Abstract
Background
Amorpha-4,11-diene synthase (ADS) is a key enzyme in the artemisinin biosynthetic pathway. ADS promotes the first step of artemisinin synthesis by cyclizing faresyl pyrophosphate to synthesize the sesquiterpene product amorpha-4,11-diene. Thanks to the continuous improvement of genomic information, its evolutionary trace can be analyzed in a genome view.
Methods
Phylogenetic analysis was used to identify ADS-like genes in other Asteraceae. Gene structure and motif analysis was used to analyze the structural similarity of these identified genes. Heterologous expression and GC–MS analysis were performed to determine whether the functions of ADS and Cna4666 are consistent. Validation of ADS genes evolutionary trajectories was achieved by selective pressure and synteny analysis.
Result
In this study, we extracted 8 ADS genes from the Artemisia annua L. genome annotation and 121 ADS similar genes from the genomes of Artemisia annua L. and other plants in the Asteraceae, and further exploring their evolutionary relationship. Phylogenetic analysis showed that the genes most closely related to ADS genes were found in the genome of Chrysanthemum nankingense. Among them, the gene structure and motif composition of Cna4666 is very similar to ADS, we wondered whether it has the potential to synthesize amorpha-4,11-diene. Therefore, we extracted the products of recombinant p0_ADS.1 and Cna4666 proteins by HS-SPME combined with GC–MS analysis, the results indicate that Cna4666 is an α-bisabolol synthase, which cannot synthesize amorpha-4,11-diene. Through synteny analysis, we did not find collinear blocks of ADS genes in the Helianthus annuus and C. nankingense genomes. Furthermore, Ka/Ks ratios indicated that the evolution of ADS genes from their similar genes principally underwent purifying selection, and there was a strong positive selection between ADS genes.
Conclusions
This study proved that ADS is a multi-copy gene in Artemisia annua L., and they are not widely distributed in Asteraceae. The data will increase our understanding of the evolutionary selection pressure on ADS genes. The results suggest that ADS genes are subject to strong positive selection internally, and it is possible that they are a recently evolved gene in the Artemisia.
Similar content being viewed by others
Background
Artemisinin is the most useful drug ingredient presently used to treat malaria. The combinational therapies based on artemisinin have been suggested by the World Health Organization (WHO) as the preferred choice of action for the treatment of malaria affected by Plasmodium since 2002 [1, 2]. Artemisinin has saved millions of lives from malaria since its discovery in 1972 [3]. To date, the source of artemisinin has been mainly extracted from Artemisia annua L [4].
Most of the synthesis pathway of artemisinin has been clearly explained and widely agreed upon, and seven related genes have been identified in the process from faresyl pyrophosphate (FPP) to artemisinin or arteannuin B (Additional file 4: Fig. S1). FPP is synthesized from isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) produced by the intracellular mevalonate (MVA) pathway or the methylerythritol 4-phosphate (MEP) pathway of plastids through farnesyl pyrophosphate (FPS) [5]. FPP is cyclized by amorpha-4,11-diene synthase to form amorpha-4,11-diene (AD) [6]. Then, AD is oxidized by cytochrome P450 monooxygenase (CYP71AV1) and alcohol dehydrogenase 1 (ADH1), catalyzed successively to form artemisinic alcohol, artemisinic aldehyde, and artemisinic acid successively [7,8,9]; Artemisinic aldehyde is oxidized by double bond reductase (DBR2) to form dihydroartemisinic aldehyde [9], which is then catalyzed by ALDH1 to form dihydroartemisinic acid [10]. Artemisinic acid and dihydroartemisinic aldehyde form arteannuin B and artemisinin, respectively, in a non-enzymatic reaction in Glandular-secreting trichomes [11,12,13]. ADS is the first step gene in the synthesis pathway of artemisinin, and is a significant component in the artemisinin biosynthesis pathway.
Terpenes mainly exist as monoterpenes, sesquiterpenes or diterpenes. Amorpha-4,11-diene belongs to sesquiterpene, so ADS is a sesquiterpene synthase. Sesquiterpene synthase catalyzes reactions with seven possible precursors, such as (E,E)-famesol, (Z,E)-famesol, (E)-α-famesol, (E)-β-famesol, (3R,6E)-nerolidol, (3S,6E)-nerolidol, etc. The initial cyclization reactions of sesquiterpene synthases can be divided into 1,10-closure and 1,11-closure of farnesyl cation or 1,6-closure, 1,7-closure, 1,10-closure, 1,11-closure of nerolidyl cation. With subsequent cyclizations, it is often difficult to determine the sequence of ring closures [14]. For example, δ-cadinene synthase from cotton undergoes 1,10-cyclization before 1,6-closure in the cyclization reaction [15, 16]; A. annua has another sesquiterpene synthase named 2-epi-cedrol synthase, which forms 1,6- cyclization followed by 6,10 and 2,11-closure [17, 18]. In addition, there are sesquiterpene synthases that do not undergo cyclization and produce acyclic sesquiterpenes, such as (E)-β-farnesene synthase from Artemisia annua [19]. A review has been published summarizing the discovery and catalytic process of ADS, it also described the application of ADS in metabolic engineering and synthetic biology and what important role sesquiterpene synthases played in the evolutionary development of artemisinin [20]. Including the following information, there was 36% protein similarity between ADS and tobacco 5-epi-aristolochene synthase (TEAS) as well as showed 41% amino acid sequence similarity with cotton ( +)-δ-cadinene synthase. And by observing the by-products catalyzed in the enzymatic reaction, it was hypothesized that amorpha-4,11-diene synthase catalyzed the 1,6-closure first followed by 1,10-closure [21], which was later confirmed [22,23,24,25,26]. The turnover rate or affinity of CYP7AV1 for ADS by-products was low, so it was pointed out that CYP71AV1 has a great catalytic specificity for AD [27]. Melissa Salmon et al. studied ADS and another FPP-based enzyme, (E)-β-farnesene synthase (BFS), a cyclized sesquiterpene synthase and a linear sesquiterpene synthase. And this study revealed that the cyclization of ADS may be due to a dominant natural mutation Y402L, or it could also be argued that Y402L has a prospective status in the functional differentiation of sesquiterpene synthases in A. annua [19]. A previous study investigated ADS homologs in 13 Artemisia species that do not produce artemisinin by PCR and showed that two new proteins, ( +)-α-bisabolol synthase and koidzumiol synthase, were cloned in three Artemisia species using ADS primers [28].
Genomics has played an important role in research fields such as plant biological characteristics, analysis of bioanabolism pathway, and molecular assisted breeding [29,30,31,32,33,34,35,36,37,38,39]. More and more plant genomes have been published, and the unique characteristics of plant genomes are becoming clearer. In addition, intraspecific and interspecific comparison of genome sequences can be used to infer species evolution and analyze phenotype-genotype relationships, which provides solid genetic support for plant research. Recently, a new A. annua genome has been published, and the assembled genome has high integrity and accurate allelic typing [40]. Therefore, we selected two haplotype genomes of A. annua and combined them with some Asteraceae plant genomes published several years ago to study ADS genes, including Cynara cardunculus var. Scolymus genome, Helianthus annuus genome, Lactuca saligna genome, Lactuca sativa genome and Chrysanthemum nankingense genome [36, 41,42,43].
As research on artemisinin and artemisinin biosynthesis genes has progressed, we know that artemisinin can be detected in many Artemisia species and that the expression of artemisinin biosynthetic genes, including ADS genes, can be detected [44,45,46,47]. Then the question of whether ADS genes are also widely present in Asteraceae has not been answered. The present study is based on the recently published chromosome-level genome of A. annua with the support of some other Asteraceae genomes. This study reveals for the first time the inconsistency of ADS gene in Asteraceae and investigates the evolution of ADS in Asteraceae.
Material and method
Identification and characterization of ADS genes and similar genes in other species
A three-stair analysis was operated to extracted ADS genes from the Artemisia annua L. database of genome. Genomic and RNA-seq data of A. annua were obtained from Global Pharmacopoeia Genome Database (GPGD, http://www.gpgenome.com/, accessed on 22 April 2022) [48]. The public reference ADS coding sequence were downloaded by accession code AF138959.1, AAF61439.1, AF327527.1, AAK15697.1, AF327526.1, AAK15696.1, AJ251751.1, CAB94691.1, AY006482.1, AAF98444.1, DQ241826.1, ABB51572.1, EF197888.1, ABM88787.1, FJ432667.1, ACL15394.1, HQ315833.1, ADU25497.1, JQ319661.1, AFA34434.1, KJ609176.1, AIC83777.1, KR445687.1, ALJ03212.1, LC106014.1, BAW34953.1, PKPP01006435.1, PWA56512.1, FJ613423.1 and ACM80358.1 from National Center for Biotechnology Information (NCBI) Nucleotide Search database(https://www.ncbi.nlm.nih.gov/nuccore/, accessed on 23 February 2022), as query sequences to blast against predictional genomic coding sequences and genome-wide sequence (identity ≥ 95%, query coverage ≥ 90%). Then, all genes were manually corrected in Apollo, according to the result of blast in transcriptome data with public reference ADS genes. In the end, only genes with complete gene structure and supported by transcriptome or full-length transcript data were retained, and incomplete prediction results were removed to finalize the position and structure of each ADS gene.
Genomic protein prediction results for five Asteraceae species, including Lactuca sativa, Helianthus annuus, Lactuca saligna, and Cynara cardunculus var. Scolymus, were downloaded from NCBI Genome Database (https://www.ncbi.nlm.nih. gov/genome, accessed on 25 April 2022) [41,42,43], and Chrysanthemum Genome Database (http://www.amwayabrc.com/download.htm, accessed on 25 April 2022) [36]. The transcriptome data of Artemisia argyi leaf were downloaded from the Sequence Read Archive (SRA) database of NCBI with the accession code PRJNA804653 and used the transcripts were assembled using Trinity without reference. After that, the Genomic protein prediction results of Artemisia annua L., Chrysanthemum nankingense, Cynara cardunculus var. scolymus, Helianthus annuus, Lactuca saligna, Lactuca sativa, and the full-length transcripts of Artemisia argyi leaf were treated as database respectively, and ADS amino acid sequences of Artemisia annua were used as a query for blastp or tblastn (identity ≥ 40%, query coverage ≥ 80%).
Prediction and calculation of protein properties such as molecular weight and isoelectric point of ADS using ProtParam online tool (http://web.expasy.org/protparam/, accessed on 29 April 2022). And Cell-PLoc (http://www.csbio.sjtu. edu.cn/bioinf/Cell-PLoc-2/, accessed on 29 April 2022) was used to predict the subcellular localization of the identified ADS proteins to demonstrate the specific location of their presence within the cell.
Phylogenetic analysis of ADS genes and similar genes
Multiple alignments of A. annua ADS protein sequences with protein sequences of similar genes of C. cardunculus, H. annuus, L. saligna, L. sativa, C. nankingense, A. argyi were performed by ClustalW of MEGA version 6 with the default parameter setting. The phylogenetic trees was built by MEGA version 6 by the Maximum likelihood (ML) or Neighbor joining method, and then the bootstrap values was set to 500 to achieve five hundred replicates.
For the analysis of the positive selection sites of the phylogenetic tree, EasyCodeML software, which is a CodeML visual analysis tool, was used [49]. The Site model was selected for analysis, and there were eight different hypothesis models in the Site model, in which M0 and M3, M1a and M2a, M7 and M8, and M8a and M8 were four pairs of Nested model, M0, M1a, M7 and M8a were Null model, and M3, M2a and M8 were Alternative model.
Protein conserved motifs and gene structure analysis
The sequence and chromosome annotation data of ADS genes were downloaded from GPGD (http://www.gpgenome.com/, accessed on 10 May 2022). While Cna4665, Cna4666, and Cna9606 were downloaded from Chrysanthemum Genome Database (http://www.amwayabrc.com/, accessed on 10 May 2022). The discovery of conserved motifs for these genes was attributed to the online MEME program (http://meme-suite.org/tools/meme, accessed on 11 May 2022). The parameter “how many motifs should MEME find” was set to 15, and leave the rest of the parameters unchanged at their default values. The NCBI CD -search Tool (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi/, accessed on 11 May 2022) was used to display their conserved domains. Besides, TBtools is a comprehensive bioinformatics tool that can be used to graphically show the motifs and structure of the above mentioned genes.
Secondary structure, tertiary structure prediction and molecular docking of ADS gene and Cna4666
The secondary structure of ADS in Artemisia annua L. and Cna4666 in Chrysanthemum nankingense were projected by an online program called SOPMA (https://npsa-prabi.ibcp.fr/cgi-bin/secpred_sopma.pl, accessed on 4 August 2022). And we used the online tool ColabFold [50], a tool combined the MMseqs2 with AlphaFold2 (https://colabresearch.google.com/github/deepmind/alphafold/blob/main/notebooks/AlphaFold.ipynb, accessed on 10 December 2022) to model the three-dimensional structural homology of the protein spatial model of ADS, Cna4666 and proteins in phylogenetic tree. Then use AutoDock software to build a molecular docking model.
Extract and analyze products of recombinant p0_ADS.1 and Cna4666 proteins using GC–MS analysis
Fllowing method draws on the earlier reaearch [22]. The coding sequences of the Cna4666 (http://www.amwayabrc.com/) gene and p0_ADS.1 (http://www.gpgenome.com/) were provided by TSINGKE Biological Technology Co., Ltd. (Beijing, China). Both genes were cloned into pET28a with BamHI and EcoRI enzyme cut sites. Then pET28a-p0_ADS.1 and pET28a-Cna4666 were transformed into E.coli BL21 (DE3) pLysS cells (TSINGKE Biotech Co., Ltd, Beijing, China). Cells grown at 37 °C and in 30 mL LB medium with 50 μg/mL Kanamycin, until OD600 reached 0.6–0.8. The induction of E. coli cells was achieved by adding 0.4 mM isopropyl-b-D-thiogalactoside (IPTG) to the bacterial solution and expressed it at 25 °C for 10 h. SPME Fiber (Merck KGaA, USA) were suspended on the bacterial solution in a 55 °C thermostatic equipment (water bath) for 30 min. Then the SPME Fiber was injected into a gas chromatograph injection unit.
In brief, the p0_ADS.1 and Cna4666 proteins was extracted using His- Tagged Protein Purification Kit (Soluble Protein, CWBIO, China). And sonication of the cells was carried out for 15 min in 5-s pulses with 5 s between pulses on ice, with the power set at 12 W. The system for the enzymatic reaction was to add 10 μg of protein to a mixture of Tris–HCl (pH = 8.0): 2-[4-(2-hydroxyethyl)piperazin-1- yl]ethanesulfonic acid at a final concentration of 30 mM, MgCl2 at a final concentration of 25 mM, dithiothreitol at a final concentration of 5 mM, and the final concentration of FPP was 60 μM. This system was placed in a glass sample vial covered with hexane and then reacted in a constant temperature water bath at 30 °C for 2 h. Then the hexane was injected into a gas chromatograph injection unit.
GC–MS analysis for product identification was performed by injecting a sample (split less mode) onto a WM-5MS column: 30 m × 0.25 μm × 0.25 mm. After an initial oven temperature of 250 °C during the injection period, the oven temperature was programmed from 50 °C (hold 5 min) to 260 °C (hold 3 min) at a rate of 8 °C/min. The carrier gas is helium, and flow rate was 1 mL/min. The column was connected to the ion source of a GCMS-QP2010 Ultra mass spectrometer, working in the 70-eV EI ionization mode and scanning from m/z 24 to 300.
Chromosomal location and gene duplication analysis of ADS genes
The position information of ADS genes was acquired from the genomic sequence annotation and the package gggenes(R) was used to map gene locations. Gene duplication analysis was performed for ADS genes using the following strategy: (1) > 85% identity of the aligned region, (2) the coverage of coding sequence which is > 90% for potentially tandem duplicated genes (3) if there were less 100 kb and 5 or fewer than 5 genes separated by two ADS genes, they were labeled as tandem duplications.
Selective pressure and synteny analysis of ADS and similar genes
We used Quick Run MCScanX Wrapper of TBtools to demonstrate the synteny relationship of the ADS orthologous genes present in Artemisia annua L. and Helianthus annuus or Chrysanthemum nankingense, the syntenic analysis plots were created by the Multiple Synteny Plot of TBtools [51, 52]. The KaKs Calculator 2.0 was used to calculate the Ks (synonymous) and Ka (non-synonymous) substitution for each ADS genes and similar genes.
Result
Identification of ADS genes in A. annua and similar genes in other species
Eight ADS genes (named p0_ADS.1, p0_ADS.2, p0_ADS.3, p0_ADS.4, p1_ADS.5, p1_ADS.6, p1_ADS.7, p1_ADS.8) were identified in the A. annua genome. Their identities between them ranged from 99 to 100%. The properties and subcellular localization of ADS genes were analyzed. The code lengths of these genes is 1641 bp and they encoded molecules of approximately 546 amino acids in length; their relative molecular masses ranged between 63.88kD (p0_ADS.4) and 63.97kD (p0_ADS.3) with a predicted isoelectric point (PI) range of 5.52 to 5.64, indicating that these ADS proteins were weakly acidic. Subcellular localization prediction showed that all 8 ADS paralogs were localized in chloroplast (Additional file 1: Table S1).
A total of 121 terpene synthase genes similar to ADS genes, with identity greater than 40% and query coverage greater than 80%, were identified in our Asteraceae plant genome dataset. There were 28 genes identified in the A. annua genome, 8 in A. argyi transcriptome, 8 in C. cardunculus genome, 23 in H. annuus genome, 13 in L. saligna genome, 12 in L. sativa genome, 29 in C. nankingense genome. These genes were renamed according to their species names and IDs, and all of them have terpene synthase family domain (pfam03936 and pfam01397) according to the analysis of the NCBI Batch CD-search online tool (Additional file 2: Table S2, Additional file 3: Table S3). And through MEME analysis, we can know that most of them have DDxxD and NSE/DTE motifs. By NCBI BLASTP analysis, some of them were identified as β-farnesene synthase, in addition to beta-caryophyllene synthase, (-)-germacrene D synthase, germacrene-A synthase, Epi-cedrol synthase (identity more than 80%).
Phylogenetic analysis of ADS and ADS-like genes
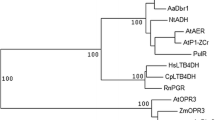
Based on the full-length ADS proteins sequences in A. annua (8), and similar proteins from the genome of A. argyi (8), C. cardunculus (8), H. annuus (23), L. saligna (13), L. sativa (12), C. nankingense (29), and A. annua (28), phylogenetic analysis were performed in a sequence alignment software, MEGA version 6, and a phylogenetic trees were built using the ML method (Fig. 1). The NJ method was then used to build phylogenetic trees to cross-validate the credibility of the clusters, the results show that the clusters of the two trees are basically the same (Additional file 5: Fig. S2). However, the clustering of the two trees resulted in different outgroups, so the conserved motif analysis of these 129 proteins proved that the outgrouping of the ML tree was more reasonable, since Hann0426, Hann1000, Hann0983 and Hann0989 have an additional characteristic motif (Additional file 6: Fig. S3). The ML tree showed that all ADS proteins from A. annua clustered in the same clades, Can4665, Can4666, and Can9606 are clustered with ADS on one branch and sharing the same node with Ccar4569. This indicated that among all homologs, Cna4665, Cna4666, Cna9606, and Ccar4569 are more closely related to ADS. Even they are more closely related to ADS genes than ADS-like genes in A. annua. While Cna4665 and Cna4666 have 82% identity with the ADS gene, Cna9606 was 72%. This gives us reason to suspect that they have the potential to synthesize amorpha-4,11-diene. In C. Cardunculus, the more closely genes related ADS is Ccar4569, in Helianthus annuus are Hann7916, Hann1259, and Hann1261; in L. saligna is Lsal2983; in L. sativa is Lsat5240; in A. argyi are Aarg8267i2 and Aarg8267i1. Furthermore, according to the result of NCBI BLASTP, Ccar4569, Hann7916, Hann1259, Hann1261, Lsat5240, Aarg8267i2 and Aarg8267i1 are more similar to beta-caryophyllene synthase [41,42,43].
Phylogenetic relationship of ADS protein sequences of A. annua and its Homologous of related species. This phylogenetic tree uses the ML method, and the different colored circular dots of each node in the phylogenetic tree indicate different sizes of bootstrap, gray indicates ≤ 50, yellow indicates 51 ~ 80, and red indicates 81 ~ 100. Different colored shapes in front of the leaf labels indicate different species, please see the illustration for details
In order to analyze which site play a role for adaptive evolution in this phylogenetic tree, positive selection was detected at the molecular level using CodeML. The site model was used to detect the positive selection effect of sites in nucleotide sequences, and the Likelihood ratio test (LRT) results after the test showed that the p-values of the three pairs of nested models, M1a vs. M2a, M7 vs. M8 and M8a vs. M8, were all smaller than 0.01, indicating that the alternative model was significantly better than the null hypothesis model, indicating the presence of positive selection effect (Additional file 7: Table S4). Further analysis using a Bayes empirical Bayes procedure identified eight sites (alignment positions 5, 14, 17, 18, 19, 20, 23 and 24) that are under positive selection with posterior probabilities ≥ 0.95, The specific site locations can be found in Table S5 (Additional file 8: Table S5). These results suggest that these eight sites may be factors that influence the function of these enzymes differently. In order to have more clarity on where these sites are located in the protein, we selected some representative proteins from the phylogenetic tree for 3D structure prediction (Additional file 9: Fig. S4). It turned out that these sites of Last8084 in the outgroup are in the α-helix, while other proteins are in the random coil.
Motif composition and gene structure of ADS genes and some similar genes
To further investigate the structural peculiarities of genes in the same branch as ADS genes, the conserved motifs of proteins and intron/exon distributions of genes were analyzed (Fig. 2). According to the results, all ADS genes contain the same number of exons and introns, i.e. seven exons and six introns, and these exons and introns are of the same lengths. Cna4665 and Cna4666 also contain seven exons and six introns, but the second intron of Cna4665 is longer than that of ADS genes, while Cna4666 is shorter (Fig. 2B). Unlike the structure of the above genes, Cna9606 possesses only six exons and five introns. Furthermore, MEME scanned a total of fifteen conserved motifs for these proteins, but not all of the proteins in the figure have these fifteen motifs. All of the ADS genes and Cna4665, Cna4666 contain motif 1–14. Cna9606 lacks motif 7, Motif 9, Motif 12, and Motif 14, but has an extra motif 15 in comparison with other genes (Fig. 2C). The results of all motif composition and gene structure analysis remind us that Cna4666 and Cna4665 are more likely to synthesize AD than Cna9606.
Gene structure, conserved motif, and genetic relationship of ADS and Cna4665, Cna4666, or Cna9606. A Phylogenetic analysis of ADS proteins and Cna4665, Cna4666, or Cna9606. B Exon/intron structure and conserved domains of ADS genes, Cna4665, Cna4666, and Cna9606. C The distribution of conserved motifs in ADS proteins, Cna4665, Cna4666, and Cna9606
Modeling of the secondary and tertiary structural homology of one ADS and Cna4666
The secondary structure of ADS and Cna4666 proteins were analyzed with SOPMA software. After sequence alignment, we learned that the DNA sequence similarity between Cna4666 and Cna4665 was 98.84%. and they are not more than 100 kb apart on the C. nankingense genome. They are a pair of tandem genes, so one of the two proteins was selected for prediction. The secondary structure of a protein refers to the regular spatial folding and coiling of the polypeptide chain backbone, which is determined by the hydrogen bonds between the non-side chain groups of amino acid residues, and it commonly includes α-helices, β-folds, random crimps, and β-turn. The results indicated that p0_ADS.1 and Cna4666 proteins share a very similar secondary structure (Table 1), and they have a higher percentage of α-helices (69.60% ~ 69.96%), a lower percentage of β-turn (2.75% ~ 3.30%), the same percentage of the extended strand (3.85%) and a slightly different proportions of the random coil (22.89% and 23.81%). The random coil is a flexible conformation that can change the direction of the peptide chain and facilitates the connection of relatively rigid α-helix and β-fold structure. It is speculated that Cna4666 may have more variations in the orientation of polypeptide chains than p0_ADS.1. In addition, to initially compare their functions, their three-dimensional structures were predicted (Fig. 3A, B). According to the predicted results, the three-dimensional structures of Cna4666 and p0_ADS.1 are extremely similar. Therefore, we used AutoDock to do the molecular docking of ADS, Cna4666 with AD, and selected the highest absolute value of Affinity in the docking results (Fig. 3C, D). We found that the docking positions of the two proteins and AD were different, and the affinities were also different (− 7.3 and − 6.4, respectively), so we speculated that the function of Cna4666 might be different from that of ADS.
Tertiary structures and molecular docking model diagram of p0_ADS.1 and Cna4666 proteins. A Tertiary structures of p0_ADS.1. B Tertiary structures of Cna4666. C The p0_ADS.1 molecular docking model with the highest absolute value of affinity. D The Cna4666 molecular docking model with the highest absolute value of affinity
Functional characterization of Cna4666 and p0_ADS.1 using GC–MS
The precursors IPP and DMAPP of terpenoids in E. coli can be used to synthesize FPP, so E. coli has the precursor FPP of AD. The Cna4666 and p0_ADS.1 genes were cloned into a plasmid pET28a, and heterologously expressed in E. coli. The volatile products in the bacterial broth were then extracted using solid-phase microextraction for GC–MS analysis. The retention time of the expression product of the Cna4666 gene was inconsistent with that of the expression product of p0_ADS.1 gene (Fig. 4A); p0_ADS.1 catalyzes the production of the AD, and the expression product of the Cna4666 gene was characterized as α-bisabolol by mass spectrum (Fig. 4B, C). In addition, we successfully achieved an enzymatic reaction using the substrate FPP and the purified protein in combination with a suitable catalytic system, with the same catalytic results as in E. coli (Additional file 10: Fig. S5). These results indicates that Cna4666 does not catalyze the production of amorpha-4,11-diene. And previous studies detected ( +)-α-bisabolol synthase in A. maritima, A. kurramensis, A. abrotanum, and A. annua, and showed that it does not synthesize AD, even in by-products [28, 53, 54]. All this reminds us that ADS may be present only in Artemisia, and that it is not widespread in Asteraceae.
Gene duplication analysis and synteny analysis of ADS genes
All of the ADS genes were evenly distributed on the Chromosomal 4 of the two haplotype genomes; p0_ADS.1, p0_ADS.2, p0_ADS.3 and p0_ADS.4 are alleles of p1_ADS.5, p1_ADS.6, p1_ADS.7 and p1_ADS.8. The duplication events of the ADS genes were analyzed, because there were no other genes separated by two ADS genes and the maximum interval between two ADS genes is 4.5 kb, we can tell that these ADS genes are tandem duplicated genes (Fig. 5A). This also indicates that ADS is a multi-copy class of genes in the genome of A. annua.
Gene duplication analysis of ADS genes and collinear association of A.annua, H. annuus, and C. nankingense. A The position of ADS genes in the two haplotype genomes. B Collinear association of A. annua haplotype1 (phase1), H. annuus, and C. nankingense. C Collinear association of A. annua haplotype0 (phase0), H. annuus and C. nankingense. Blue lines indicate collinear blocks of some ADS similar genes between A. annua, H. annuus, and C. nankingense
We further constructed a synteny analysis between ADS genes in A.annua and homologous genes in other two plants, including H.annuus and C.nankingense to infer the evolutionary relationship between ADS and homologous genes among different species(Fig. 5B, C). We detected many syntenic orthologs in their genomes, but none of ADS genes and very few ADS-like genes were detected. There were 10 pairs of collinear blocks among the ADS-like genes, and the ADS-like genes p0_5231, p0_1181, p0_7271, p0_5221, and p1_0461 of A.annua formed homologous pairs with ADS-like genes Hann3660, Hann6699, Hann8472, Hann5264 of H.annuus. And the ADS-like gene Cna1970 of C.nankingense found homologous gene pairs on chromosome 5 of two haplotype genomes of A. annua; Hann3671, Hann8472 of H.annuus found homologous gene pairs on chromosome 5 and chromosome 8 of haplotype1(phase1) of A. annua, respectively. There was no colinear block of ADS genes in the H.annuus and C.nankingense genomes, and we hypothesize that ADS genes are recently evolved functional genes.
Amino acid substitution selection pressure analysis of ADS and similar genes
Ka and Ks analysis was performed for ADS genes and ADS-like genes to estimate the selective pressure of these genes. The Ka/Ks ratio can show positive selection (Ka/Ks > 1), negative or purifying selection (Ka/Ks < 1), and neutral selection (Ka/Ks = 1) during evolution. The Ka/Ks values of ADS and these similar genes were lower than 1, indicating that purifying selection mainly contributed to the evolvement of these genes (Additional file 11: Fig. S6). In contrast, some of the Ka/Ks ratios within the ADS genes were much greater than 1 (Table 2), this result indicates that there is positive selection among the ADS genes and that they are undergoing rapid evolution, supporting the hypothesis that ADS is a recently evolved gene.
The date of the duplication events (T) was calculated using the formula T = Ks/2λ (λ represents the estimated clock-like rate of synonymous substitution, which is 9.21E-9 substitutions/synonymous site/year for A. annua) [40]. The approximate dates of the estimated divergence time are shown in Table 2. The divergence time of ADS genes in A. annua ranged from 0.002 to 1.1309 MA.
Discussion
Amorpha-4,11-diene synthase plays an important role in the synthesis pathway of artemisinin, and the gene of ADS was purified and functionally characterized for the first time in 1999 [6]. However, there are few reported studies on the relationship between ADS genes and ADS-like genes in other species. Therefore, in this study, we extracted ADS genes in the A. annua genome and ADS-like genes from the genomes of some Asteraceae, analyzed their molecular kinship, and explored whether ADS homologous genes exist in these Asteraceae plants.
In the present study, a total of 121 terpene synthase genes similar to ADS genes were identified. There were 28 ADS-like genes identified in A. annua, this number is lower than that in C. nankingense (29) but higher than that in H. annuus (23), L. saligna (13), L. sativa (12), C. cardunculus (8), A. argyi (8). Phylogenetic analysis revealed that Cna4665, Cna4666, and Cna9606 are more closely related to ADS in these similar genes. Besides, Cna4665, Cna4666, and Cna9606 are also the top three with the highest similarity to ADS sequences, with the similarity of 82.784%, 82.418%, and 72.179%, respectively. In the result of gene structural and conserved motif analysis, we found that Cna4666 and Cna4665 genes had very similar gene structures and the same motif as ADS genes. Based on the above results, we pointed out that Cna4666 has high homology with ADS, and speculated that Cna4666 may have the potential to synthesize AD. Meanwhile, we predicted the secondary and three-dimensional structures of ADS and Cna4666, and the results showed that they are very similar in both structures. So we then did a molecular docking analysis of the two proteins with AD, and the results showed that the docking models of AD and the two proteins were not the same.
In order to understand whether Cna4666 has the potential to synthesize AD, we performed gene function identification. The results showed that Cna4666 did not synthesize amorpha-4,11-diene, but synthesized another sesquiterpene, α-bisabolol, so Cna4666 was identified as α-bisabolol synthase. This suggests that an enzyme such as ADS, which has a specific function in Artemisia, does not necessarily have the same function even if there are enzymes with extremely similar sequences and structures to it. This also indirectly proves that ADS genes are not widely present in Asteraceae. The expression of genes of the artemisinin synthesis pathway, including the ADS gene, has been reported in five species of Artemisia [46]. This suggests that ADS genes are present in some Artemisia plants. One genome of Artemisia, the A. argyi, was also recently published, but we did not have the opportunity to include A. argyi genome in our analysis due to insufficient data [55]. Some Artemisia plants have very important medical and economic values, and we hope that more Artemisia genomes will be published.
However, no collinear block of the ADS genes were found in the Helianthus annuus genome (a larger, more complete genome) and the Chrysanthemum nankingense genome(the genome in which Cna4665 and Cna4666 are present). Ka and Ks analysis demonstrates the selective pressure and divergence time of ADS genes, demonstrating that ADS genes are still being affected by positive selection pressure. Combining these two results, it is reasonable to speculate that ADS is a recently evolved gene in Artemisia. However, this conclusion still needs to be confirmed.
Conclusion
Overall, this study reveals that ADS genes are inconsistency and not widespread in Asteraceae. It also explores the evolutionary trajectory of ADS in Asteraceae and draws the preliminary conclusion that ADS may be a recently evolved gene in the genus Artemisia. The exploration of ADS evolution is beneficial for a deeper understanding of artemisinin biosynthesis pathway genes, which can help in metabolic engineering of artemisinin to achieve the goal of high artemisinin production.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADS:
-
Amorpha-4,11-diene synthase
- FP:
-
Faresyl pyrophosphate
- IPP:
-
Isopentenyl pyrophosphate
- DMAPP:
-
Dimethylallyl pyrophosphate
- MVA:
-
Intracellular mevalonate
- MEP:
-
Methylerythritol 4-phosphate
- FPS:
-
Farnesyl pyrophosphate
- AD:
-
Amorpha-4,11-diene
- CYP71AV1:
-
Cytochrome P450 monooxygenase
- ADH1:
-
Alcohol dehydrogenase 1
- DBR2:
-
Double bond reductase
- GPGD:
-
Global pharmacopoeia genome database
- NCBI:
-
National Center for Biotechnology Information
- SRA:
-
Sequence read archive
- ML:
-
Maximum likelihood
References
Brian G, Theonest M. Malaria in 2002. Nature. 2002;415(6872):670–2.
Olliaro PL, Taylor WRJ. Developing artemisinin based drug combinations for the treatment of drug resistant falciparum malaria: a review. J Postgrad Med. 2004;50(1):40.
Callaway E, Cyranoski D. Anti-parasite drugs sweep Nobel prize in medicine 2015. Nature. 2015;526(7572):174–5.
Brown GD. The biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L. (Qinghao). Molecules. 2010;15(11):7603–98.
Towler MJ, Weathers PJ. Evidence of artemisinin production from IPP stemming from both the mevalonate and the nonmevalonate pathways. Plant Cell Rep. 2007;26(12):2129–36.
Bouwmeester HJ, Wallaart TE, Janssen MHA, Loo BV, Jansen BJM, Posthumus MA, et al. Amorpha-4,11-diene synthase catalyses the first probable step in artemisinin biosynthesis. Phytochemistry. 1999;52(5):843.
Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440(7086):940–3.
Paddon CJ, Westfall PJ, Pitera DJ, Benjamin K, Fisher K, McPhee D, et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496(7446):528–32.
Teoh KH, Polichuk DR, Reed DW, Nowak G, Covello PS. Artemisia annua L (Asteraceae) trichome-specific cDNAs reveal CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. Lett. 2006;580(5):1411–6.
Teoh KH, Polichuk DR, Reed DW, Covello PS. Molecular cloning of an aldehyde dehydrogenase implicated in artemisinin biosynthesis in Artemisia annuathis paper is one of a selection of papers published in a Special issue from the national research council of canada—plant biotechnology institute. Botany. 2009;87(6):635–42.
Brown GD, Sy L-K. In vivo transformations of dihydroartemisinic acid in Artemisia annua plants. Tetrahedron. 2004;60(5):1139–59.
Brown GD, Sy L-K. In vivo transformations of artemisinic acid in Artemisia annua plants. Tetrahedron. 2007;63(38):9548–66.
Czechowski T, Larson TR, Catania TM, Harvey D, Brown GD, Graham IA. Artemisia annua mutant impaired in artemisinin synthesis demonstrates importance of nonenzymatic conversion in terpenoid metabolism. Proc Natl Acad Sci. 2016;113(52):15150–5.
Degenhardt J, Kollner TG, Gershenzon J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry. 2009;70(15–16):1621–37.
Alchanati I, Patel JAA, Liu J, Benedict CR, Stipanovic RD, Bell AA, et al. The enzymatic cyclization of nerolidyl diphosphate by δ-cadinene synthase from cotton stele tissue infected with verticillium dahliae. Phytochemistry. 1998.
Benedict CR, Lu JL, Pettigrew DW, Liu J, Stipanovic RD, Williams HJ. The cyclization of farnesyl diphosphate and nerolidyl diphosphate by a purified recombinant delta-cadinene synthase. Plant Physiol. 2001;125(4):1754–65.
Hua L, Matsuda SP. The molecular cloning of 8-epicedrol synthase from Artemisia annua. Arch Biochem Biophys. 1999;369(2):208–12.
Brodelius CPE. Cloning, expression, and characterization of epi-cedrol synthase, a sesquiterpene cyclase from Artemisia annua L. Arch Biochem Biophy. 1999;369(2):213–22.
Salmon M, Laurendon C, Vardakou M, Cheema J, Defernez M, Green S, et al. Emergence of terpene cyclization in Artemisia annua. Nat Commun. 2015;6:6143.
Huang JQ, Fang X. Amorpha-4,11-diene synthase: a key enzyme in artemisinin biosynthesis and engineering. aBIOTECH. 2021;2(3):276–88.
Chang YJ, Song SH, Park SH, Kim SU. Amorpha-4,11-diene synthase of Artemisia annua: cDNA isolation and bacterial expression of a terpene synthase involved in artemisinin biosynthesis. Arch Biochem Biophys. 2000;383(2):178–84.
Mercke P, Bengtsson M, Bouwmeester HJ, Posthumus MA, Brodelius PE. Molecular cloning, expression, and characterization of amorpha-4,11-diene synthase, a key enzyme of artemisinin biosynthesis in Artemisia annua L. Arch Biochem Biophys. 2000;381(2):173–80.
Picaud S, Olofsson L, Brodelius M, Brodelius PE. Expression, purification, and characterization of recombinant amorpha-4,11-diene synthase from Artemisia annua L. Arch Biochem Biophys. 2005;436(2):215–26.
Soon-Hee Kim, Keon Heo, Yung-Jin Chang, et al. Cyclization mechanism of amorpha-4,11-diene synthase, a key enzyme in artemisinin biosynthesis. J Nat Prod. 2006;69(5):758–62.
Picaud S, Mercke P, He X, Sterner O, Brodelius M, Cane DE, et al. Amorpha-4,11-diene synthase: mechanism and stereochemistry of the enzymatic cyclization of farnesyl diphosphate. Arch Biochem Biophys. 2006;448(1–2):150–5.
Hong YJ, Tantillo DJ. A tangled web—interconnecting pathways to amorphadiene and the amorphene sesquiterpenes. Chem Sci. 2010.
Huang JQ, Li DM, Tian X, Lin JL, Yang L, Xu JJ, et al. Side products of recombinant amorpha-4,11-diene synthase and their effect on microbial artemisinin production. J Agric Food Chem. 2021;69(7):2168–78.
Paskorn M, Hikaru S, Munenori S, Aya K, Mika N, Ryota M, et al. Functional analysis of amorpha-4,11-diene synthase (ADS) homologs from non-artemisinin-producing artemisia species: the discovery of novel koidzumiol and (+)-α-bisabolol synthases. Plant Cell Physiol. 2016;8:1678–88.
Li G, Thilo W, Xiaofei Y, et al. The opium poppy genome and morphinan production. Science. 2018.
Xu J, Chu Y, Liao B, Xiao S, Yin Q, Bai R, et al. Panax ginseng genome examination for ginsenoside biosynthesis. Gigascience. 2017;6(11):1–15.
Zhao Q, Yang J, Cui MY, Liu J, Fang Y, Yan M, et al. The reference genome sequence of scutellaria baicalensis provides insights into the evolution of wogonin biosynthesis. Mol Plant. 2019;12(7):935–50.
Haberer G, Young S, Bharti AK, Gundlach H, Raymond C, Fuks G, et al. Structure and architecture of the maize genome. Plant Physiol. 2005;139(4):1612–24.
International Wheat Genome Sequencing C. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science. 2018;361(6403):7191.
Xia EH, Zhang HB, Sheng J, Li K, Zhang QJ, Kim C, et al. The tea tree genome provides insights into tea flavor and independent evolution of caffeine biosynthesis. Mol Plant. 2017;10(6):866–77.
Wei C, Yang H, Wang S, Zhao J, Liu C, Gao L, et al. Draft genome sequence of camellia sinensis var sinensis provides insights into the evolution of the tea genome and tea quality. Proc Natl Acad Sci. 2018;115(18):4151–8.
Song C, Liu Y, Song A, Dong G, Zhao H, Sun W, et al. The chrysanthemum nankingense genome provides insights into the evolution and diversification of chrysanthemum flowers and medicinal traits. Mol Plant. 2018;11(12):1482–91.
Jiang X, Shuai G, Xianmei Y, Mingqian L, He S, Xuejiao L, et al. Genomic, transcriptomic, and epigenomic analysis of a medicinal snake, bungarus multicinctus, to provides insights into the origin of elapidae neurotoxins. Acta Pharmaceutica Sinica B. 2022.
Hu H, Shen X, Liao B, Luo L, Xu J, Chen S. Herbgenomics: a stepping stone for research into herbal medicine. Sci China Life Sci. 2019;62(7):913–20.
Xu J, Liao B, Yuan L, Shen X, Liao X, Wang J et al. 50th anniversary of artemisinin: From the discovery to allele-aware genome assembly of Artemisia annua. Mol Plant. 2022;15(8):1243–46.
Liao B, Shen X, Xiang L, Guo S, Chen S, Meng Y, et al. Allele-aware chromosome-level genome assembly of Artemisia annua reveals the correlation between ADS expansion and artemisinin yield. Mol Plant. 2022;15(8):1310–28.
Scaglione D, Reyes-Chin-Wo S, Acquadro A, Froenicke L, Portis E, Beitel C, et al. The genome sequence of the outbreeding globe artichoke constructed de novo incorporating a phase-aware low-pass sequencing strategy of F1 progeny. Sci Rep. 2016;6:19427.
Badouin H, Gouzy J, Grassa CJ, Murat F, Staton SE, Cottret L, et al. The sunflower genome provides insights into oil metabolism, flowering and Asterid evolution. Nature. 2017;546(7656):148–52.
Reyes-Chin-Wo S, Wang Z, Yang X, Kozik A, Arikit S, Song C, et al. Genome assembly with in vitro proximity ligation data and whole-genome triplication in lettuce. Nat Commun. 2017;8:14953.
Arab HA, Rahbari S, Rassouli A, Moslemi MH, Khosravirad F. Determination of artemisinin in Artemisia sieberi and anticoccidial effects of the plant extract in broiler chickens. Trop Anim Health Prod. 2006;38(6):497–503.
Mannan A, Ahmed I, Arshad W, Asim MF, Qureshi RA, Hussain I, et al. Survey of artemisinin production by diverse Artemisia species in northern Pakistan. Malar J. 2010;9:310.
Salehi M, Karimzadeh G, Naghavi MR, Naghdi Badi H, Rashidi MS. Expression of key genes affecting artemisinin content in five Artemisia species. Sci Rep. 2018;8(1):12659.
Ranjbar M, Naghavi MR, Alizadeh H, Soltanloo H. Expression of artemisinin biosynthesis genes in eight Artemisia species at three developmental stages. Ind Crops Prod. 2015;76:836–43.
Liao B, Hu H, Xiao S, Zhou G, Sun W, Chu Y, et al. Global pharmacopoeia genome database is an integrated and mineable genomic database for traditional medicines derived from eight international pharmacopoeias. Sci China Life Sci. 2022;65(4):809–17.
Gao F, Chen C, Arab DA, Du Z, He Y, Ho SYW. EasyCodeML: a visual tool for analysis of selection using CodeML. Ecol Evol. 2019;9(7):3891–8.
Mirdita M, Schütze K, Moriwaki Y, Heo L, Ovchinnikov S, Steinegger M. ColabFold: making protein folding accessible to all. Nat Methods. 2022;19(6):679–82.
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13(8):1194–202.
Wang Y, Tang H, DeBarry JD, Tan X, Li J, Wang X, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40(7):e49–e49.
Li JX, Fang X, Zhao Q, Ruan JX, Yang CQ, Wang LJ, et al. Rational engineering of plasticity residues of sesquiterpene synthases from Artemisia annua: product specificity and catalytic efficiency. Biochem J. 2013;451(3):417–26.
Muangphrom P, Misaki M, Suzuki M, Shimomura M, Suzuki H, Seki H, et al. Identification and characterization of (+)-alpha-bisabolol and 7-epi-silphiperfol-5-ene synthases from Artemisia abrotanum. Phytochemistry. 2019;164:144–53.
Miao Y, Luo D, Zhao T, Du H, Liu Z, Xu Z, et al. Genome sequencing reveals chromosome fusion and extensive expansion of genes related to secondary metabolism in Artemisia argyi. Plant Biotechnol J. 2022.
Acknowledgements
The author thanks the Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences for providing the experimental platform and the group members for their support.
Funding
This work was supported by the National Science and Technology Major Project for Significant New Drugs Development (2019ZX09201005-006-001), National Natural Science Foundation of China (82204548), and the Central Public Welfare Research Institutes (ZZ13-YQ-047).
Author information
Authors and Affiliations
Contributions
CSY and LBS designed the experiment. GS managed the material. CSY performed experiments, analyzed the result and wrote the manuscripts. XJ, LBS, SXF, MY, LY revised the manuscript. All authors reviewed and approved the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Statistics of ADS gene information of Artemisia annua L.
Additional file 2: Table S2.
Gene naming and characterization of other Asteraceae species.
Additional file 3: Table S3.
Statistics of structural domains and gene families of all genes.
Additional file 4: Figure S1.
The biosynthesis pathway for artemisinin. The orange route is the main synthesis path.
Additional file 5: Figure S2.
Phylogenetic relationship of ADS protein sequences of A. annua and its Homologous of related species.
Additional file 6: Figure S3.
Conserved motif and phylogenetic relationship of 129 genes extracted from the genome.
Additional file 7: Table S4.
Results of CODEML analyses of selective pattern for ADS and ADS-like genes.
Additional file 8: Table S5.
Sequence alignment data of ADS and ADS-like genes after removal of gaps.
Additional file 9: Figure S4.
Tertiary structure of some representative proteins of the phylogenetic tree.
Additional file 10: Figure S5.
GC–MS analysis of the products formed by recombinant Cna4666 and p0_ADS.1 proteins.
Additional file 11: Figure S6.
KA and KS analysis of ADS genes and similar genes identified in Asteraceae plants.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, S., Liao, B., Guo, S. et al. Genetic analysis reveals the inconsistency of amorpha-4,11-diene synthase, a key enzyme in the artemisinin synthesis pathway, in asteraceae. Chin Med 18, 5 (2023). https://doi.org/10.1186/s13020-023-00708-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13020-023-00708-w