Abstract
Background
cblC deficiency is the most common type of methylmalonic aciduria in China. Late-onset patients present with various non-specific symptoms and are usually misdiagnosed. The purpose of this study is to investigate the clinical features of patients with late-onset cblC deficiency and explore diagnosis and management strategies around puberty.
Results
This study included 56 patients (35 males and 21 females) with late-onset cblC deficiency who were admitted to our clinic between 2002 and September 2021. The diagnosis was confirmed by metabolic and genetic tests. The clinical and biochemical features, disease triggers, outcome, and associated genetic variants were examined. The onset age ranged from 10 to 20 years (median age, 12 years). Fifteen patients (26.8%) presented with symptoms after infection or sports training. Further, 46 patients (82.1%) had neuropsychiatric diseases; 11 patients (19.6%), cardiovascular diseases; and 6 patients (10.7%), pulmonary hypertension. Renal damage was observed in 6 cases (10.7%). Genetic analysis revealed 21 variants of the MMACHC gene in the 56 patients. The top five common variants detected in 112 alleles were c.482G > A (36.6%), c.609G > A (16.1%), c.658_660delAAG (9.8%), c.80A > G (8.0%), and c.567dupT (6.3%). Thirty-nine patients carried the c.482G > A variant. Among 13 patients who exhibited spastic paraplegia as the main manifestation, 11 patients carried c.482G > A variants. Six patients who presented with psychotic disorders and spastic paraplegia had compound heterozygotic c.482G > A and other variants. All the patients showed improvement after metabolic treatment with cobalamin, l-carnitine, and betaine, and 30 school-aged patients returned to school. Two female patients got married and had healthy babies.
Conclusions
Patients with late-onset cblC deficiency present with a wide variety of neuropsychiatric symptoms and other presentations, including multiple organ damage. As a result, cb1C deficiency can easily be misdiagnosed as other conditions. Metabolic and genetic studies are important for accurate diagnosis, and metabolic treatment with cobalamin, l-carnitine, and betaine appears to be beneficial.
Similar content being viewed by others
Background
Puberty is a critical stage for manifestation of late-onset inherited metabolic disorders. cblC deficiency is the most common defect in the intracellular cobalamin metabolism pathway and is characterized by variable and non-specific symptoms [1, 2]. Methylmalonic aciduria (MMA) combined with homocystinuria caused by cblC deficiency (OMIM 277400) accounts for 70% of the cases of MMA, which is the most common organic acid metabolic disorder in China [3,4,5]. The prevalence of cb1C deficiency in Shandong province of China was 1/3,920 according to data of Newborn Screening from 2011 to 2014 [6], but the nationwide prevalence of cblC deficiency is unclear.
In patients with cblC deficiency, the age of onset ranges from the prenatal to adult stage, and the clinical manifestations vary from mild to life-threatening [1, 2, 7]. The most common phenotype is the early-onset type, which usually affects the nervous system and mainly presents with developmental delay, epilepsy, lethargy, and hypotonia. In addition, the disease is often complicated with multiple organ damage, such as visual impairment, renal damage, hematological abnormalities, and cardiovascular diseases. Neonatal-onset cb1C deficiency is associated with critical illness, rapid progression, and high mortality. In the case of infancy-onset cb1C deficiency, infection and starvation are the most common triggers of metabolic crisis. Infants usually present with developmental delays, seizures, and confusion [2, 6]. Adolescence is a high-risk period for the manifestation of cblC deficiency. Most teenagers with late-onset cblC deficiency develop behavioral abnormalities, mental regression, or movement disorders [5, 8]. These symptoms are non-specific, and patients are easily misdiagnosed. Recently, more cases of late-onset cblC deficiency have been diagnosed, with some cases being reported in adolescence or adulthood [7, 9, 10]. Plasma total homocysteine (tHCY), blood amino acid levels, acyl-carnitine analysis, and genetic study are important for obtaining a definite diagnosis [11], and patients usually recover after receiving metabolic treatment with hydroxycobalamin or adenosylcobalamin, l-carnitine, and betaine [8, 11, 12]. However, most late-onset patients exhibit non-specific symptoms that may lead to misdiagnosis and mismanagement.
In our previous study on 1,003 patients with MMA, 705 (70.3%) had MMA combined with homocystinemia caused by cb1C deficiency and 567 (80.2%) had early-onset disease (before the age of 1 year). A total of 51 patients (7.2%) had late-onset disease (after the age of 4 years) and showed significant differences in phenotypes and outcomes [4]. However, there is not enough research on the clinical presentations, diagnosis, or treatment of late-onset cb1C deficiency. Therefore, in the present study, we have investigated the clinical features, triggers, metabolic profiles, and genotypes of late-onset cblC deficiency to gain an understanding of the early interventions that may be beneficial to treat and reverse this disease.
Results
Clinical course
Age of onset
The present study cohort included 56 patients, including 35 (62.5%) males and 21 females (37.5%) (Table 1). The age of onset ranged from 10 to 20 years (median age, 12 years). A total of 31 patients (55.4%) presented with symptoms between the ages of 10 and 12 years. Fifteen patients (26.8%) presented with symptoms within the age range of 13–15 years. Five patients (8.9%) developed symptoms between the ages of 16–18 years. Five patients (8.9%) presented with symptoms in the age range of 18–20 years.
Precipitating factors
Eight patients (14.3%) presented with symptoms on the day of fever or several days after infection, and the initial diagnosis in these cases was pneumonia or encephalitis. Seven patients (12.5%) presented with neuropsychiatric symptoms, such as psychotic behavioral disorders and movement disorders, on the day of strenuous exercise or several days after a sports training program. These patients were transitioning from primary school to middle school or from middle school to high school. The parents declared that the children did not have neurological or psychiatric problems prior to disease onset.
Symptoms
Forty-five patients (80.4%) mainly presented with neuropsychiatric diseases, and 33 patients (58.9%) had movement disorders. Thirteen patients (23.2%) presented with progressive spastic paralysis, and 21 patients (37.5%) had psychotic behavioral disorders, such as short temper, speaking nonsense words, hallucination, apathy, and overeating. Further, 18 patients (32.1%) showed signs of mental regression, such as memory loss, study weariness, and decrease in grade.
Ten patients (17.9%) mainly presented with cardiovascular diseases, and five patients (8.9%), with pulmonary hypertension. Cardiomyopathy was found in 2 patients (3.6%), and 2 patients (3.6%) had arrhythmia. Further, 6 patients (10.7%) presented with proteinuria, and 1 patient developed renal insufficiency.
8 (14.3%) patients had anemia; 4 (7.1%), anorexia; 4 patients (7.1%), overeating and obesity; 1 patient (1.8%), fatty liver; 2 patients (3.5%), visual impairments (nearsightedness, strabismus, and astigmatism) (Table 1).
Misdiagnosis
Prior to being diagnosed with cblC deficiency, the 56 patients had been misdiagnosed with other diseases and had received inappropriate treatment for 2 months to 6 years. The initial diagnosis was peripheral neuropathy, depression, schizophrenia, encephalitis, primary pulmonary hypertension, and epilepsy. Eight patients (14.3%) were misdiagnosed with encephalitis. Their manifestations included mental regression (3 cases, 37.5%), depression (4 cases, 50.0%), and behavioral abnormalities (5 cases 62.5%). Five patients (8.9%) exhibited proteinuria and/or hematuria during routine urine tests and had been previously diagnosed with nephritis.
Biochemical findings
All the patients had abnormal blood amino acid and acyl-carnitine profiles before treatment (Table 1). Their plasma tHCY values (48.3–213.8 mol/L; normal control value, < 15.0 mol/L) were significantly increased. In addition, elevated blood propionyl-carnitine (2.0–14.7 mol/L; normal control values, < 5.0 mol/L), propionyl-carnitine/acetyl-carnitine ratios (0.58–0.97; normal control value, < 0.5), and propionyl-carnitine/free carnitine ratios (0.3–0.74; normal control, < 0.25) were observed. Further, 12 patients had decreased blood free carnitine levels (5.55–14.51 mol/L; normal control range, 15.0–60.0 mol/L), and 40 patients had decreased blood methionine levels (5.4–9.5 mol/L; normal control range, 12.0–50.0 mol/L). Urine methylmalonic acid concentrations in all the patients were elevated (53.1–1787.0 mmol/mol creatinine; normal control range, 0.2–3.6 mmol/mol creatinine). These biochemical findings supported a diagnosis of MMA combined with homocystinuria. In addition, decreased serum 25-OH-vitamin D levels were observed in 15 patients (26.8%).
Genetic features
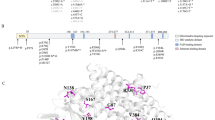
Twenty-one reported pathogenic variants were detected in the MMACHC gene of the 56 patients (Table 2, Additional file 1), and one patient got a PRDX1 variant causing secondary epimutation in MMACHC (PRDX1 c.515-48_515-47 insTT). The top five common variants were c.482G > A (36.6%), c.609G > A (16.1%), c.658_660delAAG (9.8%), c.80A > G (8.0%), and c.567dupT (6.3%). c.482G > A was the most frequent variant and was identified in 41 (36.6%) alleles. Further, 39 patients with neuropsychiatric diseases were found to have at least one allele mutated in c.482G > A. Two cases were homozygous for c.482G > A. Of 13 patients who presented with spastic paraplegia as the main manifestation, 11 had c.482G > A (84.6%). Six patients presented with both psychotic disorders and spastic paraplegia, and all of them had c.482G > A. Ten patients had compound heterozygotic variants of c.482G > A and c.658_660delAAG; seven patients displayed compound heterozygotic variants of c.482G > A and c.609G > A; and five patients had compound heterozygotic variants of c.482G > A and c.567dupT. c.609G > A was the second most common variant observed in the patients and was found in 18 cases (16.1%). Eleven patients had a c.609G > A variant and presented with neuropsychiatric diseases. Seven patients with c.80A > G and c.609G > A compound heterozygotic variants had neuropsychiatric symptoms and pulmonary hypertension.
Long-term treatment and follow-up
All 56 patients were treated with intramuscular injection of hydroxycobalamin (1 mg or 10 mg, two or three times a week) or adenosylcobalamin (1.5 or 3 mg, two or three times a week), supplemented with oral l-carnitine (1–2 g/d), betaine (3–6 g/d), and normal diet. For the patients with vitamin D deficiency, oral vitamin D supplementation was also recommended. All the patients showed significant clinical improvement after the metabolic treatment. Follow-up sessions were scheduled at 3, 6, and 12 months. Currently, the patients are 12–32 years old. Among the 33 patients who had movement disorders during the acute phase of the disease, 30 have recovered. The remaining three patients still have an unsteady walk because of spastic paralysis. Among the 30 patients with mental regression or psychotic problems, 29 have recovered and returned to school. One female patient is emotionally unstable and refuses to go back to school. Of the 12 patients who have reached adulthood, nine of them have successfully graduated from college and are working. Further, two female patients got successful pregnancies and had healthy babies [13].
Discussion
In the present study, we have described the clinical course, biochemical features, genetic findings, and the outcomes of 56 late-onset patients with combined MMA and homocystinuria caused by cblC deficiency. As there is a very little research on this topic, our findings will be valuable for both clinicians and physicians who encounter such cases in their settings.
In the present study, 56 previously healthy school children presented with varied manifestations of cb1C deficiency during adolescence. The parents declared that the children exhibited normal development without any neurological or psychiatric problems and other diseases before onset. Infection and strenuous exercise were considered to be the triggers in 15 patients who presented with movement disorders or psychotic symptoms after having infection or fatigue. Intense exercise and stress can trigger underlying metabolic diseases, so it is important to carefully investigate potential diseases in school children with exercise intolerance or training-related illnesses. Since these symptoms were non-specific, the present patients were misdiagnosed with schizophrenia, depression, autoimmune encephalitis, or neuromuscular diseases. Blood tHCY, amino acids, and acyl-carnitine profiles, and genetic analysis are important for the definite diagnosis of cblC deficiency and must be considered in previously healthy adolescents who present with such symptoms.
In the present study, 45 patients (80.4%) had neuropsychiatric symptoms and 33 patients had movement disorders. In addition, 21 patients had psychotic behavior disorders, such as bad temper, speaking nonsense word, and hallucinations, and 18 patients exhibited symptoms of mental regression (for example, their parents reported sudden onsets of inability to learn). A recently published China Mental Health Survey showed that depressive disorders have a high prevalence in adolescence [14], late-onset cblC deficiency around puberty maybe one of the causes. Five patients in the cohort presented with pulmonary hypertension, with the majority of the complaints being intolerance to sports or fainting during sport activities. Proteinuria and anemia were observed in some cases. All these findings were correlated with previously published literature [15,16,17]. Seventeen patients (29.8%) presented with other complications. Eight cases had anemia in the acute phase of the disease that improved quickly after metabolic treatment. Overeating and obesity were observed in four cases, and the patients’ physique improved gradually with the improvement of their mental symptoms. Nearsightedness, strabismus, and astigmatism were observed in two patients. However, these are common visual impairments in the general population, and it is difficult to determine whether they are related to cblC deficiency in the two cases.
Genetic study is crucial for a definite diagnosis of cblC deficiency. The mutation spectrums of the MMACHC gene vary among different populations [18, 19]. Among the 56 patients, we detected bi-allelic variants in MMACHC in 55 patients that involved 21 different types of reported pathogenic variants. The remaining patient carried a heterozygous variant in MMACHC and a PRDX1 variant causing secondary epimutation of MMACHC. The most common variant in MMACHC was c.482G > A (36.6%), and it was followed by c.609G > A (16.1%). In agreement with this finding, Lerner-Ellis et al. also found that c.482G > A was the most frequent variant in their population of late-onset cases, but the c.609G > A variant was not common in their population of late-onset cases [18]. c.609G > A is the most common variant in cblC-deficient patients in China [3, 5, 19]. In our previous study, variable phenotypes and outcomes associated with the MMACHC c.609G > A homologous mutation in 149 Chinese patients were observed. 101 (76.5%) cases had early-onset disease and 31 (23.5%) had late-onset disease [2]. However, in the patients of this study, the heterozygous c.609G > A variant was detected along with another variant in the MMACHC gene. Further, the c.658_660delAAG variant was the third and the c.80A > G variant was the fourth most common variant in this study. These variants have been reported in other studies too [15, 16].
Neuropsychiatric diseases are frequent in patients with c.482G > A or c.609G > A variants [2, 18, 19]. In 13 patients in the present cohort with spastic paraplegia, 11 (84.6%) had a c.482G > A variant. This finding suggests that the c.482G > A variant may be the most common variant in late-onset patients with neuropsychiatric presentations [18]. Six patients with the c.80A > G and c.609G > A compound heterozygotic variants displayed neuropsychiatric symptoms or pulmonary hypertension. These results suggest that diseases of the cardiovascular system should be considered in patients with a c.80A > G variant. This finding is supported by previous studies [17, 20, 21].
In this study, seven (6.3%) patients with a heterozygous c.567dupT variant presented with neuropsychiatric diseases during adolescence. Previously, c.567dupT has been detected in early-onset patients with hydrocephalus secondary to cblC deficiency [22]. c.567dupT has also been found in two alleles of 26 late-onset patients in another study [5]. The c.394C > T variant was detected in four (3.6%) cases of this cohort. Lerner-Ellis et al. reported 42 different variants in 204 patients, and c.394C > T was detected in 34 alleles [20]. Further, it has been reported that individuals with c.394C > T tend to present with late-onset disease [18]. Morel et al. studied phenotype-genotype correlations in 37 patients from published case reports and found that the c.394C > T variant is common in Asiatic-Indian/Pakistani/Middle Eastern populations. In their study, 9 out of 12 late-onset cases presented with acute neurological symptoms. Four of these nine patients were homozygous for the c.394C > T variant, and two showed compound heterozygosity for the c.271dupA and c.394C > T variants [21]. Thus, c.567dupT and c.394C > T in this study mainly related to neuropsychiatric diseases during adolescence.
In one case (P56) of the present cohort, only one heterozygous c.609G > A in the MMACHC gene was identified. Significantly elevated blood tHcy, propionyl-carnitine, and urine methylmalonic acid are indicative of cblC deficiency. A c.515-48_515-47insTTA variant of unknown pathogenicity in the PRDX1 gene, which was reported to cause MMACHC hypermethylation [23], was also found. This variant might have been associated with cb1C deficiency in this patient.
In the present study, all 56 patients were considered healthy before onset of the disease. Most cases had metabolic disturbances during the acute phase of the disease. Markedly increasing of blood propionylcarnitine, tHCY (10–14 fold), and urine methylmalonic acid were observed in most of the patients. High doses of cobalamin, l-carnitine, and betaine are administered in the acute phase to reduce the blood level of tHCY and correct the metabolic status as soon as possible [11, 24, 25]. Patients tend to regain their ability to learn and walk as the levels of their metabolic markers decrease. In this study, the problems related to the cardiovascular and pulmonary systems of the patients were reversed with this treatment regimen. Following treatment and improvement, patients gradually returned to school and started to live normal lives. Nine patients graduated and are working, and two female patients are married and have had healthy babies [13]. These results show that with appropriate treatment, patients with cblC deficiency can live normal lives [5, 26, 27].
Conclusions
Patients with late-onset cb1C deficiency present with a wide variety of nonspecific clinical features that can easily be misdiagnosed as other conditions. The findings demonstrate that physicians can determine the accurate diagnosis in such patients with biochemical and genetic analyses. Further, after metabolic treatment, most patients can fully recover and live a normal life. These results indicate that differential diagnosis of inherited metabolic disorders should be considered for previously healthy adolescent patients who present with neuropsychiatric diseases and multiple organ damage.
Methods
Patients
This study included 56 Chinese patients with late-onset cb1C deficiency who were diagnosed and followed up at Peking University First Hospital between 2002 and May 2021. Their diagnosis was confirmed by biochemical and genetic analyses.
Routine examination
Body weight, height, and secondary sex characteristics were recorded to evaluate the growth and sexual development of the patients. Blood pressure, electrocardiography, and echocardiography were used for cardiovascular monitoring. Routine examinations of blood, urine, glucose, insulin, and hepatic and renal functions were conducted in all the patients. Bone density and serum vitamin D were also measured. All the patients underwent cranial magnetic resonance imaging or computed tomography.
Biochemical assays
Amino acids, free carnitine, and acyl-carnitines in dried blood spots were analyzed by liquid chromatography-tandem mass spectrometry (API 3200, Triple Quad 4500; Applied Biosystems, CA, USA). The concentrations of the metabolites were calculated automatically using the Chemoview software [2, 28].
Gas chromatography and mass spectrometry (GC/MS) was performed with GCMS-QP 2010 (Shimadzu Corporation, Kyoto, Japan) to analyze urine organic acids, according to a previously established protocol [28,29,30]. Data were collected using the GC/MS solution software. Plasma tHCY was detected by chemiluminescence immunoassay (Abbott I2000, USA).
Genetic analysis
Peripheral blood samples were collected from the patients and their parents. DNA was extracted using a DNA Isolation Kit (AU1802; Bioteke, China). Purified DNA samples were sent to Running Gene Inc. (Beijing, China) or Berry Genomics Corporation (Beijing, China) for next-generation sequencing to screen variants in patients. Each variant was evaluated according to the Human Gene Mutation Database (HGMD, https://my.qiagendigitalinsights.com/bbp/view/hgmd/pro/gene.php?gene=MMACHC) and ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/?term=MMACHC%5Bgene%5D&redir=gene).
Treatment
For patients in the acute decompensation stage, initial therapy included adenosylcobalamin (3 mg/day) or hydroxycobalamin (10 mg/day) administered intramuscularly, l-carnitine (2–3 g/day), intravenous fluid therapy with glucose and electrolytes, oral betaine (3–9 g/day), folate (5–15 mg/day), and symptomatic treatment. After their condition stabilized, the dosages were reduced. Individual long-term metabolic treatment was adjusted according to their clinical condition [11, 24, 26].
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- MMA:
-
Methylmalonic aciduria
- cblC:
-
Cobalamin C
- LC–MS/MS:
-
Liquid chromatography-tandem mass spectrometry
- GC/MS:
-
Gas chromatography and mass spectrometry
- tHCY:
-
Total plasma homocysteine
- MRI:
-
Magnetic resonance imaging
- CT:
-
Computerized tomography
- MMACHC:
-
Cytoplasmic chaperone protein methylmalonic aciduria and homocystinuria
References
Carrillo-Carrasco N, Chandler RJ, Venditti CP. Combined methylmalonic acidemia and homocystinuria, cblC type. I. Clinical presentations, diagnosis and management. J Inherit Metab Dis. 2012;35:91–102.
He R, Mo R, Shen M, Kang L, Song J, Liu Y, et al. Variable phenotypes and outcomes associated with the MMACHC c.609G>A homologous mutation: long term follow-up in a large cohort of cases. Orphanet J Rare Dis. 2020;15(1):200.
Liu MY, Yang YL, Chang YC, Chiang SH, Lin SP, Han LS, et al. Mutation spectrum of MMACHC in Chinese patients with combined methylmalonic aciduria and homocystinuria. J Hum Genet. 2010;55(9):621–6.
Liu Y, Liu YP, Zhang Y, Song JQ, Zheng H, Dong H, et al. Heterogeneous phenotypes, genotypes, treatment and prevention of 1 003 patients with methylmalonic acidemia in the mainland of China [in Chinese]. Chin J Pediatr. 2018;56(6):414–20.
Wang SJ, Yan CZ, Wen B, Zhao YY. Clinical feature and outcome of late-onset cobalamin C disease patients with neuropsychiatric presentations: a Chinese case series. Neuropsychiatr Dis Treat. 2019;15:549–55.
Han B, Cao Z, Tian L, Zou H, Yang L, Zhu W, et al. Clinical presentation, gene analysis and outcomes in young patients with early-treated combined methylmalonic acidemia and homocysteinemia (cblC type) in Shandong province. China Brain Dev. 2016;38:491–7.
Chang KJ, Zhao Z, Shen HR, Bing Q, Li N, Guo X, et al. Adolescent/adult-onset homocysteine remethylation disorders characterized by gait disturbance with/without psychiatric symptoms and cognitive decline: a series of seven cases. Neurol Sci. 2021;42:1987–93.
Wang SJ, Zhao YY, Yan CZ. Reversible encephalopathy caused by an inborn error of cobalamin metabolism. Lancet. 2019;393:e29.
Wei Y, Zhou Y, Yuan J, Ni J, Qian M, Cui L, et al. Treatable cause of hereditary spastic paraplegia: eight cases of combined homocysteinaemia with methylmalonic aciduria. J Neurol. 2019;266:2434–9.
Beck BB, van Spronsen F, Diepstra A, Berger RM, Kömhoff M. Renal thrombotic microangiopathy in patients with cblC defect: review of an under-recognized entity. Pediatr Nephrol. 2017;32:733–41.
Huemer M, Diodato D, Schwahn B, Schiff M, Bandeira A, Benoist JF, et al. Guidelines for diagnosis and management of the cobalamin-related remethylation disorders cblC, cblD, cblE, cblF, cblG, cblJ and MTHFR deficiency. J Inherit Metab Dis. 2017;40:21–48.
Ahrens-Nicklas RC, Whitaker AM, Kaplan P, Cuddapah S, Burfield J, Blair J, et al. Efficacy of early treatment in patients with cobalamin C disease identified by newborn screening: a 16-year experience. Genet Med. 2017;19:926–35.
Liu Y, Wang Q, Li X, Ding Y, Song J, Yang Y. First Chinese case of successful pregnancy with combined methylmalonic aciduria and homocystinuria, cblC type. Brain Dev. 2015;37:286–91.
Lu J, Xu X, Huang Y, Li T, Ma C, Xu G, et al. Prevalence of depressive disorders and treatment in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2021;8(11):981–90.
Chu X, Meng L, Zhang W, Luo J, Wang Z, Yuan Y. Peripheral nervous system involvement in late-onset cobalamin C disease? Front Neurol. 2020;11:594905.
Lemoine M, François A, Grangé S, Rabant M, Châtelet V, Cassiman D, et al. Cobalamin C deficiency induces a typical histopathological pattern of renal arteriolar and glomerular thrombotic microangiopathy. Kidney Int Rep. 2018;3:1153–62.
Wen LY, Guo YK, Shi XQ. Pulmonary hypertension in late-onset methylmalonic aciduria and homocystinemia: a case report. BMC Pediatr. 2020;20:243.
Lerner-Ellis JP, Anastasio N, Liu J, Coelho D, Suormala T, Stucki M, et al. Spectrum of mutations in MMACHC, allelic expression, and evidence for genotype-phenotype correlations. Hum Mutat. 2009;30:1072–81.
Wang C, Li D, Cai F, Zhang X, Xu X, Liu X, et al. Mutation spectrum of MMACHC in Chinese pediatric patients with cobalamin C disease: a case series and literature review. Eur J Med Genet. 2019;62:103713.
Lerner-Ellis JP, Tirone JC, Pawelek PD, Doré C, Atkinson JL, Watkins D, et al. Identification of the gene responsible for methylmalonic aciduria and homocystinuria, cblC type. Nat Genet. 2006;38(1):93–100.
Morel CF, Lerner-Ellis JP, Rosenblatt DS. Combined methylmalonic aciduria and homocystinuria (cblC): phenotype-genotype correlations and ethnic-specific observations. Mol Genet Metab. 2006;88(4):315–21.
He R, Zhang H, Kang L, Li H, Shen M, Zhang Y, et al. Analysis of 70 patients with hydrocephalus due to cobalamin C deficiency. Neurology. 2020;95(23):e3129–37.
Guéant JL, Chéry C, Oussalah A, Nadaf J, Coelho D, Josse T, et al. APRDX1 mutant allele causes a MMACHC secondary epimutation in cblC patients [published correction appears in Nat Commun. 2018; 9 (1): 554]. Nat Commun. 2018;9(1):67.
Higashimoto T, Kim AY, Ogawa JT, Sloan JL, Almuqbil MA, Carlson JM, et al. High-dose hydroxocobalamin achieves biochemical correction and improvement of neuropsychiatric deficits in adults with late onset cobalamin C deficiency. JIMD Rep. 2019;51:17–24.
Djuric D, Jakovljevic V, Zivkovic V, Srejovic I. Homocysteine and homocysteine-related compounds: an overview of the roles in the pathology of the cardiovascular and nervous systems. Can J Physiol Pharmacol. 2018;96:991–1003.
Forny P, Hörster F, Ballhausen D, Chakrapani A, Chapman KA, Dionisi-Vici C, et al. Guidelines for the diagnosis and management of methylmalonic acidaemia and propionic acidaemia: first revision. J Inherit Metab Dis. 2021;44:566–92.
Gündüz M, Ekici F, Özaydın E, Ceylaner S, Perez B. Reversible pulmonary arterial hypertension in cobalamin-dependent cobalamin C disease due to a novel mutation in the MMACHC gene. Eur J Pediatr. 2014;173:1707–10.
Shibata N, Hasegawa Y, Yamada K, Kobayashi H, Purevsuren J, Yang Y, et al. Diversity in the incidence and spectrum of organic acidemias, fatty acid oxidation disorders, and amino acid disorders in Asian countries: selective screening vs. expanded newborn screening. Mol Genet Metab Rep. 2018;16:5–10.
Fu X-W, Iga M, Kimura M, Yamaguchi S. Simplified screening for organic acidemia using GC/MS and dried urine filter paper: a study on neonatal mass screening. Early Hum Dev. 2000;58:41–55.
Kimura M, Yamamoto T, Yamaguchi S. Automated metabolic profiling and in-terpretation of GC/MS data for organic acidemia screening: a personal computer-based system. Tohoku J Exp Med. 1999;188:317–34.
Hu S, Mei S, Liu N, Kong X. Molecular genetic characterization of cblC defects in 126 pedigrees and prenatal genetic diagnosis of pedigrees with combined methylmalonic aciduria and homocystinuria. BMC Med Genet. 2018;19(1):154.
Ji X, Wang H, Ye J, Qiu W, Zhang H, Liang L, et al. Prenatal diagnosis of methylmalonic aciduria from amniotic fluid using genetic and biochemical approaches. Prenat Diagn. 2019;39(11):993–7.
Acknowledgements
We would like to thank all the patients and their families who participated in this study. We thank the Translational Medicine Laboratory, Chinese People's Liberation Army General Hospital (Beijing, China), for their help with the genetic sequencing and analysis. We are greatly indebted to the team of Professor Seiji Yamaguchi (Department of Pediatrics, Shimane Medical University, Japan) and the team of Professor Kwang-Jen Hsiao (Preventive Medicine Foundation, Taipei) for their expert technical assistance in the diagnosis and treatment of methylmalonic acidemia. We also thank the science editors at Elixigen Company (Huntington Beach, California) for editing our manuscript.
Funding
This work was supported by grants from the National Key Research and Development Program of China (Nos. 2019YFC1005100, 2017YFC1001700).
Author information
Authors and Affiliations
Contributions
ZC wrote the original draft of the manuscript; HD, RH, YL, XL, HY, JQ, FW, HX, HZ, LK, DL, and YL collected the clinical data and followed up the patients; YJ, ML, and JS performed the metabolic assays; YZ and YY designed the study and supervised the clinical work. All the authors have read and approved of the final manuscript.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Detailed clinical information for each enrolled subject.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, Z., Dong, H., Liu, Y. et al. Late-onset cblC deficiency around puberty: a retrospective study of the clinical characteristics, diagnosis, and treatment. Orphanet J Rare Dis 17, 330 (2022). https://doi.org/10.1186/s13023-022-02471-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-022-02471-x