phagocytosis [123]. Mechanistically, ABCA7 and low-density lipoprotein receptor–related protein 1 (LRP1) colocalize at the cell surface of macrophages after stimulation by apoptotic cells. Interaction of LRP1 with apoptotic cells induces extracellular signal–regulated kinase (ERK) signaling, which is required for phagocytosis. Abca7-deficiency abolishes ERK signaling by a decreased trafficking of ABCA7 and LRP1 to the cell surface [122]. Of note, LRP1 also interacts with APP to regulate its processing and subsequent Aβ production [124]. Microglia from heterozygous Abca7 knockout AD mice show an abnormal accumulation of Aβ, likely due to a disturbed endosomal-lysosomal trafficking [125]. These data indicate that, unlike its closest homolog ABCA1, the primary function of ABCA7 is more likely in phagocytosis than in HDL metabolism. In fact, highest ABCA7 gene expression in primary human brain cells is found in microglia, consistent with a role in phagocytosis [55].
Functional evidence for a role in AD
Investigation of the cognitive phenotype of Abca7 knockout mice showed sex-specific differences, revealing impaired novel object recognition in males and impaired spatial reference memory in females [126]. Both in vitro and mice studies show that ABCA7 deficiency results in an increased Aβ load, which can be explained by changes in APP processing [127,128,129] and/or a reduction in phagocytosis of Aβ by microglia and macrophages [130, 131]. Conversely, AD mice overexpressing ABCA7 show a reduced Aβ expression compared to control AD mice expressing only endogenous ABCA7, as well as an improvement in cognitive function [129]. Expression of murine ABCA7 was also identified in an in vitro BBB model, consisting of primary mouse brain capillary endothelial cell cultures, and was found to regulate the efflux of cholesterol as well as Aβ across the BBB [132]. In addition, primary cultured mice hippocampal neurons show a reduced cell viability and activation of ER stress in the presence of Aβ1-42, while overexpression of ABCA7 eliminates these effects [129]. A recent study by Lyssenko and colleagues found reduced ABCA7 protein levels in individuals who developed AD neuropathology at a younger age compared to those who develop it at a later age [133]. These data provide evidence that ABCA7 plays a protective role against AD.
Genetics of ABCA7
ABCA7 was cloned from human macrophages in the year 2000 [84]. A decade later, ABCA7 was first linked to a human disease after GWAS found a strong association between common SNPs in ABCA7 and LOAD in Caucasian and African American (AA) cohorts [15, 28, 134,135,136]. Resequencing studies of ABCA7 in AD patient and control cohorts identified a significant enrichment in patients of rare (minor allele frequency (MAF) ≤ 1%) heterozygous variants, predicted to lead to a PTC. However, multiple studies in Caucasian cohorts show that the association of PTC mutations with AD is independent of the GWAS hits initially identified [79,80,81, 137,138,139]. Rare PTC mutations include nonsense, frameshift and splice site mutations as well as the noncanonical c.5570 + 5G > C mutation, which leads to a stop codon due to aberrant splicing of exon 41, and are found in up to 5% of the Caucasian AD patients [140]. Instead, in African Americans, a PTC mutation (rs142076058; p.R587fs), which explains an AA-specific GWAS hit, was identified in over 15% of the patients [141]. In addition, CpG methylation in the ABCA7 locus is significantly associated with AD [142].
PTC mutations are expected to lead to a loss-of-function due to nonsense-mediated decay (NMD), a quality control mechanism that removes transcripts containing a premature stop codon. However, ABCA7 transcript analysis revealed not only escape from NMD, but also transcript rescue, with high variability between the different PTC mutations [80]. Further, a strong association was identified between a GWAS hit and a variable number tandem repeat (VNTR) in intron 18 [143]. Longer VNTR alleles are enriched in AD patients and correlate with a decrease in overall ABCA7 expression and an increase in alternative splicing leading to in-frame skipping of exon 19, which results in a partial loss of the first NBD [143]. Moreover, rare missense mutations are enriched in patients compared to healthy controls [142]. Recent work shows that predicted deleterious ABCA7 missense mutations cause subcellular protein mislocalization as demonstrated in HeLa cells. Wild type ABCA7 is predominantly expressed at the plasma membrane [78, 113]. However, the mutated protein is not able to reach the plasma membrane and is retained in the ER instead, resulting in a loss of functional protein [78]. Not surprisingly, these findings are in line with data from ABCA1, 3 and 4. Disease-associated missense mutations in these ABCA genes are found to lead to protein mislocalization or to impact the function of the protein by abolishing substrate binding and/or ATPase activity [144,145,146]. Interestingly, functional and subcellular localization studies on disease-causing ABCA1 mutations and their corresponding ABCA4 mutations show similar effects, implying a comparable structure–function relationship [147]. Consequently, pathogenic missense mutations in ABCA1, 3, 4, 5 and 12, which are implicated in human diseases, might pinpoint amino acid residues that are essential for the correct functioning of ABCA transporters in general. Alignment of the ABCA amino acid sequences shows that several published ABCA7 missense mutations affect amino acid residues which are conserved between ABCA7 and ABCA1, 4 and 12 and that these residues, when mutated in ABCA1, 4 and 12, are disease-causing (Fig. 2). Additionally, a protective effect is observed for a common ABCA7 missense variant (rs72973581; p.G215S), suggesting a bidirectional effect of missense mutations [82]. A high-throughput assay to assess the functional impact of ABCA7 variants is currently not available. Characterization of subcellular localization and functional effects of ABCA7 missense mutations is needed to discriminate pathogenic variants from neutral or protective variants. Recently, a cryo-electron microscopy structure of ABCA7 was announced (PDB ID: 7KQC) which will advance our understanding of the mechanistic consequences of missense mutations [83].
Bi-allelic mutations i.e., homozygous or compound heterozygous mutations, in ABCA1, 3, 4, 5 and 12 are implicated in recessive disorders. Monoallelic mutations are linked to increased disease risk or milder phenotypes [54, 148, 149]. Earlier, we explored the role of rare homozygous and compound heterozygous mutations in ABCA7 and identified cis and trans compound heterozygous mutations but no rare homozygous mutations [78]. Although presently understudied, deep-intronic and common variants in ABCA4 play a role in disease as well and can contribute to recessive inheritance [150, 151]. Likewise, investigating the contribution of common and noncoding variants in ABCA7 is important since they might influence disease risk or act as a modifier in carriers of a PTC or deleterious missense mutation.
Clinicopathological phenotype of ABCA7 mutation carriers
ABCA7 was initially associated with LOAD in GWAS, while PTC mutations are enriched in both LOAD and EOAD patients [79,80,81]. PTC carriers present with a very wide range in onset age of 46–90 years old [81, 140]. Onset age is independent of the type of PTC mutation, since variable onset age is observed between carriers of the same PTC mutation, suggesting the involvement of modifying factors [140]. However, presence of the APOE ε4 allele does not have a significant impact on onset age in these carriers [140]. Investigation of larger cohorts of carriers is of high importance to study the co-occurrence with APOE isoforms or other potential genetic and environmental modifiers [140]. Besides the possible presence of genetic modifiers, NMD-efficiency and ABCA7 protein expression may play a role in age-related penetrance of PTC mutations. In fact, NMD efficiency and protein expression differ between individual PTC mutations, whereas transcript rescue differs even between carriers of the same PTC mutation [80]. Carriers of an expanded VNTR allele also present with widely variable onset ages, ranging from 44–90 years old, although no association is observed between VNTR length and onset age [143].
In PTC mutation carriers, clear clinically defining characteristics are absent, and most carriers present with a classical amnestic AD phenotype albeit with a higher vascular involvement (Hendrickx Van de Craen et al., unpublished) [152]. Brain autopsy of 10 PTC mutation carriers (five frameshift, three splice and two nonsense mutations) and six missense mutation carriers shows hallmark AD pathology (i.e., senile plaques and neurofibrillary tangles), as well as CAA in all carriers plus capillary (type 1) CAA in all but one nonsense mutation (p.W1336*) carrier (Hendrickx Van de Craen et al., unpublished) [78, 152]. A genetic association study exploring the association of AD risk loci with AD neuropathological features in 256 participants aged ≥ 85 years identified a significant association between the ABCA7 locus and Braak stage as well as CAA, but not capillary CAA [153]. Cerebrospinal fluid biomarker analysis revealed a negative correlation between Aβ1–42 and VNTR length [143].
Familial clustering is higher in PTC carriers compared to the overall AD cohort, with a positive familial history found in up to 77% of PTC carriers [80, 140, 152]. Apparent co-segregation of missense mutations (p.R880Q, p.G1820S) [78, 154] and PTC mutations (p.E709fs, p.R578fs, c.3578-2A > C and p.L1043fs) [138, 141, 154, 155] has been described in six pedigrees, all mimicking an autosomal dominant inheritance pattern. However, co-segregation in these pedigrees is not significant, because the pedigrees are too small to reach a significant logarithm of the odds (LOD) score. Zhao and colleagues developed a rare variant non-parametric linkage analysis method to detect rare variants contributing to complex diseases segregating in families and applied this method to whole genome sequencing data of 107 LOAD pedigrees with Caribbean Hispanic and European ancestry [156]. Nominal significant linkage was observed for ABCA7, with 13 rare missense variants segregating in 20 Caribbean Hispanic families [156].
The increased disease risk and familial clustering associated with ABCA7 PTC mutations suggest that these carriers might represent a genetic subtype of AD. In the future, screening of ABCA7 PTC mutations in clinical practice might improve AD diagnosis and risk prediction, mainly in familial AD patients negative for mutations in APP, PSEN1 and PSEN2. However, further research is necessary to elucidate both the pathogenicity and the disease penetrance of individual ABCA7 PTC mutations.
Conclusions
Functional studies suggest a central role for ABCA transporters in the maintenance of the cholesterol-homeostasis in the brain. Dysregulation of these transporters might result in cholesterol accumulation, leading to toxic effects and neurodegeneration. ABCA7 was first associated with AD by GWAS. Resequencing studies have identified an enrichment of PTC mutations, missense mutations and an expanded VNTR in AD patients. Recently, ABCA1 was also linked to AD in GWAS, although the underlying functional variants are currently unknown. In total, genetic and functional studies link four members of the ABCA subfamily (ABCA1, 2, 5 and 7) to AD. All four members were found to modulate Aβ deposition, one of the neuropathological hallmarks of AD, albeit in different directions. Disease-modelling using patient-derived iPSCs can allow to identify and study the cellular mechanisms disturbed by mutations in ABCA genes in human brain cells. A better understanding of the function of ABCA transporters in physiological and pathophysiological conditions will help to better understand their role in the etiopathogenesis of AD and might aid the development of new therapeutic strategies as well as contribute to the genetic diagnosis of AD.
Availability of data and materials
Not applicable.
Abbreviations
- AA:
-
African American
- ABCA:
-
ATP-binding cassette transporter member A
- ABC:
-
ATP-binding cassette transporter
- AD:
-
Alzheimer’s disease
- APOE:
-
Apolipoprotein E
- Aβ:
-
Amyloid β
- APP:
-
Amyloid precursor protein
- BBB:
-
Blood brain barrier
- CAA:
-
Cerebral amyloid angiopathy
- EOAD:
-
Early-onset Alzheimer’s disease
- ERK:
-
Extracellular signal–regulated kinase
- GWAS:
-
Genome-wide association study
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- LOAD:
-
Late-onset Alzheimer’s disease
- LRP1:
-
Low-density lipoprotein receptor–related protein 1
- LXR:
-
Liver X receptor
- NBD:
-
Nucleotide binding domain
- NKT:
-
Natural-killer T cell
- NMD:
-
Nonsense-mediated mRNA decay
- PSEN1/2:
-
Presenilin 1/2
- PTC:
-
Premature termination codon
- SNP:
-
Single nucleotide polymorphism
- SREBP2:
-
Sterol regulatory element-binding protein 2
- TD:
-
Tangier disease
- TMD:
-
Transmembrane domain
- VNTR:
-
Variable number tandem repeat
References
Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17(3):327–406.
Schellenberg GD, Montine TJ. The genetics and neuropathology of Alzheimer’s disease. Acta Neuropathol. 2012;124(3):305–23.
Cacace R, Sleegers K, Van Broeckhoven C. Molecular genetics of early-onset Alzheimer’s disease revisited. Alzheimers Dement. 2016;12(6):733–48.
Wingo TS, et al. Autosomal Recessive Causes Likely in Early-Onset Alzheimer Disease. Arch Neurol. 2012;69(1):59–64.
Goate A, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349(6311):704–6.
Rogaev EI, et al. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature. 1995;376(6543):775–8.
Levy-Lahad E, et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269(5226):973–7.
Zhang Y-W, et al. APP processing in Alzheimer’s disease. Mol Brain. 2011;4(1):3.
Oikawa N, Walter J. Presenilins and γ-Secretase in Membrane Proteostasis. Cells. 2019;8(3):209.
Gatz M, et al. Role of Genes and Environments for Explaining Alzheimer Disease. Arch Gen Psychiatry. 2006;63(2):168–74.
Pericak-Vance MA, et al. Linkage studies in familial Alzheimer disease: evidence for chromosome 19 linkage. Am J Hum Genet. 1991;48(6):1034–50.
Strittmatter WJ, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90(5):1977–81.
Husain MA, Laurent B, Plourde M. APOE and Alzheimer’s Disease: From Lipid Transport to Physiopathology and Therapeutics. Front Neurosci. 2021;15:85.
Bellenguez, C., et al., New insights on the genetic etiology of Alzheimer’s and related dementia. medRxiv, 2020: p. 2020.10.01.20200659.
Hollingworth P, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43(5):429–35.
Dean M, Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet. 2005;6:123–42.
Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42(7):1007–17.
Linton KJ, Higgins CF. Structure and function of ABC transporters: the ATP switch provides flexible control. Pflügers Arch Eur J Physiol. 2007;453(5):555–67.
Peelman F, et al. Characterization of the ABCA Transporter Subfamily: Identification of Prokaryotic and Eukaryotic Members, Phylogeny and Topology. J Mol Biol. 2003;325(2):259–74.
Kaminski WE, Piehler A, Wenzel JJ. ABC A-subfamily transporters: Structure, function and disease. Biochimica et Biophysica Acta (BBA) - Mol Basis Dis. 2006;1762(5):510–24.
Kelsell DP, et al. Mutations in ABCA12 underlie the severe congenital skin disease harlequin ichthyosis. Am J Hum Genet. 2005;76(5):794–803.
DeStefano GM, et al. Mutations in the cholesterol transporter gene ABCA5 are associated with excessive hair overgrowth. PLoS Genet. 2014;10(5):e1004333–e1004333.
Shulenin S, et al. ABCA3 Gene Mutations in Newborns with Fatal Surfactant Deficiency. N Engl J Med. 2004;350(13):1296–303.
Allikmets R, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15(3):236–46.
Bodzioch M, et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22(4):347–51.
Knight HM, et al. A Cytogenetic Abnormality and Rare Coding Variants Identify ABCA13 as a Candidate Gene in Schizophrenia, Bipolar Disorder, and Depression. Am J Human Gen. 2009;85(6):833–46.
Nakato M, et al. ABCA13 dysfunction associated with psychiatric disorders causes impaired cholesterol trafficking. J Biol Chem. 2021;296:100166.
Naj AC, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43(5):436–41.
Cheng X, Smith JC. Biological Membrane Organization and Cellular Signaling. Chem Rev. 2019;119(9):5849–80.
Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12(2):105–12.
Pfrieger FW, Ungerer N. Cholesterol metabolism in neurons and astrocytes. Prog Lipid Res. 2011;50(4):357–71.
Pfrieger FW. Outsourcing in the brain: do neurons depend on cholesterol delivery by astrocytes? BioEssays. 2003;25(1):72–8.
Wahrle SE, et al. ABCA1 Is Required for Normal Central Nervous System ApoE Levels and for Lipidation of Astrocyte-secreted apoE*. J Biol Chem. 2004;279(39):40987–93.
Fagan AM, Holtzman DM. Astrocyte lipoproteins, effects of apoE on neuronal function, and role of apoE in amyloid-beta deposition in vivo. Microsc Res Tech. 2000;50(4):297–304.
Lütjohann D, et al. Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc Natl Acad Sci USA. 1996;93(18):9799–804.
Chang, T.-Y., et al., Acyl-coenzyme A:cholesterol acyltransferases. American journal of physiology. Endocrinology and metabolism, 2009. 297(1): p. E1-E9.
Gosselet F, et al. Transcriptional profiles of receptors and transporters involved in brain cholesterol homeostasis at the blood–brain barrier: Use of an in vitro model. Brain Res. 2009;1249:34–42.
Cho YY, Kwon O-H, Chung S. Preferred Endocytosis of Amyloid Precursor Protein from Cholesterol-Enriched Lipid Raft Microdomains. Mol (Basel, Switzerland). 2020;25(23):5490.
Kojro E, et al. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the α-secretase ADAM 10. Proc Natl Acad Sci. 2001;98(10):5815–20.
Anstey KJ, Ashby-Mitchell K, Peters R. Updating the Evidence on the Association between Serum Cholesterol and Risk of Late-Life Dementia: Review and Meta-Analysis. J Alzheimer’s Dis : JAD. 2017;56(1):215–28.
Torrandell-Haro G, et al. Statin therapy and risk of Alzheimer’s and age-related neurodegenerative diseases. Alzheimers Dement (N Y). 2020;6(1):e12108.
Björkhem I. Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med. 2006;260(6):493–508.
Mahley RW, Rall SC Jr. Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–37.
Rannikmäe K, et al. APOE associations with severe CAA-associated vasculopathic changes: collaborative meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85(3):300–5.
Castellano JM, et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Trans Med. 2011;3(89):89ra57-89ra57.
Wisniewski T, et al. Acceleration of Alzheimer’s fibril formation by apolipoprotein E in vitro. Am J Pathol. 1994;145(5):1030–5.
LaDu MJ, et al. Isoform-specific binding of apolipoprotein E to β-amyloid. J Biol Chem. 1994;269(38):23403–6.
Tokuda T, et al. Lipidation of apolipoprotein E influences its isoform-specific interaction with Alzheimer’s amyloid beta peptides. Biochem J. 2000;348 Pt 2(Pt 2):359–65.
Michikawa M, et al. Apolipoprotein E exhibits isoform-specific promotion of lipid efflux from astrocytes and neurons in culture. J Neurochem. 2000;74(3):1008–16.
Jansen IE, et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat Genet. 2019;51(3):404–13.
Kunkle BW, et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet. 2019;51(3):414–30.
Tall AR, Wang N. Tangier disease as a test of the reverse cholesterol transport hypothesis. J Clin Investig. 2000;106(10):1205–7.
Ohashi R, et al. Reverse cholesterol transport and cholesterol efflux in atherosclerosis. QJM: An Int J Med. 2005;98(12):845–56.
Brooks-Wilson A, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22(4):336–45.
Kim WS, et al. Quantitation of ATP-binding cassette subfamily-A transporter gene expression in primary human brain cells. Neuroreport. 2006;17(9):891–6.
Do TM, et al. Direct evidence of abca1-mediated efflux of cholesterol at the mouse blood-brain barrier. Mol Cell Biochem. 2011;357(1–2):397–404.
Fitz NF, et al. ABCA1 Deficiency Affects Basal Cognitive Deficits and Dendritic Density in Mice. J Alzheimer’s Dis : JAD. 2017;56(3):1075–85.
Zhao J, et al. APOE ε4/ε4 diminishes neurotrophic function of human iPSC-derived astrocytes. Hum Mol Genet. 2017;26(14):2690–700.
Koldamova R, Staufenbiel M, Lefterov I. Lack of ABCA1 considerably decreases brain ApoE level and increases amyloid deposition in APP23 mice. J Biol Chem. 2005;280(52):43224–35.
Wahrle SE, et al. Deletion of Abca1 Increases Aβ Deposition in the PDAPP Transgenic Mouse Model of Alzheimer Disease*. J Biol Chem. 2005;280(52):43236–42.
Lefterov I, et al. Memory deficits in APP23/Abca1+/- mice correlate with the level of Aβ oligomers. ASN neuro. 2009;1(2):e00006.
Fitz NF, et al. Abca1 deficiency affects Alzheimer’s disease-like phenotype in human ApoE4 but not in ApoE3-targeted replacement mice. J Neurosci. 2012;32(38):13125–36.
Wahrle SE, et al. Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J Clin Invest. 2008;118(2):671–82.
Boehm-Cagan A, et al. ABCA1 Agonist Reverses the ApoE4-Driven Cognitive and Brain Pathologies. J Alzheimers Dis. 2016;54(3):1219–33.
Rawat V, et al. ApoE4 Alters ABCA1 Membrane Trafficking in Astrocytes. J Neurosci. 2019;39(48):9611–22.
de Leeuw SM, et al. APOE2, E3, and E4 differentially modulate cellular homeostasis, cholesterol metabolism, and inflammatory response in isogenic iPSC-derived astrocytes. Stem cell reports. 2022;17(1):110–26.
Costet P, et al. Sterol-dependent Transactivation of theABC1 Promoter by the Liver X Receptor/Retinoid X Receptor*. J Biol Chem. 2000;275(36):28240–5.
Fitz NF, et al. Therapeutic targeting of nuclear receptors, liver X and retinoid X receptors, for Alzheimer’s disease. Br J Pharmacol. 2019;176(18):3599–610.
Fitz NF, et al. Liver X receptor agonist treatment ameliorates amyloid pathology and memory deficits caused by high-fat diet in APP23 mice. J Neurosci. 2010;30(20):6862–72.
Donkin JJ, et al. ATP-binding cassette transporter A1 mediates the beneficial effects of the liver X receptor agonist GW3965 on object recognition memory and amyloid burden in amyloid precursor protein/presenilin 1 mice. J Biol Chem. 2010;285(44):34144–54.
Koldamova RP, et al. The Liver X Receptor Ligand T0901317 Decreases Amyloid β Production in Vitro and in a Mouse Model of Alzheimer’s Disease*. J Biol Chem. 2005;280(6):4079–88.
Ho Hong S, et al. Novel ABCA1 compound variant associated with HDL cholesterol deficiency. Biochim Biophys Acta. 2002;1587(1):60–4.
Blacker D, et al. Results of a high-resolution genome screen of 437 Alzheimer’s Disease families. Hum Mol Genet. 2003;12(1):23–32.
Pericak-Vance MA, et al. Identification of novel genes in late-onset Alzheimer’s disease. Exp Gerontol. 2000;35(9–10):1343–52.
Nordestgaard LT, et al. Loss-of-function mutation in ABCA1 and risk of Alzheimer’s disease and cerebrovascular disease. Alzheimers Dement. 2015;11(12):1430–8.
Beecham GW, et al. Rare genetic variation implicated in non-Hispanic white families with Alzheimer disease. Neurology Genetics. 2018;4(6):e286–e286.
Landry YD, et al. ATP-binding Cassette Transporter A1 Expression Disrupts Raft Membrane Microdomains through Its ATPase-related Functions*. J Biol Chem. 2006;281(47):36091–101.
Bossaerts, L., et al., Rare missense mutations in ABCA7 might increase Alzheimer’s disease risk by plasma membrane exclusion. Acta Neuropathologica Communications, Preprint side Research Square, 2022.
Bellenguez C, et al. Contribution to Alzheimer’s disease risk of rare variants in TREM2, SORL1, and ABCA7 in 1779 cases and 1273 controls. Neurobiol Aging. 2017;59:220.e1-220.e9.
De Roeck A, et al. Deleterious ABCA7 mutations and transcript rescue mechanisms in early onset Alzheimer’s disease. Acta Neuropathol. 2017;134(3):475–87.
Le Guennec K, et al. ABCA7 rare variants and Alzheimer disease risk. Neurology. 2016;86(23):2134.
Sassi C, et al. ABCA7 p.G215S as potential protective factor for Alzheimer’s disease. Neurobiol Aging. 2016;46:235.e1-235.e2359.
My Le, L.T., et al., Cryo-EM structure of lipid embedded human ABCA7 at 3.6Å resolution. bioRxiv, 2021: p. 2021.03.01.433448.
Kaminski WE, et al. Identification of a Novel Human Sterol-Sensitive ATP-Binding Cassette Transporter (ABCA7). Biochem Biophys Res Commun. 2000;273(2):532–8.
Kaminski WE, et al. Complete Coding Sequence, Promoter Region, and Genomic Structure of the Human ABCA2 Gene and Evidence for Sterol-Dependent Regulation in Macrophages. Biochem Biophys Res Commun. 2001;281(1):249–58.
Luciani MF, et al. Cloning of Two Novel ABC Transporters Mapping on Human Chromosome 9. Genomics. 1994;21(1):150–9.
Vulevic B, et al. Cloning and Characterization of Human Adenosine 5′-triphosphate-binding Cassette, Sub-family A, Transporter 2 (<em>ABCA2</em>). Can Res. 2001;61(8):3339.
Broccardo C, et al. ABCA2 is a marker of neural progenitors and neuronal subsets in the adult rodent brain. J Neurochem. 2006;97(2):345–55.
Davis W Jr, et al. Human ATP-binding cassette transporter-2 (ABCA2) positively regulates low-density lipoprotein receptor expression and negatively regulates cholesterol esterification in Chinese hamster ovary cells. Biochim Biophys Acta. 2004;1683(1–3):89–100.
Kim WS, et al. Role of ABCG1 and ABCA1 in Regulation of Neuronal Cholesterol Efflux to Apolipoprotein E Discs and Suppression of Amyloid-β Peptide Generation*. J Biol Chem. 2007;282(5):2851–61.
Davis W Jr. The ATP-binding cassette transporter-2 (ABCA2) regulates cholesterol homeostasis and low-density lipoprotein receptor metabolism in N2a neuroblastoma cells. Biochim Biophys Acta. 2011;1811(12):1152–64.
Tanaka Y, et al. Temporal and spatial profiles of ABCA2-expressing oligodendrocytes in the developing rat brain. J Comp Neurol. 2003;455(3):353–67.
Zhou CJ, et al. ATP-binding cassette transporter ABCA2 (ABC2) expression in the developing spinal cord and PNS during myelination. J Comp Neurol. 2002;451(4):334–45.
Mack JT, et al. “Skittish” Abca2 knockout mice display tremor, hyperactivity, and abnormal myelin ultrastructure in the central nervous system. Mol Cell Biol. 2007;27(1):44–53.
Sakai H, et al. ABCA2 Deficiency Results in Abnormal Sphingolipid Metabolism in Mouse Brain*. J Biol Chem. 2007;282(27):19692–9.
Chen ZJ, et al. Association of ABCA2 expression with determinants of Alzheimer’s disease. Faseb j. 2004;18(10):1129–31.
Davis W Jr. The ATP-binding cassette transporter-2 (ABCA2) increases endogenous amyloid precursor protein expression and Aβ fragment generation. Curr Alzheimer Res. 2010;7(7):566–77.
Michaki V, et al. Down-regulation of the ATP-binding cassette transporter 2 (Abca2) reduces amyloid-β production by altering Nicastrin maturation and intracellular localization. J Biol Chem. 2012;287(2):1100–11.
Grbovic OM, et al. Rab5-stimulated up-regulation of the endocytic pathway increases intracellular beta-cleaved amyloid precursor protein carboxyl-terminal fragment levels and Abeta production. J Biol Chem. 2003;278(33):31261–8.
Hu W, et al. ATP Binding Cassette Subfamily A Member 2 (ABCA2) Expression and Methylation are Associated with Alzheimer’s Disease. Medical Science Monit. 2017;23:5851–61.
Macé S, et al. ABCA2 is a strong genetic risk factor for early-onset Alzheimer’s disease. Neurobiol Dis. 2005;18(1):119–25.
Wollmer MA, et al. Ethnicity-dependent genetic association of ABCA2 with sporadic Alzheimer’s disease. Am J Med Genet B Neuropsychiatr Genet. 2006;141b(5):534–6.
Minster RL, DeKosky ST, Kamboh MI. No association of DAPK1 and ABCA2 SNPs on chromosome 9 with Alzheimer’s disease. Neurobiol Aging. 2009;30(11):1890–1.
Xu X, et al. Meta-analyses of 8 polymorphisms associated with the risk of the Alzheimer’s disease. PLoS ONE. 2013;8(9):e73129–e73129.
Arnould I, et al. Identifying and characterizing a five-gene cluster of ATP-binding cassette transporters mapping to human chromosome 17q24: a new subgroup within the ABCA subfamily. Genescreen. 2001;1(3):157–64.
Fu Y, et al. ABCA5 regulates amyloid-β peptide production and is associated with Alzheimer’s disease neuropathology. J Alzheimers Dis. 2015;43(3):857–69.
Ray AG, et al. Novel Mechanism of Cholesterol Transport by ABCA5 in Macrophages and Its Role in Dyslipidemia. J Mol Biol. 2020;432(17):4922–41.
Kim WS, Halliday GM. Changes in sphingomyelin level affect alpha-synuclein and ABCA5 expression. J Parkinsons Dis. 2012;2(1):41–6.
Kubo Y, et al. ABCA5 resides in lysosomes, and ABCA5 knockout mice develop lysosomal disease-like symptoms. Mol Cell Biol. 2005;25(10):4138–49.
Simón-Sánchez J, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41(12):1308–12.
Kim WS, et al. Increased ATP-binding cassette transporter A1 expression in Alzheimer’s disease hippocampal neurons. J Alzheimers Dis. 2010;21(1):193–205.
Abe-Dohmae S, et al. Human ABCA7 Supports Apolipoprotein-mediated Release of Cellular Cholesterol and Phospholipid to Generate High Density Lipoprotein*. J Biol Chem. 2004;279(1):604–11.
Ikeda Y, et al. Posttranscriptional regulation of human ABCA7 and its function for the apoA-I-dependent lipid release. Biochem Biophys Res Commun. 2003;311(2):313–8.
Hayashi M, et al. Heterogeneity of high density lipoprotein generated by ABCA1 and ABCA7. J Lipid Res. 2005;46(8):1703–11.
Linsel-Nitschke P, et al. Potential role of ABCA7 in cellular lipid efflux to apoA-I. J Lipid Res. 2005;46(1):86–92.
Kim WS, et al. Abca7 Null Mice Retain Normal Macrophage Phosphatidylcholine and Cholesterol Efflux Activity despite Alterations in Adipose Mass and Serum Cholesterol Levels*. J Biol Chem. 2005;280(5):3989–95.
Tanaka N, et al. Helical apolipoproteins of high-density lipoprotein enhance phagocytosis by stabilizing ATP-binding cassette transporter A7. J Lipid Res. 2010;51(9):2591–9.
Iwamoto N, et al. ABCA7 expression is regulated by cellular cholesterol through the SREBP2 pathway and associated with phagocytosis. J Lipid Res. 2006;47(9):1915–27.
Abe-Dohmae S, Ueda K, Yokoyama S. ABCA7, a molecule with unknown function. FEBS Lett. 2006;580(4):1178–82.
Nowyhed HN, et al. ATP Binding Cassette Transporter ABCA7 Regulates NKT Cell Development and Function by Controlling CD1d Expression and Lipid Raft Content. Sci Rep. 2017;7(1):40273.
Wu Y-C, Horvitz H.R. The C. elegans Cell Corpse Engulfment Gene ced-7 Encodes a Protein Similar to ABC Transporters. Cell. 1998;93(6):951–60.
Jehle AW, et al. ATP-binding cassette transporter A7 enhances phagocytosis of apoptotic cells and associated ERK signaling in macrophages. J Cell Biol. 2006;174(4):547–56.
Tanaka N, et al. HMG-CoA reductase inhibitors enhance phagocytosis by upregulating ATP-binding cassette transporter A7. Atherosclerosis. 2011;217(2):407–14.
Shinohara M, et al. Role of LRP1 in the pathogenesis of Alzheimer’s disease: evidence from clinical and preclinical studies: Thematic Review Series: ApoE and Lipid Homeostasis in Alzheimer’s Disease. J Lipid Res. 2017;58(7):1267–81.
Aikawa T, et al. ABCA7 haplodeficiency disturbs microglial immune responses in the mouse brain. Proc Natl Acad Sci USA. 2019;116(47):23790–6.
Logge W, et al. Role of Abca7 in mouse behaviours relevant to neurodegenerative diseases. PLoS ONE. 2012;7(9):e45959–e45959.
Satoh K, et al. ATP-binding cassette transporter A7 (ABCA7) loss of function alters Alzheimer amyloid processing. J Biol Chem. 2015;290(40):24152–65.
Sakae N, et al. ABCA7 Deficiency Accelerates Amyloid-β Generation and Alzheimer’s Neuronal Pathology. J Neurosci. 2016;36(13):3848–59.
Li M, et al. Study on Lentivirus-Mediated ABCA7 Improves Neurocognitive Function and Related Mechanisms in the C57BL/6 Mouse Model of Alzheimer’s Disease. J Mol Neurosci. 2017;61(4):489–97.
Kim WS, et al. Deletion of Abca7 increases cerebral amyloid-β accumulation in the J20 mouse model of Alzheimer’s disease. J Neurosci. 2013;33(10):4387–94.
Fu Y, et al. ABCA7 Mediates Phagocytic Clearance of Amyloid-β in the Brain. J Alzheimers Dis. 2016;54:569–84.
Lamartinière Y, et al. ABCA7 Downregulation Modifies Cellular Cholesterol Homeostasis and Decreases Amyloid-β Peptide Efflux in an in vitro Model of the Blood-Brain Barrier. J Alzheimers Dis. 2018;64:1195–211.
Lyssenko NN, Praticò D. ABCA7 and the altered lipidostasis hypothesis of Alzheimer’s disease. Alzheimer’s Dementia. 2021;17(2):164–74.
Lambert J-C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45(12):1452–8.
Logue MW, et al. A Comprehensive Genetic Association Study of Alzheimer Disease in African Americans. Arch Neurol. 2011;68(12):1569–79.
Reitz C, et al. Variants in the ATP-Binding Cassette Transporter (ABCA7), Apolipoprotein E ϵ4, and the Risk of Late-Onset Alzheimer Disease in African Americans. JAMA. 2013;309(14):1483–92.
Del-Aguila JL, et al. Role of ABCA7 loss-of-function variant in Alzheimer’s disease: a replication study in European-Americans. Alzheimer’s research & therapy. 2015;7(1):73–73.
Cuyvers E, et al. Mutations in ABCA7 in a Belgian cohort of Alzheimer’s disease patients: a targeted resequencing study. Lancet Neurology. 2015;14(8):814–22.
Steinberg S, et al. Loss-of-function variants in ABCA7 confer risk of Alzheimer’s disease. Nat Genet. 2015;47(5):445–7.
Bossaerts L, Hens E, Hanseeuw B, Vandenberghe R, Cras P, De Deyn PP, Engelborghs S, Van Broeckhoven C; BELNEU Consortium. Premature termination codon mutations in ABCA7 contribute to Alzheimer's disease risk in Belgian patients. Neurobiol Aging. 2021;106:307.e1–307.e7. https://doi.org/10.1016/j.neurobiolaging.2021.04.023.
Cukier HN, et al. ABCA7 frameshift deletion associated with Alzheimer disease in African Americans. Neurol Gen. 2016;2(3):e79–e79.
De Roeck A, Van Broeckhoven C, Sleegers K. The role of ABCA7 in Alzheimer’s disease: evidence from genomics, transcriptomics and methylomics. Acta Neuropathol. 2019;138(2):201–20.
De Roeck A, et al. An intronic VNTR affects splicing of ABCA7 and increases risk of Alzheimer’s disease. Acta Neuropathol. 2018;135(6):827–37.
Matsumura Y, et al. Characterization and Classification of ATP-binding Cassette Transporter ABCA3 Mutants in Fatal Surfactant Deficiency *. J Biol Chem. 2006;281(45):34503–14.
Singaraja RR, et al. Specific mutations in ABCA1 have discrete effects on ABCA1 function and lipid phenotypes both in vivo and in vitro. Circ Res. 2006;99(4):389–97.
Garces FA, Scortecci JF, Molday RS. Functional Characterization of ABCA4 Missense Variants Linked to Stargardt Macular Degeneration. Int J Mol Sci. 2020;22(1):185.
Quazi F, Molday RS. Differential phospholipid substrates and directional transport by ATP-binding cassette proteins ABCA1, ABCA7, and ABCA4 and disease-causing mutants. J Biol Chem. 2013;288(48):34414–26.
Allikmets R. Further evidence for an association of ABCR alleles with age-related macular degeneration. The International ABCR Screening Consortium. Am J Human Gen. 2000;67(2):487–91.
Westeneng-van Haaften SC, et al. Clinical and genetic characteristics of late-onset Stargardt’s disease. Ophthalmology. 2012;119(6):1199–210.
Runhart EH, et al. The Common ABCA4 Variant p.Asn1868Ile Shows Nonpenetrance and Variable Expression of Stargardt Disease When Present in trans With Severe Variants. Invest Ophthalmol Visual Sci. 2018;59(8):3220–31.
Sangermano R, et al. Deep-intronic ABCA4 variants explain missing heritability in Stargardt disease and allow correction of splice defects by antisense oligonucleotides. Genet Med. 2019;21(8):1751–60.
Van den Bossche T, et al. Phenotypic characteristics of Alzheimer patients carrying an ABCA7 mutation. Neurology. 2016;86(23):2126–33.
Mäkelä M, et al. Alzheimer risk loci and associated neuropathology in a population-based study (Vantaa 85+). Neurol Genet. 2018;4(1):e211.
May P, et al. Rare ABCA7 variants in 2 German families with Alzheimer disease. Neurology Genetics. 2018;4(2):e224.
Kunkle BW, et al. Targeted sequencing of ABCA7 identifies splicing, stop-gain and intronic risk variants for Alzheimer disease. Neurosci Lett. 2017;649:124–9.
Zhao L, et al. A Rare Variant Nonparametric Linkage Method for Nuclear and Extended Pedigrees with Application to Late-Onset Alzheimer Disease via WGS Data. Am J Human Gen. 2019;105(4):822–35.
Acknowledgements
Not applicable
Funding
The research was in part supported by the Flemish Government initiated Methusalem excellence program and the Flanders Impulse Program on Networks for Dementia Research.
Author information
Authors and Affiliations
Contributions
LB and CVB conceived the manuscript. LB and RC wrote the manuscript. RC and CVB edited the manuscript. LB prepared figures. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bossaerts, L., Cacace, R. & Van Broeckhoven, C. The role of ATP-binding cassette subfamily A in the etiology of Alzheimer’s disease. Mol Neurodegeneration 17, 31 (2022). https://doi.org/10.1186/s13024-022-00536-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13024-022-00536-w