Abstract
Background
Osteoporosis is a common disease in aging populations. However, osteoporosis treatment is still challenging. Here, we aimed to investigate the role of neohesperidin (NEO) in osteoporosis progression and the potential mechanism.
Methods
Bone mesenchymal stem cells (BMSCs) were isolated and treated with different concentrations of NEO (0, 10, 30, 100 μM). Cell proliferation was analyzed by cell count kit-8 (CCK-8) assay. RNA-sequencing was performed on the isolated BMSCs with control and NEO treatment. Differentially expressed genes were obtained by R software. Alkaline phosphatase (ALP) staining and Alizarin red staining (ARS) were performed to assess the osteogenic capacity of the NEO. qRT-PCR was used to detect the expression of osteoblast markers.
Western blot was used to evaluate the protein levels in BMSCs.
Results
NEO treatment significantly improved hBMSC proliferation at different time points, particularly when cells were incubated with 30 μM NEO (P < 0.05). NEO dose-dependently increased the ALP activity and calcium deposition than the control group (P < 0.05). A total of 855 differentially expressed genes were identified according to the significance criteria of log2 (fold change) > 1 and adj P < 0.05. DKK1 partially reversed the promotion effects of NEO on osteogenic differentiation of BMSCs. NEO increased levels of the β-catenin protein in BMSCs.
Conclusion
NEO plays a positive role in promoting osteogenic differentiation of BMSCs, which was related with activation of Wnt/β-catenin pathway.
Similar content being viewed by others
Background
Osteoporosis is common in women, which is characterized mainly by degeneration of microarchitecture of the trabeculae [1]. Osteoporosis patients have a greater risk of osteoporosis-related fracture [2]. One of the critical causes of bone loss is the imbalance between osteoclast (OC)-related bone resorption and osteoblast-related bone reformation [3]. Estrogen replacement treatment (ERT) is considered an effective therapy for menopausal osteoporosis, but it increases the risk of endometrial cancer, breast cancer, and asthma [4]. Bisphosphonates are the current mainstay for the treatment of postmenopausal osteoporosis [5]. However, bisphosphonates exhibit nephrotoxicity, hepatic toxicity, and alimentary canal toxicity.
As such, new safe and affordable drugs are required for the continued treatment and control of osteoporosis [4]. Neohesperidin (NEO) is citrus aurantium extract and belongs to the flavonoids [6]. Flavonoids are well known to exhibit several biological and pharmacological activities including has anti-inflammatory, anti-tumor, and antiviral effects [7, 8]. However, the role of NEO on osteogenic differentiation of BMSCs was unknown. RNA-sequencing technology is a high-throughput sequencing technology which has developed rapidly in recent years [9]. RNA-sequencing technology can be used to detect the differentially expressed genes between different treatment groups [10]. The Wnt/β-catenin signaling pathway serves a vital role in regulating osteoblastic differentiation of BMSCs [11, 12]. Moreover, Wnt/β-catenin pathway antagonists can be used for the treatment of osteoporosis [13, 14]. However, whether NEO activates the Wnt/β-catenin pathway was unknown.
In our study, we found for the first time that NEO promoted osteogenesis by promoting the Wnt/β-catenin signaling pathway. The expression of osteogenic-related genes and proteins was measured to evaluate the influence of this pharmacological intervention of NEO on osteogenic differentiation. Our work indicates that NEO could be a promising therapeutic option for osteolytic bone diseases. Additionally, the therapeutic effect of NEO on preventing bone loss in vivo was confirmed by animal experiments. Data from estrogen deficiency-induced osteoporosis mouse models indicated that the NEO might be a potential curing option for osteoporosis. Although several biological effects of NEO have been reported, its effects on promoting osteogenesis have yet to be discovered.
Therefore, in the present study, we aimed to investigate the effects of NEO on promoting osteogenic differentiation and exact mechanisms in human bone mesenchymal stem cells.
Methods and materials
Cell culture
BMSCs were harvested from the human bone marrow as previously described [15]. BMSCs were cultured into alpha-modified minimal essential medium (α-MEM) containing 10% FBS and 1% antibiotics. Then, α-MEM which was changed per 2 days. Osteogenic induction was performed by replacing the medium with an osteogenic medium (containing 10-nM dexamethasone, 10-mM β-glycerophosphate, and 50 μg/mL vitamin C) (Sigma, USA).
The efficacy and toxicity evaluation of NEO
BMSCs were seeded in plates (96-well plate) at a concentration of 5×103 cells per well and cultured in α-MEM medium (the component of α-MEM medium has mentioned above). Meantime, NEO was added to the cultured cells at varying concentrations (0, 10, 30, and 100 μM). The medium was changed per 2 days. BMSCs were treated with varying concertation of NEO (0, 10, 30, and 100 μM) for 1, 3, 5, and 7 days, and then, a 10-μl CCK-8 solution (Solarbio, Beijing, China) was added into wells, then cells were incubated for another 4 h. The absorbance at 450 nm was measured by the enzyme-linked immunosorbent assay (Thermo Fisher Scientific, Waltham, MA, USA).
ALP and ARS
ALP staining was monitored using an ALP histochemical diagnostic kit (Beyotime, China) according to the manufacturer’s protocol. In brief, BMSCs were fixed by 4% paraformaldehyde after 7 days of osteogenic induction. After three washes with phosphate-buffered saline (PBS), staining was performed using NBT/BCIP. Then, an equal volume of distilled water was added to abort the reaction. ARS was performed as previously described [16]. BMSCs were fixed by 4% paraformaldehyde after 14 days of osteogenic induction. After three washes with PBS, staining was performed using 0.1% ARS solution (Solarbio, Beijing, China). The results were photographed under a Zeiss microscope (Zeiss, Oberkochen, Germany).
RNA sequencing
BMSCs were cultured and treated with control or NEO (30 μM) as described above. RNA-sequencing analysis was performed by the Shanghai Biotechnology Corporation (Shanghai, China). In brief, the total RNA was extracted using an RNeasy Mini kit (QIAGEN, Hilden, Germany) and was reverse transcribed into complementary DNA (cDNA). Paired-end libraries were synthesized by using a TruSeq RNA Sample Preparation Kit (Illumina, USA) according to the TruSeq RNA Sample Preparation Guide.
The cleaved RNA fragments are copied into the first-strand cDNA using reverse transcriptase and random primers. Differential expression gene (DEG) analysis for mRNA was performed using R package edge R [17]. DEGs with log2(fold change) value >1 and adj p value <0.05, considered as significantly modulated, were retained for further analysis. A volcano plot and heatmap for the differentially expressed genes were generated with “ggplot2” and “heatmap” in R respectively [18]. Next, the enriched Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were performed by using the clusterProfiler R package [19]. To predict protein-protein interactions, Search Tool for the Retrieval of Interacting Genes (STRING) database was used (https://string-db.org) [20]. Also, Molecular Complex Detection (MCODE), one plug-in unit of Cytoscape, was set to screen the models of PPI network.
Quantitative RT-PCR
TRIzol reagent (Invitrogen) was used to extract total RNA from the cells, and the RNA concentration and purity were determined by ND-1000 spectrophotometer. Revert Aid First-Strand cDNA Synthesis Kit (Fermentas, USA) was used to reverse sample RNA from total RNA into cDNA and RT-PCR. Primer list as follow: GAPDH, forward: 3′-GGAGAAAGCTGCTAA-5′, Reverse: 3′-ACGACCTGGTCCTCGGTGTA-5′; β-catenin, Forward: 3′-GTGTGGAGTGCCTGATGTG-153 5′, Reverse: 3′-CTGCTTGACCCTTGGAGAC-5′; TCF7, Forward: 3′–TTCCAACCCTGCTACTGC-5′, Reverse: 3′-TGCCTGTCACCTCCAAG-5′; Lef1, Forward: 3′-CTTCTGGCTCACGCTTTTC-5′, Reverse: 3′-TTGGGGTCTTCATCTCCTG-5′. c-myc, Forward: 3′-GAAGTGGCTCACGCTTTTC-′, Reverse: 3′-AAGVGGTCTACTACGCCAG-5′, RUNX2, 5′-AAGGAAGCTTGGCGTTGTGA-3′; reverse 5′-GAGAGGTGAGGAGTCTTATG-3′, BMP2, Forward: 5′-AACACGCTGCCTGTCTACACT-3′; Reverse: 5′-CAGTGCAGGGTCCGAGGT-3 and Osteocalcin, Forward: 5′-GACCCCTTTACTCTGACCCC-3′, Reverse: 5′-AGGCTCCAGTGAATTCGGAA-3′. The relative expression of each gene was detected and calculated.
Western Blot analysis
The intracellular protein was collected by digestion and centrifugation, washed twice with PBS, and the cell pellet was collected by centrifugation. A certain amount of lysate was added and lysed on ice for 30 min. After lysing, the total protein was collected by centrifugation at 12000 r/min for 15 min at 131 4°C. A certain amount of protein loading buffer was added and the mixture was denatured at 100°C for 15 min, and then, polyacrylamide gel electrophoresis (12%, SDS-PAGE) was performed. After electrophoresis, the proteins were transferred to a 4.5-μm polyvinylidene fluoride (PVDF) membrane. The PVDF membrane was blocked with 5% skimmed milk powder for 1 h at room temperature, and a primary antibody β-catenin (Abcam, 1:1000), p-β-cateinin (Abcam, 1:1000), and GAPDH (Abcam, 1:1000) were added to incubate overnight. After incubation, the PVDF membrane was washed 3 times with TBST buffer (TRIS-HCl balanced salt buffer + Tween) and then incubated with the secondary antibody (IgG H&L (HRP), Abcam, 1:2000) at room temperature in the dark for 1 h. The bands were then washed three times with TBST buffer, the membrane was scanned with an ODYSSEY infrared imaging system, and the molecular weight and optical density values of the target bands were analyzed using a gel image processing system.
Statistical analysis
In the results section, all of the quantitative data are presented as the mean ± standard deviation. Statistical analyses were conducted using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test with GraphPad Prism 8. All data are demonstrated as the means ± SDs; *P< 0.05 comparing to the control.
Results
NEO increased hBMSC proliferation and osteogenic differentiation
BMSCs were incubated in an osteogenic induction medium with or without NEO at different concentrations (0, 10, 30, and 100 μM) for 1, 2, 3, and 7 days. According to the results of the CCK-8 assay, the NEO treatment significantly improved hBMSC proliferation at different time points, particularly when cells were incubated with 30-μM NEO (P < 0.05) (Fig. 1b). Then, ALP and Alizarin red staining were used to determine the effect of NEO on the osteogenic differentiation of hBMSCs. Results showed that NEO dose-dependently increased the ALP activity and calcium deposition than the control group (Fig. 1c).
The expression levels of osteogenesis-related genes (Runx2, Osteocalcin, and BMP2) were assessed by real-time PCR. NEO dose-dependently increased the osteogenesis-related genes (Runx2, Osteocalcin, and BMP2) of hBMSCs than that of the control group (Fig. 2a–c). Moreover, NEO significantly increased the β-catenin expression, which suggested that NEO could activate the Wnt-β-catenin signaling pathway.
A NEO dose-dependently increased the osteogenesis-related genes. a Relative expression of RUNX2 expression in control and NEO treatment groups. b Relative expression of BMP2 expression in control and NEO treatment groups. c Relative expression of osteocalcin expression in control and NEO treatment groups. d Relative expression of β-catenin expression in control and NEO treatment groups
Differentially expressed genes between NEO and control
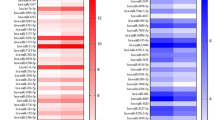
The raw data was normalized and the final expression value in each group was in a identical level (Fig. 3a). A total of 83 differentially expressed genes were identified according to the significance criteria of log2 (fold change) > 1 and adj P < 0.05.
Among these, 58 mRNAs were upregulated and 25 were downregulated. The volcano plot and heatmap of the differentially expressed mRNAs can be seen in Fig. 3b, c, respectively.
Upregulated expressed genes were mainly enriched into Wnt/β-catenin signaling pathway, Thyroid hormone synthesis, Staphylococcus aureus infection, Retinol metabolism, and the Renin-angiotensin system (Fig. 4). Downregulated expressed genes were mainly enriched into transcriptional misregulation in cancer, TGF-beta signaling pathway, signaling pathway regulation pluripotency of stem cells, Relaxin signaling pathway, Protein digestion, and absorption and platelet activation (Fig. 4).
Upregulated expressed genes were mainly enriched in response to oxygen levels, response to nutrient, response to hypoxia, response to decreased oxygen levels, and regulation of wound healing (Fig. 4).
Downregulated expressed genes were mainly enriched into visual system development, urogenital system development, transmembrane receptor protein serine/threonine kinase signaling pathway, transforming growth factor-beta receptor signaling pathway, and sequestering of the extracellular ligand from the receptor (Fig. 4).
DKK1 partially reversed the promotion effects of NEO on osteogenic differentiation of BMSCs
Consistent with previous results, NEO significantly increased the ALP activity and calcium deposition, while these effects of NEO were partially blocked by Wnt-β-catenin signaling pathway inhibitor, DKK1 (Fig. 5a).
Then, we assessed the osteogenesis-related genes (Runx2, osteocalcin, and BMP2) in control, NEO, and NEO+DKK1 groups. We found that NEO significantly increased osteogenesis-related genes (Runx2, osteocalcin, and BMP2) expression, while these upregulated expressions were partially reversed by DKK1 (Fig. 5b).
NEO increased levels of the β-catenin protein in BMSCs
qRT-PCR was performed to detect β-catenin, TCF7, Lef1, and c-myc mRNA levels in control, NEO, and NEO+DKK1 groups. Results found that NEO significantly increased β-catenin, TCF7, Lef1, and c-myc expression than the control group (Fig. 6a–d, P<0.05), while these effects could be partially reversed by DKK1 (Fig. 6a–d, P<0.05).
DKK1 partially reversed the promotion effects of NEO on β-catenin expression. a Relative expression of β-catenin expression in the control, NEO, and NEO+DKK1 groups. b Relative expression of TCF7 expression in the control, NEO, and NEO+DKK1 groups. c Relative expression of Lef1 expression in the control, NEO, and NEO+DKK1 groups. d Relative expression of c-myc expression in the control, NEO, and NEO+DKK1 groups, *P<0.05
We then conducted Western Blotting to examine the levels of the β-catenin and p-β-catenin proteins and investigate whether NEO increased hBMSC osteogenesis via the Wnt/β-catenin pathway. NEO increased β-catenin levels on days 3, 7, and 14 compared to the control group (Fig. 7a).
Moreover, p-β-catenin levels showed the opposite trend to β-catenin levels, as expected (Fig. 7b).
Discussion
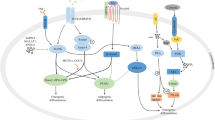
In this study, we firstly revealed that NEO could promote osteogenic differentiation of BMSCs through Wnt/β-catenin signaling pathway. Moreover, we performed a differentially expressed gene between NEO and control groups from the RNA-sequencing data. NEO may be an effective addition to osteoporotic therapy.
CCK-8 assay was carried out to determine the optimum concentration of NEO. The most suitable final concentration of NEO was found to be 30 μM. To demonstrate the function of NEO in osteogenic differentiation, we performed ALP and ARS staining assays. Results found that NEO significantly increased the ALP activity and calcium deposition. It is known that ALP activity and calcium deposition are osteogenic markers indicating cell differentiation ability [21].
NEO, which is a natural flavanone glycoside with numerous pharmacological properties, including antiviral, antioxidant, anti-inflammation, and antitumor [22, 23]. However, the role of NEO on osteogenic differentiation of BMSCs was unknown. BMSCs are a kind of adult stem cells derived from mesoderm with strong proliferation, expansion ability, and multidirectional differentiation potential [24, 25]. They exist in the bone marrow and have the adherent ability and can differentiate into osteoblasts, adipocytes, and other cells [26, 27] through the menopausal transition in women exhibited by an increase in fat accumulation as well as impaired osteogenic differentiation potentials [28]. Therefore, the promotion of osteogenic differentiation can help inhibit the development of osteoporosis.
In order to explore the mechanisms of NEO during the osteogenic differentiation of BMSCs, we performed RNA sequencing analysis to determine differentially expressed genes affected by NEO. A total of 855 differentially expressed genes were identified according to the significance criteria of log2 (fold change) > 1 and adj P < 0.05. KEGG pathway enrichment analysis revealed that these differentially expressed genes mainly enriched in Wnt-β-catenin signaling pathway.
Several signaling pathways have been identified that participated in osteogenic differentiation of BMSCs, such as PI3K/Akt signaling and Wnt/beta-catenin pathway [29,30,31,32,33]. Loss of function mutations in the Wnt co-receptor LRP5 results in severe osteoporosis [34].
In this study, NEO activated β-catenin and induce osteogenic differentiation of BMSCs, which in turn promote the expression of osteoblast markers. This NEO-induced osteogenic activity was obviously blocked by DKK1 or XAV939. Wnt/beta-catenin pathway not only stimulates the osteogenic differentiation of BMSCs, but also induces the maturation of BMSCs into osteoblasts [35]. Previous studies suggested that inactivation of Wnt/beta-catenin will switch the osteogenesis of MSCs to chondrogenesis [36]. Taken together, we firstly identified that NEO stimulated osteogenic differentiation of BMSCs through Wnt/beta-catenin pathway. NEO has no toxicities or adverse effects at the animal level [37].
Several important limitations in this study are worth mentioning. First, we did not perform in vivo animal study to identify the role of NEO to prevent the osteoporosis progression. Second, the optimal dose of NEO needed for more studies to identify. Last, the receptor of NEO needed for more studies to identify.
Conclusion
In conclusion, NEO enhanced the proliferation and osteogenic differentiation of BMSCs. NEO promoted the osteogenesis of BMSCs by activating the Wnt signaling pathway. The current study describes a promising therapeutic agent for patients with osteoporosis.
Availability of data and materials
All the data pertaining to the present study are willing to share upon reasonable request.
Abbreviations
- NEO:
-
Neohesperidin
- BMSCs:
-
Bone mesenchymal stem cells
- CCK-8:
-
Cell count kit-8
- ALP:
-
Alkaline phosphatase
- ARS:
-
Alizarin red staining
- OC:
-
Osteoclast
- ERT:
-
Estrogen replacement treatment
- α-MEM:
-
Alpha-modified minimal essential medium
- PBS:
-
Phosphate-buffered saline
- DEGs:
-
Differential expression genes
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- STRING:
-
Search Tool for the Retrieval of Interacting Genes
- MCODE:
-
Molecular Complex Detection
- PVDF:
-
Polyvinylidene fluoride
- ANOVA:
-
One-way analysis of variance
References
Srivastava M, Deal C. Osteoporosis in elderly: prevention and treatment. Clin Geriatr Med. 2002;18(3):529–55. https://doi.org/10.1016/s0749-0690(02)00022-8.
Prestwood KM, Pilbeam CC, Raisz LG. Treatment of osteoporosis. Annu Rev Med. 1995;46(1):249–56. https://doi.org/10.1146/annurev.med.46.1.249.
Miller PD. Management of severe osteoporosis. Expert Opin Pharmacother. 2016;17(4):473–88. https://doi.org/10.1517/14656566.2016.1124856.
Baccaro LF, Conde DM, Costa-Paiva L, Pinto-Neto AM. The epidemiology and management of postmenopausal osteoporosis: a viewpoint from Brazil. Clin Interv Aging. 2015;10:583–91. https://doi.org/10.2147/cia.s54614.
Bianchi G, Sambrook P. Oral nitrogen-containing bisphosphonates: a systematic review of randomized clinical trials and vertebral fractures. Curr Med Res Opin. 2008;24(9):2669–77. https://doi.org/10.1185/03007990802370912.
Guo J, Fang Y, Jiang F, Li L, Zhou H, Xu X, et al. Neohesperidin inhibits TGF-β1/Smad3 signaling and alleviates bleomycin-induced pulmonary fibrosis in mice. Eur J Pharmacol. 2019;864:172712. https://doi.org/10.1016/j.ejphar.2019.172712.
Zhao Z, Ma X, Ma J, Sun X, Li F, Lv J. Naringin enhances endothelial progenitor cell (EPC) proliferation and tube formation capacity through the CXCL12/CXCR4/PI3K/Akt signaling pathway. Chem Biol Interact. 2018;286:45–51. https://doi.org/10.1016/j.cbi.2018.03.002.
Song N, Zhao Z, Ma X, Sun X, Ma J, Li F, et al. Naringin promotes fracture healing through stimulation of angiogenesis by regulating the VEGF/VEGFR-2 signaling pathway in osteoporotic rats. Chem Biol Interact. 2017;261:11–7. https://doi.org/10.1016/j.cbi.2016.10.020.
Zhang H, He L, Cai L. Transcriptome sequencing: RNA-Seq. Methods Mol Biol. 2018;1754:15–27. https://doi.org/10.1007/978-1-4939-7717-8_2.
Byron SA, Van Keuren-Jensen KR, Engelthaler DM, et al. Translating RNA sequencing into clinical diagnostics: opportunities and challenges. Nat Rev Genet. 2016;17(5):257–71. https://doi.org/10.1038/nrg.2016.10.
Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–205. https://doi.org/10.1016/j.cell.2012.05.012.
Guo Y, Xiao L, Sun L, et al. Wnt/beta-catenin signaling: a promising new target for fibrosis diseases. Physiol Res. 2012;61:337–46. https://doi.org/10.33549/physiolres.932289.
Wu W, Xiao Z, Chen Y, Deng Y, Zeng D, Liu Y, et al. CD39 produced from human GMSCs regulates the balance of osteoclasts and osteoblasts through the Wnt/β-catenin pathway in osteoporosis. Mol Ther. 2020;28(6):1518–32. https://doi.org/10.1016/j.ymthe.2020.04.003.
Rossini M, Gatti D, Adami S. Involvement of WNT/β-catenin signaling in the treatment of osteoporosis. Calcif Tissue Int. 2013;93(2):121–32. https://doi.org/10.1007/s00223-013-9749-z.
Chu DT, Phuong TNT, Tien NLB, Tran DK, Thanh VV, Quang TL, et al. An update on the progress of isolation, culture, storage, and clinical application of human bone marrow mesenchymal stem/stromal cells. Int J Mol Sci. 2020;21(3). https://doi.org/10.3390/ijms21030708.
Yu L, Qu H, Yu Y, Li W, Zhao Y, Qiu G. LncRNA-PCAT1 targeting miR-145-5p promotes TLR4-associated osteogenic differentiation of adipose-derived stem cells. J Cell Mol Med. 2018;22(12):6134–47. https://doi.org/10.1111/jcmm.13892.
Li Z, Lin Y, Cheng B, Zhang Q, Cai Y. Identification and analysis of potential key genes associated with hepatocellular carcinoma based on integrated bioinformatics methods. Front Genet. 2021;12:571231. https://doi.org/10.3389/fgene.2021.571231.
Trubl G, Roux S, Solonenko N, Li YF, Bolduc B, Rodríguez-Ramos J, et al. Towards optimized viral metagenomes for double-stranded and single-stranded DNA viruses from challenging soils. PeerJ. 2019;7:e7265. https://doi.org/10.7717/peerj.7265.
Núñez-Enríquez JC, Bárcenas-López DA, Hidalgo-Miranda A, Jiménez-Hernández E, Bekker-Méndez VC, Flores-Lujano J, et al. Gene expression profiling of acute lymphoblastic leukemia in children with very early relapse. Arch Med Res. 2016;47(8):644–55. https://doi.org/10.1016/j.arcmed.2016.12.005.
Zhu GD, Cao XJ, Li YP, Li JX, Leng ZJ, Xie LM, et al. Identification of differentially expressed genes and signaling pathways in human conjunctiva and reproductive tract infected with Chlamydia trachomatis. Hum Genomics. 2021;15(1):22. https://doi.org/10.1186/s40246-021-00313-8.
Chen S, Tang Y, Liu Y, Zhang P, Lv L, Zhang X, et al. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019;52(5):e12669. https://doi.org/10.1111/cpr.12669.
Chakraborty S, Rakshit J, Bandyopadhyay J, Basu S. Multi-target inhibition ability of neohesperidin dictates its neuroprotective activity: implication in Alzheimer’s disease therapeutics. Int J Biol Macromol. 2021;176:315–24. https://doi.org/10.1016/j.ijbiomac.2021.02.073.
Xia N, Wan W, Zhu S, Liu Q. Synthesis of hydrophobic propionyl neohesperidin ester using an immobilied enzyme and description of its anti-proliferative and pro-apoptotic effects on MCF-7 human breast cancer cells. Front Bioeng Biotechnol. 2020;8:1025. https://doi.org/10.3389/fbioe.2020.01025.
Li N, Liu L, Liu Y, Luo S, Song Y, Fang B. miR-144-3p suppresses osteogenic differentiation of BMSCs from patients with aplastic anemia through repression of TET2. Mol Ther Nucleic Acids. 2020;19:619–26. https://doi.org/10.1016/j.omtn.2019.12.017.
Yu J, Jiang L, Gao Y, Sun Q, Liu B, Hu Y, et al. Interaction between BMSCs and EPCs promotes IUA angiogenesis via modulating PI3K/Akt/Cox2 axis. Am J Transl Res. 2018;10(12):4280–9.
Yu X, Quan J, Long W, Chen H, Wang R, Guo J, et al. LL-37 inhibits LPS-induced inflammation and stimulates the osteogenic differentiation of BMSCs via P2X7 receptor and MAPK signaling pathway. Exp Cell Res. 2018;372(2):178–87. https://doi.org/10.1016/j.yexcr.2018.09.024.
Liu M, Ding H, Wang H, Wang M, Wu X, Gan L, et al. Moringa oleifera leaf extracts protect BMSC osteogenic induction following peroxidative damage by activating the PI3K/Akt/Foxo1 pathway. J Orthop Surg Res. 2021;16(1):150. https://doi.org/10.1186/s13018-021-02284-x.
Kong J, Wan LP, Liu ZM, et al. MiR-1301 promotes adipogenic and osteogenic differentiation of BMSCs by targeting Satb2. Eur Rev Med Pharmacol Sci. 2020;24:3501–8. https://doi.org/10.26355/eurrev_202004_20809.
Zhao SJ, Kong FQ, Jie J, Li Q, Liu H, Xu AD, et al. Macrophage MSR1 promotes BMSC osteogenic differentiation and M2-like polarization by activating PI3K/AKT/GSK3β/β-catenin pathway. Theranostics. 2020;10(1):17–35. https://doi.org/10.7150/thno.36930.
Xiong Y, Zhao B, Zhang W, et al. Curcumin promotes osteogenic differentiation of periodontal ligament stem cells through the PI3K/AKT/Nrf2 signaling pathway. Iran J Basic Med Sci. 2020;23:954–60. https://doi.org/10.22038/ijbms.2020.44070.10351.
Wang Y, Zhang X, Shao J, Liu H, Liu X, Luo E. Adiponectin regulates BMSC osteogenic differentiation and osteogenesis through the Wnt/β-catenin pathway. Sci Rep. 2017;7(1):3652. https://doi.org/10.1038/s41598-017-03899-z.
Zhang G, Chen X, Cheng X, Ma W, Chen C. BMSC seeding in different scaffold incorporation with hyperbaric oxygen treats seawater-immersed bony defect. J Orthop Surg Res. 2021;16(1):249. https://doi.org/10.1186/s13018-021-02368-8.
Li L, Zhou X, Zhang JT, Liu AF, Zhang C, Han JC, et al. Exosomal miR-186 derived from BMSCs promote osteogenesis through hippo signaling pathway in postmenopausal osteoporosis. J Orthop Surg Res. 2021;16(1):23. https://doi.org/10.1186/s13018-020-02160-0.
Langhans MT, Yu S, Tuan RS. Stem cells in skeletal tissue engineering: technologies and models. Curr Stem Cell Res Ther. 2016;11(6):453–74. https://doi.org/10.2174/1574888x10666151001115248.
Chen XJ, Shen YS, He MC, Yang F, Yang P, Pang FX, et al. Polydatin promotes the osteogenic differentiation of human bone mesenchymal stem cells by activating the BMP2-Wnt/β-catenin signaling pathway. Biomed Pharmacother. 2019;112:108746. https://doi.org/10.1016/j.biopha.2019.108746.
Wang M, Li J, Ye Y, He S, Song J. SHED-derived conditioned exosomes enhance the osteogenic differentiation of PDLSCs via Wnt and BMP signaling in vitro. Differentiation. 2020;111:1–11. https://doi.org/10.1016/j.diff.2019.10.003.
Lina BA, Dreef-van der Meulen HC, Leegwater DC. Subchronic (13-week) oral toxicity of neohesperidin dihydrochalcone in rats. Food Chem Toxicol. 1990;28(7):507–13. https://doi.org/10.1016/0278-6915(90)90121-3.
Acknowledgments
Not applicable.
Funding
There is no funding for this article.
Author information
Authors and Affiliations
Contributions
XWC, WJZ, and WG designed the study and conducted the experiments. JS and ZQL performed the statistical analyses. XWW, XWC, and WJZ wrote the draft. XWW, XWC, and WJZ edited and approved the final manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chang, Yw., Zhu, Wj., Gu, W. et al. Neohesperidin promotes the osteogenic differentiation of bone mesenchymal stem cells by activating the Wnt/β-catenin signaling pathway. J Orthop Surg Res 16, 334 (2021). https://doi.org/10.1186/s13018-021-02468-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-021-02468-5