Abstract
Background
The essential oil is one of the main active ingredients of Amomum villosum Lour. However, volatile compounds are easily lost during the drying, storage and even sample preparation procedure. Therefore, using fresh samples can obtain more accurately data for qualitative and comparative analysis.
Methods
In this study, the volatile compounds in different parts of fresh A. villosum from different origins were systemic analyzed and compared by using cryogenic grinding combined HS–SPME–GC–MS for the first time. GC–MS analyses were performed on a 6890 Series GC instrument coupled to a 5973 N mass spectrometer. The volatile compounds were extracted by the SPME fiber (100 μm PDMS). Analytes separation was achieved on a HP-5MS capillary column. The oven temperature was initially programmed at 70 °C, then raised 4 °C/min to reach 125 °C and then programmed at 0.5 °C/min to 133 °C, then at 6 °C/min to 170 °C and finally, at 20 °C/min to 280 °C held for 2 min. The temperatures of the injection port, ion source and transfer line were set at 250 °C, 230 °C and 280 °C, respectively.
Results
Forty-eight main compounds were identified in different parts of fresh A. villosum. The most abundant components in fresh fruit samples were camphor (3.91%), bornyl acetate (10.53%), caryophyllene (8.70%), β-bisabolene (11.50%), (E)-nerolidol (14.82%) and cubenol (10.04%). This is quite different with that of dried samples analyzed in our previous work. As different parts of the same plant, many common components with biological activities were detected in fruit and other parts. In principle components analysis (PCA) and hierarchical clustering analysis (HCA), four parts of A. villosum were divided into different groups clearly. Additionally, fruit and root samples also could be divided into two subgroups (HCA) in accordance with their regions.
Conclusion
The developed method was successfully used for qualitative and comparative analysis of volatile compounds in fresh A. villosum samples. Additionally, using fresh samples can obtain much more information which is helpful for their performance in the fields of functional foods, agriculture and biomedical industry. Furthermore, our research is helpful for comprehensive utilization and quality control of A. villosum.
Similar content being viewed by others
Introduction
Amomum villosum Lour., belongs to the Zingiberaceae family, is wildly cultivated in southern China, especially in Yangchun, Guangdong province (111° 16′ 27″ to 112° 09′ 22″ E, 21° 50′ 36″ to 22° 41′ 01″ N) [1, 2]. The dried ripe fruit of A. villosum, also named Amomi Fructus, has been used as traditional medicine to treat gastritis, stomachache, and digestive diseases for hundreds of years [3]. Recently, Amomi Fructus is also authorized as a food by the China Food and Drug Administration [4]. As the main active ingredient in Amomi Fructus, essential oil possesses many biological activities such as antimicrobial, anti-inflammation and analgesic effects [5,6,7]. Due to the excellent functions and special aroma of its oil, Amomi Fructus presents great economic values in the functional foods and biomedical industry. Nowadays, it has been used as natural raw materials for wine, tea, candies and cosmetics. Additionally, Amomi Fructus is also one of the food spices in China [8]. With the development of Amomi Fructus related industry, the natural resource of Amomi Fructus is hard to meet the increasing demand [1, 9]. Generally, different parts of the plant have similar secondary metabolites. Expand the application parts are helpful for making up the shortages of this resource. Therefore, qualitative and comparative analysis of the essential oil in different parts of A. villosum from different geographical areas is very necessary, which is beneficial for comprehensive utilization of this these resources as well as quality control of A. villosum.
The chemical composition of essential oil in A. villosum has been reported by several papers [10,11,12,13,14]. However, due to the fresh A. villosum samples is difficult to collect and store, most of these studies were focused on dried samples. Actually, volatile compounds are easily lost during the drying, storage and even sample preparation procedure. Therefore, using fresh samples can obtain more accurately data for qualitative and comparative analysis. Conventional techniques such as steam distillation and supercritical CO2 extraction coupled with gas chromatography–mass spectrometry (GC–MS) have been employed to investigate the essential oils in fresh plants [15,16,17,18]. However, these techniques either need a tedious and time-consuming sample preparation procedure or require special and expensive equipment. Headspace solid-phase microextraction (HS-SPME) is a convenient alternative to traditional essential oil extraction methods. It allows extraction and simultaneous concentration of analytes in one single step. Furthermore, no organic solvents were needed in the whole extraction process [19, 20]. In recent years, HS-SPME coupled GC-MS has gained wide applications in volatile compounds analysis especially in case on natural plants [21,22,23,24].
The sample preparation methods for HS–SPME–GC–MS analysis of herbs usually contain pulping, milling and so on. However, the frictional forces arising during the milling process result in the temperature increasing which is enhancing the losses of essential oil and thermo-sensitive components through evaporation and oxidative reactions [25]. As the literature report, the losses of essential oil in nutmeg and cinnamon are about 37% and 17%, respectively [26]. Therefore, a volatile compounds saver sample preparation method is very necessary. Cryogenic grinding technique using liquid nitrogen that provides extremely low temperature needed to pre-cool the sample. During this process, the moisture and oil are solidified so that the sample becomes brittle. Additionally, to maintain the low temperature, the liquid nitrogen vaporized to gaseous state and creates an inert and dry atmosphere which further protects the sample [27, 28]. As the literature report, cryogenic grinding obtained 29.5% more volatile oil in cloves than that of ambient grinding [28].
In this work, the volatile compounds in 72 fresh A. villosum samples were analyzed and compared by using cryogenic grinding combined HS–SPME–GC–MS. As far as we are aware, this is the first time for systemic qualitative and comparative analysis of volatile compounds in different parts of fresh A. villosum from different geographical areas. Additionally, this work is helpful for comprehensive utilization and quality control of this plant.
Materials and methods
Materials and chemicals
Yangchun is located in southwest of Guangdong province of China (111° 16′ 27″ to 112° 09′ 22″ E, 21° 50′ 36″ to 22° 41′ 01″ N), which is the geo-authentic habitats of A. villosum. Seventy-two batches of fresh A. villosum samples containing fruits (1–18), roots (19–36), leaves (37–54) and stems (55–72) were obtained from different places of Yangchun. The detailed characteristics are presented in Table 1. Species identification was performed by Dr. Jing Zhao and Dr. Hao Hu. The voucher specimens of these samples were deposited at the Institute of Chinese Medical Sciences, University of Macau, Macao SAR, China.
All other chemicals and reagents were of analytical grade. The extraction fibers: 100 μm polydimethylsiloxane (PDMS), 65 μm polydimethylsiloxane/divinylbenzene (PDMS/DVB), 85 μm polyacrylate and 75 μm carboxen poly(dimethylsiloxane) (CAR/PDMS) were purchased from Supelco (Bellefonte, PA, USA). Headspace vials (20 mL) and accessories were obtained from Agilent (Palo Alto, CA, USA).
Experimental procedures for cryogenic grinding of fresh A. villosum samples
In order to reduce the loss of volatile compounds in the sample preparation procedure, cryogenic grinding was employed. Fresh A. villosum samples (5 g) were frozen in liquid nitrogen, then volatilization of the liquid nitrogen and quickly crushed the sample in airtight grinding bowl. The procedure was repeated until the sample milling to fine powder (20 mesh). Then 50 mg sample powder was transferred to a 20 mL headspace vials for SPME analysis.
Instrumentation and GC–MS conditions
The incubation, equilibrium, extraction and desorption of volatile components were carried out automatically by a Combi-Pal autosampler (CTC Analytics, Zwingen, Switzerland). Specifically, the headspace vials were incubated and equilibrated at 80 °C for 10 min, under continuous agitation (500 rpm). And then the volatile compounds were extracted by the SPME fiber (100 μm PDMS) for 30 min at the same condition. The desorption was performed in the injector at 250 °C for 5 min.
GC–MS analyses were performed on a 6890 Series GC instrument coupled to a 5973 N mass spectrometer and a ChemStation software (Agilent Technologies, Palo Alto, CA). Analytes separation was achieved on a HP-5MS capillary column (30 m × 0.25 mm i.d.) coated with 0.25 μm film (5% phenyl methyl siloxane). The splitless injection mode was used. High-purity helium was used as carrier gas with flow rate of 1 mL/min. The oven temperature was initially programmed at 70 °C, then raised 4 °C/min to reach 125 °C and then programmed at 0.5 °C/min to 133 °C, then at 6 °C/min to 170 °C and finally, at 20 °C/min to 280 °C held for 2 min. The temperatures of the injection port, ion source and transfer line were set at 250 °C, 230 °C and 280 °C, respectively. The mass spectrometer was operated in electron-impact mode (EI).
Statistical data analysis
The principal components analysis (PCA) and hierarchical clustering analysis (HCA) analyses were performed in software R for windows based on the 72 A. villosum samples with 48 identified volatile compounds and their Comparative index. Specifically, the PCA was conducted using R package, gmodels. And the analysis results were visualized using R packages, ‘ggplot2’ and ‘scatterplot3d’. The heatmap analysis and HCA were performed using R package, ‘pheatmap’.
Results and discussion
Optimization of the SPME conditions
For volatile compounds analysis, selected the appropriate SPME fibers is the first step. In this study, 65 μm PDMS/DVB, 100 μm PDMS, 85 μm polyacrylate and 75 μm CAR/PDMS were used to evaluate the effect of fiber types on the extraction of volatile compounds in fresh A. villosum. (Sample 1). The relative peak area (RPA) was employed to evaluate the content of volatile compounds under different SPME conditions. The RPA was calculated as: RPA (B) % = (B/A) *100%; (A was the total peak area of volatile compounds under the optimum condition; B was the total peak area of volatile compounds under other conditions) [29]. As shown in Fig. 1a, 100 μm PDMS achieved the highest extraction of the volatile compounds than other fibers. This indicated that the retention ability of this fiber for the volatile compounds in fresh A. villosum is much stronger than the other fibers. Additionally, the 65 μm PDMS/DVB absorbed more volatile compounds than 85 μm polyacrylate and 75 μm CAR/PDMS. Actually, the 100 μm PDMS, 65 μm PDMS/DVB are non-polar fiber and semi-polar fiber, respectively. Otherwise, 85 μm polyacrylate and 75 μm CAR/PDMS are polar fibers. The main volatile compounds in A. villosum such as bornyl acetate, borneol and camphor are low-polar compounds which are effective absorbed by the non-polar fiber or semi-polar fiber [30]. In addition, the fiber coating thickness also affects the extraction process. Usually, the small molecules are absorbed more efficiently by a thicker fiber [30]. The extraction temperature (60, 70, 80, 90 °C) at four levels were investigated. The results (Fig. 1b) showed that the content of volatile compounds increased steadily with temperature (60–80 °C). Actually, the extraction temperature had a significant influence on the adsorption process because it can influence the distribution coefficients of the volatile compounds among sample, headspace and fiber. When the temperature was higher than 80 °C, the content of volatile compounds was stable. However, higher temperature may also cause compound degradation. Therefore 80 °C was selected as the optimum temperature. The extraction time ranges from 10 to 40 min was investigated (Fig. 1c). When the extraction time increased from 10 to 30 min, the total content of volatile compounds was increased correspondingly. When the extraction time was higher than 30 min, the total content of volatile compounds was stable. Therefore 30 min was selected as the optimum extraction time. Particle size is also an important factor affecting the extraction efficiency. Usually, small particle size is easier for essential oil volatilize. However, conventional sample preparation methods for HS-SPME GC–MS analysis containing cut, pulping, milling and so on. All these methods may cause the loss of volatile compounds. In this study, cryogenic grinding was employed and the particle size (20, 40, 60, 80 mesh) was optimized. As shown in Fig. 1d, when the particle size was smaller than 40 mesh, the total content of volatile compounds was decreased significantly. This result indicated that the volatile compounds are easily lost in sample preparation procedure. The samples with particle size bigger than 20 mesh was not investigated in this study, because the large particle size might affect sampling uniformity. The total content of volatile compounds detected at 20 and 40 mesh was similar, but the particle size at 20 mesh requires less sample preparation time and lower cost. Therefore, 20 mesh was selected as optimum particle size.
Volatile compounds in different parts of A. villosum
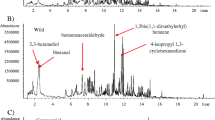
The volatile compounds in different parts of A. villosum were analyzed by the developed cryogenic grinding combined HS–SPME–GC–MS method. Volatile compounds were identified based on the National Institute of Standards and Technology (NIST) 2.0 Mass Spectra Database and the published literatures [1,2,3,4,5,6,7,8, 11,12,13,14]. The identified compounds were listed in Table 2 and Additional file 1: Table S1, and chromatograms of representative samples were shown in Fig. 2. (The chromatograms of all analyzed samples were shown in Additional file 1: Fig. S1.). As shown in Table 2, a total 48 main compounds were identified in different parts of A. villosum samples, accounting for 81.28–98.80% of the total detected essential oil components. Specifically, 33 compounds accounting for average 95.35% (range 90.86–98.80%) of the total detected essential oil components in fruit samples were identified, 27 compounds (93.60%, 89.79–98.67%) were identified in root samples, 19 compounds (86.00%, 81.28–93.78%) were identified in leaf samples and 21 compounds (92.72%, 90.33–98.51%) were identified in stem samples.
The volatile compounds from fruits of A. villosum were characterized by high amounts of sesquiterpenes hydrocarbons (44.89%, 34.4–50.98%) followed by oxygenated sesquiterpenes (29.81%, 21.3–36.02%), oxygenated monoterpenes (15.51%, 11.77–19.95%) and monoterpene hydrocarbons (3.37%, 1.81–6.09%). The most abundant components were camphor (3.91%, 2.44–6.32%), bornyl acetate (10.53%, 8.14–14.27%), caryophyllene (8.70%, 6.64–10.99%), β-bisabolene (11.50%, 9.00–14.05%), (E)-nerolidol (14.82%, 10.14–18.66%) and cubenol (10.04%, 7.19–12.99%).
In our previous work, the volatile compounds in 12 batches of the dried fruit of A. villosum were evaluated and 11 main components were determined. Camphor, borneol and bornyl acetate were the most abundant compounds, accounting for almost 98% of the determined compounds [1]. This result was in accordance with that previously reported by other authors. Zeng et al. (2010) analyzed the volatile compounds in different varieties of Amomi Fructus and found camphor (27.81%), borneol (2.11%) and bornyl acetate (59.60%) as the major components in dried fruit of A. villosum [31]. However, in fresh fruits of A. villosum, the average content of camphor, borneol and bornyl acetate was 3.91%, 1.06% and 10.53%, respectively. This result is quite different with that of dried fruits of A. villosum. The reason might be that many volatile components are lost or degraded during drying and storage. (E)-nerolidol, the most abundant component in fresh fruits of A. villosum, exhibits many biological functions such as antifungal [32] and antiulcer [33] effects. However, this compound was great lost in dried samples. Therefore, qualitative and comparative analysis of volatile compounds in fresh A. villosum samples can obtain much more information which is helpful for their performance in the fields of functional foods, agriculture and biomedical industry.
The volatile compounds from root samples showed a higher amount of sesquiterpene hydrocarbons (67.21%, 61.31–73.02%). Oxygenated sesquiterpenes (11.58%, 8.22–15.2%) represented the second most abundant chemical classes of this part. The most abundant components were caryophyllene (8.67%, 5.53–11.27%), allo-aromadendrene (4.21%, 2.86–5.87%), chamigrene (27.88%, 22.26–36.38%), β-guaiene (9.85%, 7.27–13.71%), β-bisabolene (6.26%, 3.97–10.97%), δ-cadinene (5.94%, 3.97–8.28%) and eremophilene (3.98%, 2.46–6.07%) as the major component. Monoterpene hydrocarbons (5.81%, 4.03–8.90%) and oxygenated monoterpenes (4.30%, 2.83–8.45%) gave a minor contribution.
The volatile compounds from leaf samples consisted mostly of sesquiterpene hydrocarbons (60.34%, 52.85–68.94%) and monoterpene hydrocarbons (13.29%, 5.60–23.9%) followed by oxygenated sesquiterpenes (7.75%, 5.19–14.76%). The most abundant components were (-)-β-pinene (7.41%, 2.66–17.96%), d-limonene (5.87%, 2.64–8.60%), elixene (4.20%, 2.48–6.05%), caryophyllene (27.67%, 21.49–35.15%), α-caryophyllene (4.03%, 3.06–5.88%), α-curcumene (9.76%, 6.52–12.77%), δ-cadinene (5.47%, 3.00–13.52%).
High content of sesquiterpene hydrocarbons (41.70%, 25.26–49.12%) were detected in stem samples, followed by oxygenated sesquiterpenes (17.66%, 12.69–21.50%), oxygenated monoterpenes (15.97%, 11.74–22.37%), monoterpene hydrocarbons (11.62%, 7.80–16.32%). d-limonene (8.62%, 4.82–13.12%), camphor (8.08%, 4.97–11.59%), 4-terpineol (3.99%, 1.08–6.49%), allo-aromadendrene (8.09%, 5.70–10.83%), α-curcumene (8.73%, 5.11–11.81%), β-guaiene (6.79%, 1.20–9.99%), δ-cadinene (8.07%, 1.49–10.29%), γ-eudesmol (12.84%, 9.42–15.4%) and eremophilene (5.94%, 4.25–8.64%) were the most abundant components.
For further evaluation, the comparative index was used for comparative analysis of volatile compounds in different parts of A. villosum samples. The comparative index was calculated as: comparative index (A/SI) = P(A/SI)/P(AMax.)*100; (comparative index (A/SI) was the comparative index of compound A in sample SI; P(A/SI) was the peak area of compound A in sample SI; P(AMax.) was the maximum peak area of compound A in different parts of A. villosum samples). As shown in Table 2, the fruits of A. villosum showed high amounts of volatile compounds (average 77.81, range 55.84–100) followed by roots (47.95, 35.68–68.76), leaves (39.88, 29.49–56.66) and stems (32.32, 16.64-57.90). Heatmap analysis was performed on 72 A. villosum samples with 48 identified volatile compounds and their comparative index (Fig. 3). As different parts of the same plant, there are many common components between fruit and other parts (Fig. 3). The bioactive compounds such as (-)-β-pinene, d-limonene and caryophyllene were existed in four parts of A. villosum. Additionally, the main pharmacological active ingredients camphor can be detected in root and stem samples, and bornyl acetate can be detected in root samples. These results indicated that other parts of A. villosum have potential for partial replacement of the fruit. In addition, there are also many characteristic components detected in root, leaf and stem of A. villosum. Higher content of chamigrene (55.5, 37.63–100.00) and β-guaiene (59.75, 37.91–100.00) were detected in root samples. Elixene (50.26, 26.48–100.00), α-caryophyllene (74.81, 41.95–10.00) and α-curcumene (61.83, 33.69–100.00) were mostly existed in leaf samples. The stem samples contain higher amounts of allo-aromadendrene (61.25, 25.84–100.00) and γ-eudesmol (52.87, 28.04–100.00). These results are beneficial for development of the unique applications of different parts of A. villosum in biomedical and functional foods industry. As sample distance assessment characterized by the volatile compounds profile of each sample is a useful way to depict the relationships between samples, PCA and HCA were performed to determine the similarity among samples derived from different parts of A. villosum. The PCA and HCA were performed on 72 A. villosum samples with 48 identified volatile compounds and their comparative index. As shown in Fig. 4a, three principal components (PC) explained 83.5% of the total variance. Specifically, PC 1, 2 and 3 were account for 44.4%, 22.8% and 16.3, respectively. All the A. villosum samples could be divided into four groups, which were corresponding to sample sets collected from fruit, root, leaf and stem, respectively. The HCA (Fig. 4b) was in accordance with that of PCA, four parts of A. villosum samples were divided into four subgroups clearly. These results indicated that the pattern of the content of volatile compounds detected in different organs of A. villosum were so divergent that a sample could easily be assigned to its sample set basing on this kind of pattern.
Comparison of volatile compounds in fresh A. villosum from different geographical areas
As shown by the results of HCA (Fig. 4b), samples collected from fruits could be divided into two groups although two of the samples (sample 1 and 9) was misassigned. Samples in group I-1 were all from Pingxi village, Yangchun and samples in group I-2 were from Shiwanzai village, Yangchun (exclude sample 1 and 9). This result indicated that the volatile compounds in fruit samples may be affected by the growing region. The average comparative index of five chemical classes (monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpene hydrocarbons, oxygenated sesquiterpenes and others) of group I–1 and I–2 were shown in Fig. 5a. The results showed that great difference was observed in four chemical classes (oxygenated monoterpenes, sesquiterpene hydrocarbons, oxygenated sesquiterpenes and others). This indicated that these four chemical classes in fruit of A. villosum might be more susceptible to the growing environment. Similarly, many root samples from different regions were also divided into two groups (group II-1 and group II-2). As shown in Fig. 5b great difference was observed in sesquiterpene hydrocarbons and other compounds. Leaf and stem samples could not be clustered into any subgroups that related to the geographic distribution of the corresponding samples. These results indicated that the fruits and roots of A. villosum produced by distinct areas, at least the areas analyzed here, showed a difference in the content of volatile compounds. However, for further investigate, much more samples are needed to investigate the most suitable growth environment, cultivating mode and collection time for A. villosum with high quality.
Comparative analysis of five chemical classes of A. villosum samples from different regions. a Fruit samples b root samples. M: monoterpene hydrocarbons; OM: oxygenated monoterpenes; SE: sesquiterpene hydrocarbons; OSE: oxygenated sesquiterpenes; PX: Pingxi village; SWZ, Shiwanzai village. All data was presented as average of two determinations, their relative average deviations were less than 5.3%
Conclusions
In this study, the volatile compounds in different parts of fresh A. villosum from different origins were systemic analyzed and compared by using cryogenic grinding combined HS–SPME–GC–MS for the first time. The type and content of main volatile compounds in fresh fruits of A. villosum is quite different with that of dried samples (reported in previous studies). This indicated that systemic analysis of fresh A. villosum can obtain much more information which is helpful for their development and application. Other parts of A. villosum have potential for partial replacement of the fruit, due to many common components with biological activities were detected in fruits and other parts of A. villosum. In addition, many characteristic components were also detected in different parts of A. villosum. These results are beneficial for development of the unique applications of different parts of A. villosum. Additionally, fruit and root samples also could be divided into two subgroups (HCA) in accordance with their regions. Furthermore, our research is helpful for comprehensive utilization and quality control of A. villosum.
Availability of data and materials
The research data generated from this study is included within the article.
Abbreviations
- CAR/PDMS:
-
Carboxen poly(dimethylsiloxane)
- EI:
-
Electron-impact mode
- HS-SPME:
-
Headspace solid-phase micro-extraction
- HCA:
-
Hierarchical clustering analysis
- RPA:
-
Relative peak area
- GC–MS:
-
Gas chromatography–mass spectrometry
- PCA:
-
Principle components analysis
- PDMS:
-
Polydimethylsiloxane
- PDMS/DVB:
-
Polydimethylsiloxane/divinylbenzene
References
Lai YF, Chen LX, Chen YN, Zhao J, Leong F, Li XW, Yang Q, Li P, Hu H. Sustainable development of Amomum villosum: a systematic investigation on three different production modes. Afr J Tradit. Complem. 2016;13(4):97–104.
Ao H, Wang J, Chen L, Li S, Dai C. Comparison of Volatile Oil between the Fruits of Amomum villosum Lour. and Amomum villosum Lour. var. xanthioides T. L. Wu et Senjen Based on GC-MS and Chemometric Techniques. Molecules. 2019;24:1163.
Suo S, Lai Y, Li M, Song Q, Cai J, Zhao J, Yang Q, Ung COL, Hu H. Phytochemicals, pharmacology, clinical application, patents, and products of Amomi Fructus. Food Chem Toxicol. 2018;119:31–6.
Zhang T, Lu S, Bi Q, Liang L, Wang Y, Yang X, Gu W, Yu J. Volatile oil from Amomi Fructus attenuates 5-fluorouracil-induced intestinal mucositis. Front Pharmacol. 2017;8:786.
Zhang ST, Wang ZY, Wang TS, Li MX, Lin JM. Composition and Antimicrobial Activities of Essential Oil of Fructus Amomi. Nat Prod Res Dev. 2011;23:464–72.
Wu X, Li X, Xiao F, Zhang Z, Xu Z, Wang H. Studies on the analgesic and anti-inflammatory effect of bornyl acetate in volatile oil from Amomum villosum. J Chin Med Mater. 2004;27(6):438–9.
Chen Z, Ni W, Yang C, Zhang T, Lu S, Zhao R, Mao X, Yu J. Therapeutic effect of Amomum villosum on inflammatory bowel disease in rats. Front Pharmacol. 2018;9:639.
Deng C, Yao N, Wang A, Zhang X. Determination of essential oil in a traditional Chinese medicine, Fructus amomi by pressurized hot water extraction followed by liquid-phase microextraction and gas chromatography–mass spectrometry. Anal Chim Acta. 2005;536(1–2):237–44.
Tang L, He G, Su J, Xu H. The Strategy to promote the development of industry of genuine medicinal material of Amomum villosum. Chin Agr Sci Bull. 2012;28:94–9.
Deng C, Liu N, Gao M, Zhang X. Recent developments in sample preparation techniques for chromatography analysis of traditional Chinese medicines. J Chromatogr A. 2007;1153(1–2):90–6.
Yu JG, Sun L, Zhou LD, Luo XZ, Guo J, Liu CY, Cong PZ. Studies on the chemical constituents of Fructus Amomi. China J Chin Mater Med. 1997;4:231–2.
Kang W, Zhang F, Su Y, Guo Y. Application of gas chromatography-quadrupole-time-of-flight-mass spectrometry for post-target analysis of volatile compounds in Fructus Amomi. Eur J Mass Spectrom. 2013;19(2):103–10.
Shen S, Sha Y, Deng C, Fu D, Chen J, Zhang X. Comparison of solid-phase microextraction, supercritical fluid extraction, steam distillation, and solvent extraction techniques for analysis of volatile consituents in Fructus amomi. J AOAC Int. 2005;88:418–23.
Dai DN, Huong LT, Thang TD, Ogunwande IA, Eresanya OI. Chemical constituents of essential oil from the stem of Amomum villosum Lour. Trends Phytochem Res. 2018;2(1):61–4.
Cassel E, Vargas RMF, Martinez N, Lorenzo D, Dellacassa E. Steam distillation modeling for essential oil extraction process. Ind Crop Prod. 2009;29(1):171–6.
Kimbaris AC, Siatis NG, Daferera DJ, Tarantilis PA, Pappas CS, Polissiou MG. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum). Ultrason Sonochem. 2006;13(1):54–60.
Qiao XG, Sun AD. Study on the supercritical CO2 extraction of volatile compounds of garlic. J Shandong Agric Univ. 2006;37:17–20.
Roldán-Gutiérrez JM, Ruiz-Jiménez J, LuquedeCastro MD. Ultrasound-assisted dynamic extraction of valuable compounds from aromatic plants and flowers as compared with steam distillation and superheated liquid extraction. Talanta. 2008;75(5):1369–75.
Mohammadhosseini M. Chemical Composition of the volatile fractions from flowers, leaves and stems of Salvia mirzayanii by HS-SPME-GC-MS. J Essent Oil Bear Pl. 2015;18(2):464–76.
Filipiak W, Bojko B. SPME in clinical, pharmaceutical, and biotechnological research—how far are we from daily practice? Trac-Trend Anal Chem. 2019;115:203–13.
Chen Q, Song J, Bi J, Meng X, Wu X. Characterization of volatile profile from ten different varieties of Chinese jujubes by HS-SPME/GC–MS coupled with E-nose. Food Res Int. 2018;105:605–15.
Calvi L, Pentimalli D, Panseri S, Giupponi L, Gelmini F, Beretta G, Vitali D, Bruno M, Zilio E, Pavlovic R, Giorgi A. Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC–MS and LC-HRMS (q-exactive orbitrap®) approach. J Pharm Biomed. 2018;150:208–19.
Liu ZC, Wang LP, Liu YM. Rapid differentiation of Chinese hop varieties (Humulus lupulus) using volatile fingerprinting by HS-SPME-GC-MS combined with multivariate statistical analysis. J Sci Food Agric. 2018;98(10):3758–66.
Wang C, Lv S, Wu Y, Lian M, Gao X, Meng Q. Study of aroma formation and transformation during the manufacturing process of Biluochun green tea in Yunnan Province by HS-SPME and GC-MS. J Sci Food Agric. 2016;96(13):4492–8.
Ghodki BM, Goswami T. Optimization of cryogenic grinding process for cassia (Cinnamomum loureirii Nees L.). J Food Process Eng. 2016;39(6):659–75.
Andres C. Grinding spices at cryogenic temperatures retains volatiles and oils. Food Proc. 1976;37:52–3.
Saxena S, Sharma Y, Rathore S, Singh K, Barnwal P, Saxena R, Upadhyaya P, Anwer M. Effect of cryogenic grinding on volatile oil, oleoresin content and anti-oxidant properties of coriander (Coriandrum sativum L.) genotypes. J Food Sci Tech. 2015;52:568–73.
Singh K, Goswami T. Cryogenic grinding of cloves. J Food Process Pres. 2000;24(1):57–71.
Yang ZY, Wu DT, Chen CW, Cheong KL, Deng Y, Chen LX, Han BX, Chen NF, Zhao J, Li SP. Preparation of xylooligosaccharides from xylan by controlled acid hydrolysis and fast protein liquid chromatography coupled with refractive index detection. Sep Purif Technol. 2016;171:151–6.
Mani V. Properties of commercial SPME coatings. In: Pawliszyn J, editor. applications of solid phase microextraction. Cambridge: The Royal Society of chemistry; 1999. p. 57–108.
Zeng Z, Xi ZC, Meng SJ, Pang SM, Xie RQ, Ye XN, Zhang YQ. Study on volatile constitutions and quality evaluation of different varieties of Fructus Amomis. J Instrum Anal. 2010;29:701–6.
Lee SJ, Han JI, Lee GS, Park MJ, Choi IG, Na KJ, Jeung EB. Antifungal effect of eugenol and nerolidol against Microsporum gypseum in a guinea pig model. Biol Pharm Bull. 2007;30:184–8.
Klopell FC, Lemos M, Sousa JPB, Comunello E, Maistro EL, Bastos JK, De Andrade SF. Nerolidol, an antiulcer constituent from the essential oil of Baccharis dracunculifolia DC (Asteraceae). Zeitschrift für Naturforschung C. 2007;62(7–8):537–42.
Acknowledgements
Not applicable.
Funding
The research was partially funded by grants from the National Natural Science Foundation of China (No. 81673389), the National Key R&D Program of China (2019YFC1711300), the Science and Technology Development Fund, Macau SAR (File no. 0075/2018/A2, 034/2017/A1 and 0017/2019/AKP), the Guangdong Key Project for Modernization of Lingnan herbs and the University of Macau (File no. MYRG2018-00083-ICMS/MYRG2019-00128-ICMS/CPG2020-00021-ICMS).
Author information
Authors and Affiliations
Contributions
LC carried out the experiment, drafted the manuscript and analyzed the data; YL collect the samples; WZ analyzed the data; HH, JC, YW, JZ and SL contributed to the design and interpretation of the research; HH, JZ and SL revised the manuscript and conceived of the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Fig.S1.
The chromatogram of A. villosum samples. I, fruit (S1-S18); II, root (S19-S36); III, leaf (S37-S54); IV, stem (S55-S72). Table S1. The main volatile compounds in 72 A. villosum samples.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, LX., Lai, YF., Zhang, WX. et al. Comparison of volatile compounds in different parts of fresh Amomum villosum Lour. from different geographical areas using cryogenic grinding combined HS–SPME–GC–MS. Chin Med 15, 97 (2020). https://doi.org/10.1186/s13020-020-00377-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13020-020-00377-z