Abstract
Background
Soil CO2 efflux is considered to mainly derive from biotic activities, while potential contribution of abiotic processes has been mostly neglected especially in productive ecosystems with highly active soil biota. We collected a subtropical forest soil to sterilize for incubation under different temperature (20 and 30 °C) and moisture regimes (30%, 60 and 90% of water holding capacity), aiming to quantify contribution of abiotic and biotic soil CO2 emission under changing environment scenarios.
Main findings:
Results showed that abiotic processes accounted for a considerable proportion (15.6−60.0%) of CO2 emission in such a biologically active soil under different temperature and moisture conditions, and the abiotic soil CO2 emission was very likely to derive from degradation of soil organic carbon via thermal degradation and oxidation of reactive oxygen species. Furthermore, compared with biotically driving decomposition processes, abiotic soil CO2 emission was less sensitive to changes in temperature and moisture, causing reductions in proportion of the abiotic to total soil CO2 emission as temperature and moisture increased.
Conclusions
These observations highlight that abiotic soil CO2 emission is unneglectable even in productive ecosystems with high biological activities, and different responses of the abiotic and biotic processes to environmental changes could increase the uncertainty in predicting carbon cycling.
Similar content being viewed by others
Background
Soil constitutes to the greatest terrestrial carbon (C) pool, with a size > 2000 Pg organic C in upper 2 m depth of land [1], which is around three times the atmospheric C pool. Soil CO2 emission, often termed as soil respiration, may substantially affect CO2 concentration in the atmosphere and then the global climate system [2], since it is the largest terrestrial C source to the atmosphere [3]. Soil respiration is generally separated into two components, i.e., autotrophic and heterotrophic respirations, and on global scale the latter contributes an increasing proportion of the total soil respiration in recent decades [4, 5]. The global heterotrophic respiration is estimated to be 50.3 ± 25.0 Pg C yr− 1 during 1982–2012, a figure that is much higher than its autotrophic counterpart (35.2 Pg C yr− 1) [6], therefore highlighting a necessity to deepen our understanding on the sources and variations of soil heterotrophic respiration.
Soil organic C (SOC) decomposition is traditionally regarded to derive mainly from biotic activities (especially microbial activities, Rbiotic) and be pooled as heterotrophic respiration in most of previous studies, although it is widely accepted that soil CO2 may be produced by both the biotic and abiotic processes. In addition to microbial driven SOC decomposition, diverse abiotic pathways, such as photodegradation, thermal degradation, oxidation of reactive oxidative species (ROSs), extracellular oxidative metabolism, and inorganic chemistry reactions, may also contribute to CO2 emission from the soil (Rsoil) and therefore produce soil abiotic CO2 emission (Rabiotic) [7, 8]. These abiotic C decomposition pathways have been recognized especially in litter decomposition studies, because a considerable number of studies have reported photodegradation of litter under different conditions [9,10,11]. However, soil Rabiotic has been scarcely quantified, except few studies conducted in harsh environments such as arid and polar soils where biotic activities are extremely low [12, 13]; the abiotic processes may contribute up to 99.5% of the total soil CO2 emission, e.g., in an alkaline soil of the southern Gurbantunggut Desert region, China [12]. In those biologically active ecosystems, contribution of the abiotic to total soil CO2 emission is assumed tiny and often overlooked, although recent studies highlighted the importance of nonmicrobial CO2 emission and possible production pathways in the soil [7, 8].
Environmental factors including temperature and moisture regimes may play critical roles to regulate the rate of soil CO2 emission. A consensus has been almost reached that temperature can exponentially affect soil CO2 efflux rate, and the global average temperature sensitivity is estimated to be 3.0 ± 1.1 over air temperature ranging 0–20 ℃ [14]. Soil moisture could significantly affect soil CO2 emission, especially the heterotrophic component [15, 16]. These are attributable to the fact that biotic activities are to a great extent supported by supplies of energy and water. However, chemical reactions will also be accelerated by warming temperature, as Arrhenius theory demonstrates, and soil moisture could potentially influence abiotic SOC decomposition by adjusting supplies of substrates and oxygen [15]. Therefore, changes in environmental conditions probably induce variations in the Rabiotic. Nevertheless, this topic to the best of our knowledge is still virgin, despite we are facing scenarios of global warming and precipitation changes.
In this study, we sterilized a productive soil with high biotic activities for incubation to investigate the contribution of abiotic and biotic soil CO2 emission, and different regimes of incubation temperature (20 and 30 °C) and moisture (30%, 60 and 90% of water holding capacity [WHC]) were set up as experimental factors. The soil microbial biomass and activity of the studied soil are high as observed in previous studies [17, 18]. This experiment was conducted with aims: (1) to quantify what were the proportions of Rabiotic and Rbiotic to the total soil CO2 emission, in order to verify whether abiotic soil CO2 emission is neglectable in ‘biologically active’ soils; and (2) to investigate how the rate and proportion of Rabiotic and Rbiotic would vary under changing temperature and moisture scenarios.
Results and discussion
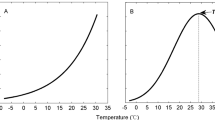
We observed that Rsoil ranged from 0.061 ± 0.0069 (under 20 ℃ & 30% WHC) to 0.25 ± 0.027 mg kg− 1 soil day− 1 (under 30 ℃ & 90% WHC) under different combinations of temperature and moisture regimes, and increasing temperature or moisture significantly or tended to promote Rsoil in this study (Fig. 1A). It is not surprising because such positive relationships in a certain temperature or moisture range have been frequently reported for soil CO2 emission, although the range may be variable depending on soil type and properties [15, 16, 19].
Total, abiotic and biotic soil CO2 efflux rate under different temperature and moisture regimes. In each panel, different lowercase letters indicate significant differences among moisture regimes, and asterisks (*) indicate significant differences between the soil CO2 efflux rate between high and low temperatures
Interestingly, both the Rabiotic and Rbiotic contributed obviously to Rsoil under each treatment (Fig. 1; Table 1). Under 20 °C, Rabiotic was 0.036 ± 0.0027, 0.037 ± 0.0065 and 0.045 ± 0.011 mg kg− 1 soil day− 1 at 30%, 60 and 90% of WHC, respectively, and the rate at 30 °C was 0.076 ± 0.0044, 0.074 ± 0.0087 and 0.039 ± 0.0045 mg kg− 1 soil day− 1 correspondingly (Fig. 1B, C). This produces a considerable proportion (on average 36.7%) of Rabiotic to Rsoil in the studied soil (Table 1), highlighting a necessity to investigate abiotic soil CO2 emission even in those fertile soils with relatively high biotic activities. This observation may be supported by the result that the studied soil contains a considerable proportion of chemical readily-oxidizable SOC that may be easily degraded by abiotic processes [20, 21].
Abiotic soil CO2 efflux may derive from solar-induced organic matter degradation (i.e., photodegradation of the functional group of carboxyl and glucose), thermal degradation by dissociating chemical bonds of the SOC, oxidation of ROSs that could be produced by biotic or photochemical processes, extracellular oxidative metabolism, and inorganic chemistry reactions [8]. Photodegradation and inorganic chemistry reactions have been reported, though limited, to contribute to the total soil CO2 emission in previous studies [9, 12, 13]. In this study, however, the mechanisms of photodegradation, extracellular oxidative metabolism and inorganic chemical reactions may not contribute obviously to the observed high proportion of Rabiotic, due to (i) dark incubation used, (ii) high temperature (121 ℃ in the process of sterilization) resulting in inactivation of exoenzymes, (iii) low carbonate content in such a strongly acidic soil (pHwater = 3.6 ± 0.07).
Thermal degradation and ROSs oxidization could mainly account for the Rabiotic component of the tested soil in the present study. Regarding thermal degradation of SOC, most studies have employed high temperatures that are well above the ignition temperature of organic materials (often higher than 140 °C) [22, 23], and therefore it seems that thermal degradation might not have functioned under relatively low temperatures, e.g., 20 and 30 °C as used in this study. However, previous studies have reported obvious CO2 emission due to thermal degradation of organic materials derived from plant residues under relatively low temperature conditions, and the CO2 emission rate exponentially increased with the increase of incubation temperature ranging from 25 to 55 °C [24]. This suggests that SOC, of which a considerable portion derives from plant residues and excretions, may also be thermally degraded under relatively low temperatures below the ignition point of organic materials, although we could not quantify the exact proportion of contribution of thermal degradation to the total soil CO2 emission. Similarly, we also observed obviously higher abiotic soil CO2 emission rate under 30 °C than under 20 °C, especially at the water regimes of 30 and 60% WHC (Fig. 1B).
Besides the thermal degradation pathway, the ROSs oxidation pathway could also contribute substantially to the soil abiotic CO2 emission. As previous studies demonstrated, existence of iron (Fe) and magnesium (Mn) may substantially contribute to soil CO2 emission via producing ROSs (such as hydroxyl radical and hydrogen peroxide) to oxidize SOC [25,26,27]. The studied soil is a type of highly weathered soil that is rich in the Fe content and contains detectable Mn content [28]. These soil compounds, probably some others not analyzed in our studies, can produce a considerable amount of ROSs in oxidation-reduction reactions [25, 29], consequently contributing to the abiotic soil CO2 emission as observed in this study (Fig. 1). Despite the abiotic oxidation potential of Fe and Mn, we also recognize that existence of soil organisms may substantially accelerate the Fe and Mn mediated abiotic oxidation process, since production of Fe (II) and Mn (III) are predominantly regulated by soil microorganisms [27].
In this study, Rbiotic was significantly higher under high than low temperatures (Fig. 1C), consistently among the soil moisture treatments (interactive effects of two-way ANOVAs: F = 0.707, df = 2, p = 0.507), since metabolic reactions in soil organisms could be accelerated as temperature increased [30]. This is not controversial. Unlike on biotic components, however, temperature effects on Rabiotic were not consistent under different soil moisture conditions, as indicated by the significant interactive effect between incubation temperature and moisture treatments (interactive effects of two-way ANOVAs: F = 6.577, df = 2, p = 0.007). Under 30 and 60% of WHC, Rabiotic was also significantly higher under high than low temperatures, whereas it was comparable between the high and low temperature treatments of this study under 90% WHC treatment (Fig. 1B). This might be associated with limited movement and supply of oxygen under the water-rich soil where water could occupy most space in the soil aggregates [15]. In this condition, chemical reaction rate could not be promoted with increasing temperature due to lack of oxygen as a necessary reactant. Further studies to partition contribution of the abiotic processes would be helpful to clearly understand such inconsistent temperature effects on Rabiotic among varying soil moisture conditions.
Similar with rate changes, the proportion of Rbiotic to Rsoil significantly increased with temperature increases (Table 1). For Rabiotic, however, its proportion significantly or tended to decrease as temperature increased (Table 1), despite warming temperature could promote it under 30 and 60% of WHC conditions (Fig. 1B). This result implies that biological processes are more sensitive to warming than abiotic processes, which is also evidenced by the greater Q10 (rate of change of soil CO2 emission with temperature increases by 10 °C) of Rbiotic than Rabiotic (Fig. 2). Therefore, compared with abiotic processes such as solar-induced and thermal degradation, C emission related metabolic activities may be more promoted by global warming. Considering abiotic soil CO2 emission and its response to temperature changes could result in lower positive feedbacks of soil C emission to temperature warming than that projected previously while the abiotic soil CO2 emission was not considered.
Noteworthily, soil moisture affected Rabiotic and Rbiotic in inverse ways (Fig. 1; Table 1). Increases in soil moisture did not significantly alter or even significantly reduced (under 30 ℃ & 90%WHC) Rabiotic within the investigation period (Fig. 1B). The reduction in Rabiotic may be attributable to the fact that soil abiotic CO2 emission driven by the ROSs pathway has been affected by changes in soil moisture via regulating soil oxygen availability [31, 32]. Contrastingly, increasing soil moisture significantly increased Rbiotic, which is consistent under the two incubation temperatures (Fig. 1C). As a result, the proportion of Rbiotic to Rsoil was significantly higher under high soil moisture conditions and that of Rabiotic significantly decreased with the increasing soil moisture, regardless of incubation temperatures (Table 1). Abiotic processes contributed greatest proportion to soil CO2 emission under low temperature and low moisture conditions (i.e., 20 °C & 30% WHC in this study), under which Rbiotic was low (Fig. 1A). Likewise in a previous study, Ma et al. found that abiotic components dominated soil CO2 efflux on an alluvial plain in a temperate arid desert where biological activities were weak in the soil [12]. It has been recognized that abiotic decompositions are important for litter decomposition [9, 24], and that abiotic soil CO2 emission may dominate soil CO2 efflux in harsh environments such as several arid or tundra ecosystems [12, 13]. However, the potential importance of abiotic decomposition of SOC has been largely overlooked in previous studies, especially in biologically active ecosystems. Our result indicates that abiotic processes may contribute a considerable proportion of total soil CO2 emission in (sub)tropical forests which have high soil biotic activities. Therefore, more attention is requested to clarify the variations and mechanisms of such abiotic CO2 productions and effluxes from the soil.
Moreover, we also calculated the Q10 index to indicate apparent temperature sensitivity of soil CO2 emission [30]. Within the temperature range between 20 and 30 ℃, Q10 of Rsoil was 2.57 ± 0.12, a value that is a bit lower than the global average Q10 (3.0 ± 1.1) of soil CO2 efflux (including autotrophic respiration) over 0–20 ℃ range of air temperature [14]. This may be associated with relatively higher temperature sensitivity of autotrophic than heterotrophic respiration [33] or/and thermal acclimation of SOC decomposition, i.e., higher temperature sensitivity under low than high temperatures [19, 30, 34]. Air temperature in the study site had been increasing by 1.0 ± 0.1 ℃ during past five decades (1954–2009) [35] and the lasting increase in temperature may have consequently reduced the magnitude of C sink [36], due to extra soil C loss induced by warming [37].
Furthermore, Q10 of Rabiotic and Rbiotic were 1.75 ± 0.21 and 3.20 ± 0.34, respectively (Fig. 2B, C). Such a high Q10 of Rbiotic, compared with Rsoil and Rabiotic, indicates high sensitivity of biotic decomposition activity to warming and therefore raising temperature could increase the proportion of biotic to total SOC decomposition (Table 1). Under high soil moisture conditions (such as 90% WHC in this study), however, Q10 (1.01 ± 0.12) of Rabiotic was not significantly different from one (One-Sample t = 0.065, df = 3, p = 0.952), suggesting Rabiotic to be independent of temperature increases in water-rich soil conditions. Moreover, high soil moisture (i.e., 90% WHC) could significantly reduce Q10 of both Rabiotic and Rbiotic, consequently declining Q10 of Rsoil. This may be linked to limited oxygen supply under high soil moisture [15], because oxygen availability is important to controlling temperature sensitivity of soil CO2 emission [38]. As observed in previous studies, oxygen availability may positively affect not only the microbial driving SOC decomposition [29, 39], but also the abiotic SOC degradation, since the ROSs pathway that produces soil abiotic CO2 emission could be regulated by oxygen availability [26, 31]. As a reactant, insufficient oxygen supply under high soil moisture condition could reduce the temperature sensitivity of soil CO2 emission and resultantly cause the observed pattern in this study.
Despite the interesting observations, it is notable that two drawbacks exist in the present study. First, this study is a laboratory incubation experiment, in which the intact soil structure and properties may have been substantially disturbed by pretreating processes [40], e.g., sieving and adjusting the soil water content before incubation. This could on the one hand simplify experimental treatments and decrease the heterogeneity of samples and therefore make easier to compare treatment effects [41], which is much useful in such an exploratory study. On the other hand, however, such disturbances are most likely to make observation values away from ‘true values’ [42] and therefore paralleling field observations may be needed to verify the observations. Second, we investigated abiotic soil CO2 emission rate after sterilizing soils under high temperature (121 ℃) and biotic component is quantified by subscripting the soil CO2 emission rate in the sterilized soil from that in the corresponding unsterilized soil. Despite widely used in related studies, this simplification runs an assumption that the total production of soil CO2 is a result of linearly adding up the abiotic and biotic components, which may be not the case under natural conditions since biotic and abiotic C degradation processes are often closely coupled [43]. Therefore, developing advanced methodologies remains needed to improve the estimates of these two components.
Conclusions
In this study, we observed that abiotic processes contributed a considerable proportion of soil CO2 emission in the subtropical forest, with thermal degradation and ROSs oxidation being probably the underlying mechanisms, and abiotic and biotic soil CO2 emissions showed different responses to changes in temperature and moisture in this study. Abiotic soil CO2 emission has been evidenced to be important in harsh environments such as arid or arctic ecosystems, while it is often considered to be minor and overlooked in ecosystems with high biotic activities. Our observations highlight that the abiotic soil C degradation processes may remain unneglectable even in biologically active ecosystems. As the best of our knowledge, almost all previous modelling studies have overlooked such an important component, which is likely to contribute to the uncertainty of predictions in the ecosystem C cycling under environmental changes. However, the present study has been designed to exploratorily investigate the contribution, as well as temperature and moisture sensitivities, of abiotic and biotic processes to the total soil CO2 emission in a biologically active ecosystem, with underlying mechanisms rarely revealed by our observations in this study. It is necessary and much urgent to further explore the mechanisms by which the abiotic soil CO2 emission is produced and how it would vary in the changing environmental conditions, especially in the biologically active ecosystems.
Methods
Incubation experiment
The studied soil was collected from the surface (0–10 cm) of a subtropical old-growth forest that experienced the subtropical monsoon climate. The forest is the regional climax, with dominate tree species being Castanopsis chinensis,Cryptocarya chinensis, Cryptocarya concinna, and Erythrophleum fordii etc. [44]. The soil is lateritic red earth that could be classified as Oxisol according to the US Soil Taxonomy [45]. The soil pH (water extracted) was 3.57 ± 0.073, the SOC content was 36.27 ± 6.07 g kg− 1, and the total nitrogen content was 2.00 ± 0.15 g kg− 1. The contents of sand, silt and clay are 6.9%, 39.7 and 53.4%, respectively. After collection, the soil samples were immediately transferred with ice bags into lab to sieve for uses.
The sieved soils were separated into two parts. The one was sterilized twice with an interval of 24 h under 121 ℃ to obtain sterile soil, which was incubated to investigate the soil abiotic CO2 emission. Two methods, including plate culture and PCR methods, were employed to check the efficiency of soil sterility at the end of incubation experiment and results evidenced no observable soil bacteria grew in the sterilized soils. The other one was not sterilized for incubation to investigate the total soil CO2 emission. The difference in the total and abiotic soil CO2 emission rate was calculated as soil biotic CO2 emission. Both the sterilized and non-sterilized soils were incubated for 64 days under different temperature (20 or 30 ℃) and moisture regimes (30%, 60 or 90% of WHC), with aims to quantify the rate and proportions of Rabiotic and Rbiotic and their response to changes in temperature and moisture conditions in such a biologically active soil. The accumulated CO2 concentration released from the soils was determined at days 3, 6, 9, 12, 16, 20, 24, 28, 34, 40, 52, and 64, by using the alkali (NaOH) absorption method.
Data processes and statistics
The soil CO2 efflux rate under each treatment was calculated by fitting the accumulated CO2 emission with the incubation period, with the slope of linear regression (for the non-sterilized soil the R2s > 0.98, while for the sterilized soil the R2s > 0.85) indicating the average efflux rate during the incubation period. The temperature sensitivity index (Q10) was calculated by dividing the rate under 30 °C by that under 20 °C [46]. After normality test, the rate of Rabiotic, Rbiotic and Rsoil, as well as the proportion of Rabiotic or Rbiotic to Rsoil, were compared between different incubation temperatures by the Paired-Samples T test or among different moisture regimes by the one-way Analysis of Variances (ANOVAs). Two-way ANOVAs were employed to reveal the significance level of interactive effects on the soil CO2 emission rate and proportion between incubation temperature and moisture treatments. Tukey Post Hoc Multiple Comparisons were employed to compare the average values between each two groups of the moisture regimes when one-way ANOVAs revealed significant differences. The rate calculations were conducted in R software (version 2.15.2) and all the statistics and figures were finished in IBM SPSS Statistics 22.
Availability of data and materials
No restrictions are placed on materials, such as materials transfer agreements. Details of all data and materials used in the analysis are available in the main text or on request of the corresponding authors.
Abbreviations
- CO2 :
-
Carbon dioxide
- C:
-
Carbon
- Pg:
-
Petagram
- yr:
-
Year
- SOC:
-
Soil organic carbon
- Rbioti :
-
Soil biotic CO2 emission
- ROSs:
-
Reactive oxidative species
- Rtotal :
-
Total soil CO2 emission
- Rabiotic :
-
Soil abiotic CO2 emission
- WHC:
-
Water holding capacity
- Fe:
-
Iron
- Mn:
-
Magnesium
- ANOVA:
-
Analysis of variances
- Q10 :
-
Temperature sensitivity index
- PCR:
-
Polymerase chain reaction
References
Batjes NH. Harmonized soil property values for broad-scale modelling (WISE30sec) with estimates of global soil carbon stocks. Geoderma. 2016;269:61–8.
Melillo JM, Frey SD, DeAngelis KM, Werner WJ, Bernard MJ, Bowles FP, et al. Long-term pattern and magnitude of soil carbon feedback to the climate system in a warming world. Science. 2017;358(6359):101.
Bond-Lamberty B, Thomson A. Temperature-associated increases in the global soil respiration record. Nature. 2010;464(7288):579–82.
Bond-Lamberty B, Bailey VL, Chen M, Gough CM, Vargas R. Globally rising soil heterotrophic respiration over recent decades. Nature. 2018;560(7716):80–3.
Lei J, Guo X, Zeng Y, Zhou J, Gao Q, Yang Y. Temporal changes in global soil respiration since 1987. Nat Commun. 2021;12(1):403.
Lu H, Li S, Ma M, Bastrikov V, Chen X, Ciais P, et al. Comparing machine learning-derived global estimates of soil respiration and its components with those from terrestrial ecosystem models. Environ Res Lett. 2021;16:054048.
Liu J, Hartmann SC, Keppler F, Lai DYF. Simultaneous abiotic production of greenhouse gases (CO2, CH4, and N2O) in subtropical soils. J Geophys Res Biogeosci. 2019;124(7):1977–87.
Wang B, Lerdau M, He Y. Widespread production of nonmicrobial greenhouse gases in soils. Global Change Biol. 2017;23(11):4472–82.
Rutledge S, Campbell DI, Baldocchi D, Schipper LA. Photodegradation leads to increased carbon dioxide losses from terrestrial organic matter. Global Change Biol. 2009;16:3065–74.
Austin AT, Mendez MS, Ballare CL. Photodegradation alleviates the lignin bottleneck for carbon turnover in terrestrial ecosystems. Proc Natl Acad Sci United States of Am. 2016;113(16):4392–7.
Brandt LA, King JY, Hobbie SE, Milchunas DG, Sinsabaugh RL. The role of photodegradation in surface litter decomposition across a grassland ecosystem precipitation gradient. Ecosystems. 2010;13(5):765–81.
Ma J, Li Y, Liu R. The abiotic contribution to total CO2 flux for soils in arid zone. Biogeosci Discussions. 2015;12(14):11217–44.
Shanhun FL, Almond PC, Clough TJ, Smith CMS. Abiotic processes dominate CO2 fluxes in Antarctic soils. Soil Biol Biochem. 2012;53:99–111.
Bond-Lamberty B, Thomson A. A global database of soil respiration data. Biogeosciences. 2010;7(6):1915–26.
Yan Z, Bond-Lamberty B, Todd-Brown KE, Bailey VL, Li S, Liu C, et al. A moisture function of soil heterotrophic respiration that incorporates microscale processes. Nat Commun. 2018;9(1):2562.
Moyano FE, Manzoni S, Chenu C. Responses of soil heterotrophic respiration to moisture availability: an exploration of processes and models. Soil Biol Biochem. 2013;59:72–85.
Huang W, Han T, Liu J, Wang G, Zhou G, Niu S. Changes in soil respiration components and their specific respiration along three successional forests in the subtropics. Funct Ecol. 2016;30(8):1466–74.
Wei H, Chen X, Xiao G, Guenet B, Vicca S, Shen W. Are variations in heterotrophic soil respiration related to changes in substrate availability and microbial biomass carbon in the subtropical forests? Sci Rep. 2015;5:18370.
Lloyd J, Taylor JA. On the temperature dependence of soil respiration. Funct Ecol. 1994:315–23.
Chen X, Deng Q, Lin G, Lin M, Wei H. Changing rainfall frequency affects soil organic carbon concentrations by altering non-labile soil organic carbon concentrations in a tropical monsoon forest. Sci Total Environ. 2018;644:762–9.
Chen XM, Li YL, Mo JM, Otieno D, Tenhunen J, Yan JH, et al. Effects of nitrogen deposition on soil organic carbon fractions in the subtropical forest ecosystems of Southern China. J Plant Nutr Soil Sci. 2012;175(6):947–53.
Barreto MSC, Ramlogan M, Oliveira DMS, Verburg EEJ, Elzinga EJ, Rouff AA, et al. Thermal stability of soil organic carbon after long-term manure application across land uses and tillage systems in an oxisol. Catena. 2021;200:105164.
Peltre C, Fernández JM, Craine JM, Plante AF. Relationships between biological and thermal indices of soil organic matter stability differ with soil organic carbon level. Soil Sci Soc Am J. 2013;77(6):2020–8.
Lee H, Rahn T, Throop H. An accounting of C-based trace gas release during abiotic plant litter degradation. Global Change Biol. 2012;18(3):1185–95.
Hall SJ, Silver WL. Iron oxidation stimulates organic matter decomposition in humid tropical forest soils. Glob Chang Biol. 2013;19(9):2804–13.
Bhattacharyya A, Campbell AN, Tfaily MM, Lin Y, Kukkadapu RK, Silver WL, et al. Redox fluctuations control the coupled cycling of iron and carbon in tropical forest soils. Environ Sci Technol. 2018;52(24):14129–39.
Jones ME, LaCroix RE, Zeigler J, Ying SC, Nico PS, Keiluweit M. Enzymes, manganese, or iron? Drivers of oxidative organic matter decomposition in soils. Environ Sci Technol. 2020;54(21):14114–23.
Li D, Mo J, Fang Y, Xue J. Study on availability of micronutrients in soils under three different forests of Dinghushan Nuture Reserve. Guihaia. 2004;24(6):529–34.
Li Y, Shahbaz M, Zhu Z, Deng Y, Tong Y, Chen L, et al. Oxygen availability determines key regulators in soil organic carbon mineralisation in paddy soils. Soil Biol Biochem. 2021;153:108106.
Davidson EA, Janssens IA. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature. 2006;440:165–73.
Zhao Q, Dunham-Cheatham S, Adhikari D, Chen C, Patel A, Poulson SR, et al. Oxidation of soil organic carbon during an anoxic-oxic transition. Geoderma. 2020;377:114584.
Barcellos D, O’Connell C, Silver W, Meile C, Thompson A. Hot spots and hot moments of soil moisture explain fluctuations in iron and carbon cycling in a humid tropical forest soil. Soil Systems. 2018;2(4):59.
Boone RD, Nadelhoffer KJ, Canary JD, Kaye JP. Roots exert a strong influence on the temperature sensitivity of soil respiration. Nature. 1998;396(6711):570–2.
Wei H, Guenet B, Vicca S, Nunan N, AbdElgawad H, Pouteau V, et al. Thermal acclimation of organic matter decomposition in an artificial forest soil is related to shifts in microbial community structure. Soil Biol Biochem. 2014;71:1–12.
Zhou G, Wei X, Wu Y, Liu S, Huang Y, Yan J, et al. Quantifying the hydrological responses to climate change in an intact forested small watershed in Southern China. Global Change Biol. 2011;17:3736–46.
Piao S, Fang J, Ciais P, Peylin P, Huang Y, Sitch S, et al. The carbon balance of terrestrial ecosystems in China. Nature. 2009;458(7241):1009–13.
Nottingham AT, Meir P, Velasquez E, Turner BL. Soil carbon loss by experimental warming in a tropical forest. Nature. 2020;584(7820):234–7.
Blagodatskaya Е, Zheng X, Blagodatsky S, Wiegl R, Dannenmann M, Butterbach-Bahl K. Oxygen and substrate availability interactively control the temperature sensitivity of CO2 and N2O emission from soil. Biol Fert Soils. 2014;50(5):775–83.
Walz J, Knoblauch C, Böhme L, Pfeiffer E-M. Regulation of soil organic matter decomposition in permafrost-affected Siberian tundra soils - Impact of oxygen availability, freezing and thawing, temperature, and labile organic matter. Soil Biol Biochem. 2017;110:34–43.
Wagg C, Schlaeppi K, Banerjee S, Kuramae EE, van der Heijden MGA. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat Commun. 2019;10(1):4841.
Benton TG, Solan M, Travis JM, Sait SM. Microcosm experiments can inform global ecological problems. Trends Ecol Evol. 2007;22(10):516–21.
Schjønning P. Soil mechanical properties of seven Danish Soils. 1991;95:458.
Ward CP, Nalven SG, Crump BC, Kling GW, Cory RM. Photochemical alteration of organic carbon draining permafrost soils shifts microbial metabolic pathways and stimulates respiration. Nat Commun. 2017;8(1):772.
Zhou G, Guan L, Wei X, Zhang D, Zhang Q, Yan J, et al. Litterfall production along successional and altitudinal gradients of subtropical monsoon evergreen broadleaved forests in Guangdong, China. Plant Ecol. 2006;188(1):77–89.
Tang X, Liu S, Zhou G, Zhang D, Zhou C. Soil-atmospheric exchange of CO2, CH4, and N2O in three subtropical forest ecosystems in southern China. Global Change Biol. 2006;12(3):546–60.
Qin SQ, Chen LY, Fang K, Zhang QW, Wang J, Liu FT, et al. Temperature sensitivity of SOM decomposition governed by aggregate protection and microbial communities. Sci Adv. 2019;5(7):eaau1218.
Acknowledgements
Miss Xiaoran Shan and Dr. Guojun Lin are grateful for their helps on experimental work and data processes.
Funding
This study was supported by National Natural Science Foundation of China [32071641], Guangzhou Municipal Science and Technology Bureau [2018040110159], and Guangzhou University [YG2020018].
Author information
Authors and Affiliations
Contributions
HW designed the study; XC, ML, and ZX conducted the experiments and collected the data; XC analyzed and visualized the data; HW and XC wrote the first draft of the manuscript; all authors contributed substantially to revisions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, X., Liu, M., Xu, Z. et al. Influences of temperature and moisture on abiotic and biotic soil CO2 emission from a subtropical forest. Carbon Balance Manage 16, 18 (2021). https://doi.org/10.1186/s13021-021-00181-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13021-021-00181-8