Abstract
Background
The management of articular cartilage defects presents many clinical challenges due to its avascular, aneural and alymphatic nature. Bone marrow stimulation techniques, such as microfracture, are the most frequently used method in clinical practice however the resulting mixed fibrocartilage tissue which is inferior to native hyaline cartilage. Other methods have shown promise but are far from perfect. There is an unmet need and growing interest in regenerative medicine and tissue engineering to improve the outcome for patients requiring cartilage repair. Many published reviews on cartilage repair only list human clinical trials, underestimating the wealth of basic sciences and animal studies that are precursors to future research. We therefore set out to perform a systematic review of the literature to assess the translation of stem cell therapy to explore what research had been carried out at each of the stages of translation from bench-top (in vitro), animal (pre-clinical) and human studies (clinical) and assemble an evidence-based cascade for the responsible introduction of stem cell therapy for cartilage defects.
Main body of abstract
This review was conducted in accordance to PRISMA guidelines using CINHAL, MEDLINE, EMBASE, Scopus and Web of Knowledge databases from 1st January 1900 to 30th June 2015. In total, there were 2880 studies identified of which 252 studies were included for analysis (100 articles for in vitro studies, 111 studies for animal studies; and 31 studies for human studies). There was a huge variance in cell source in pre-clinical studies both of terms of animal used, location of harvest (fat, marrow, blood or synovium) and allogeneicity. The use of scaffolds, growth factors, number of cell passages and number of cells used was hugely heterogeneous.
Short conclusions
This review offers a comprehensive assessment of the evidence behind the translation of basic science to the clinical practice of cartilage repair. It has revealed a lack of connectivity between the in vitro, pre-clinical and human data and a patchwork quilt of synergistic evidence. Drivers for progress in this space are largely driven by patient demand, surgeon inquisition and a regulatory framework that is learning at the same pace as new developments take place.
Similar content being viewed by others
Background
Articular cartilage is a highly specialised tissue acting as a shock absorber, enabling synovial joints to articulate with low frictional forces. Due to its avascular, aneural and alymphatic state, it has a limited repair potential [1]. Surgical options to manage damaged articular cartilage include arthroscopic debridement [2–5], bone marrow stimulation techniques [6–8], chondrocyte implantation [9–13], osteochondral autografts (mosaicplasty) [2, 14, 15], osteochondral allograft [16–18] and, in the presence of osteoarthritis, joint replacement [19].
Bone marrow stimulation techniques, such as microfracture, are the most frequently used method in clinical practice for treating small symptomatic lesions of the articular cartilage [6–8]. However, the resulting tissue has shown to be a mixed fibrocartilage tissue [20–22] with varying amounts of type II collagen [8, 21, 23, 24] and inferior to native hyaline cartilage. Fibrocartilage is vulnerable to shear stresses and prone to breaking down over time [20]. Subchondral osseous overgrowth has also been reported after microfracture [25, 26]. Osteochondral grafts can lead to donor site morbidity and healing seams at the recipient site [27, 28]. Autologous chondrocyte implantation (ACI) [9, 10] and its later evolution, matrix-induced autologous chondrocyte implantation (MACI), offered great promise with 80% of patients showing good or excellent results at 10 years [29] but at best results in hyaline-like repair and has experienced complications such as graft failure, periosteal hypertrophy and delamination [30, 31]. In addition, it has also been reported that cells may lose their phenotype during expansion [32, 33].
There is therefore a growing interest in regenerative medicine, which can broadly be thought of as two main types: cell therapy, where cells are injected directly into the blood or into tissues, and tissue engineering, where cell-scaffold combinations are used to repair or regenerate tissues.
Stem cells are cells that have the ability to divide and develop into many different cell types in the body and can be categorised as pluripotent and multipotent. Pluripotent stem cells are often harvested from embryonic sources and can develop into any type of cell in the body whereas multipotent stem cells are generally taken from adults and can divide and develop into a more limited range of cell types. When stem cells divide, the new cells can either remain stem cells or develop into a new type of cell with a more specific function (Table 1).
Mesenchymal stem cells (MSCs) are a form of multipotent cells that may offer an alternative to cartilage repair techniques not hampered by availability and donor site morbidity.
The introduction of stem cell therapies into clinical practice however is a form of translational research, which as per any “bench-to-bedside” pathway now has enormous governance issues [34, 35] and is highly regulatory across four phases (Table 2) and by the Tissues and Cells Directive (2004/23/EC) https://www.hta.gov.uk/policies/eu-tissue-and-cells-directives.
Many published reviews on cartilage repair only list human clinical trials [13, 36–46], underestimating the wealth of basic sciences and animal studies that are precursors to future research and may be relevant in clinical practice further down the line. In addition, true translation would imply that all of the clinical studies would have supporting pre-clinical data.
We therefore set out to perform a systematic review of the literature to assess the translation of stem cell therapy to explore what research had been carried out at each of the stages of translation from bench-top (in vitro), animal (pre-clinical), and human studies (clinical) and assemble an evidence-based cascade for the responsible introduction of stem cell therapy for cartilage defects. In particular, we wanted to focus on the key burning questions pertaining to cartilage repair such as cell source, dosage (how many cells should be used), requirement for scaffolds and the role for extrinsic growth factors.
Main text
Search methodology
This review was conducted in accordance to PRISMA guidelines [47] using CINHAL, MEDLINE, EMBASE, Scopus and Web of Knowledge databases from 1st January 1900 to 30th June 2015.
The keywords used in the selection were “(“mesenchymal stem cells”[All Fields] OR “mesenchymal stem cells”[MeSH Terms] OR “mesenchymal”[All Fields] OR “stem cells”[All Fields] OR “Stem Cells”[MeSH Terms] OR “MSC”[All Fields]) AND (“Articular Cartilage”[MeSH Terms] OR “articular”[All Fields] OR “cartilage”[All Fields] OR “cartilage”[MeSH Terms]) AND (“healing”[All Terms] OR “repair”[All Terms] OR “Regeneration”[MeSH Terms] OR “regeneration”[All Fields] OR “tissue engineering”[MeSH Terms] OR “tissue engineering”[All Fields]) AND (“defect”[All Terms]) AND (“chond*”[All Terms])”.
All review and non-English studies were excluded. For analysis, only original research studies were included. Any duplicates were excluded. Initially, KM and JS independently screened studies’ title and abstract. Those included had the full text reviewed. Any disparities were discussed with the senior author (AJG). The references of eligible studies were also searched and included where relevant.
Unpublished trial databases (e.g. ClinicalTrials.gov) were reviewed as the grey literature using popular search engines, including Google. The keywords used for registered clinical trials in clinical trial databases were “stem cells”, “cartilage” and “orthopaedics”.
Eligible studies were drafted into tables tabulating the key data.
Results
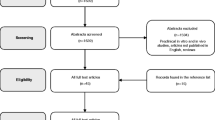
The initial search identified 2880 study articles, of which 239 were included for analysis. The PRISMA flow diagram is shown in Fig. 1.
In vitro studies
MSC source
A list of cell sources used in the in vitro studies is shown in Table 3. The commonest being human MSCs (66%) followed by rabbit MSCs (15%). The majority of the studies used bone marrow-derived MSCs (63%) followed by adipose tissue (33%). Two studies used commercial cell lines [48, 49].
Scaffold
Within the in vitro studies, 26 different types of natural scaffold and 9 types of synthetic scaffolds were identified with a further 18 different types of hybrids, the most popular being a fibrin-polyurethane scaffold (Table 4).
Growth factors
The commonest used growth factors were TGF-β and the bone morphogenetic protein (BMP) family. A list of growth factors used can be seen in Table 5.
Cell seeding and passage
There was wide heterogeneity in cell seeding density and there appeared to be no standard form of measurement. Li et al. [50] examined three different seeding densities: 2, 5 and 10 × 106 cells/scaffold, and found that scaffolds seeded with 5 × 106 cells per scaffold induced the highest chondrogenesis; however, other groups [51–53] found that a higher seeding density results in better chondrogenesis.
Apart from 26 studies which did not state cell passage number, most studies used MSC of an early passage, anything between uncultured fresh (passage zero (P0) and five times passaged cells (P5). One study used cells of P6 [54], and another study used cells between P4 and P7 [48]. No relationship was apparent between chondrogenesis and number of passages.
Length of study
The length of each in vitro study can be seen in Table 6. The majority of studies were short-term models; 27 studies (25%) ended between 1 and 2 weeks, 35 studies (33%) ended between 2 and 3 weeks and 15 studies (14%) ended between 3 and 4 weeks.
Method of assessment
A range of techniques was used to assess chondrogenesis within the in vitro studies. These techniques consisted of histology, immunohistochemistry, qPCR, biochemical analysis, imagery and mechanical testing. The techniques used are summarised in Table 7.
Animal studies (pre-clinical)
One hundred eleven animal studies were included of which 109 were controlled laboratory studies, one was a pilot study [49] and one was a longitudinal case study on a race horse [55]. The commonest animal studied with 59 studies was rabbit (53%). The different species of animals studied is shown in Table 8.
Defect
The size of the defect varied from 2 to 25 mm2 in the smaller animals and from 1 to 64 mm2 in the larger animals. All but two studies [56, 57] used the knee for defect creation.
Stem cell type
Bone marrow-derived stem cells were used in 84 studies (75%). Thirteen studies (11%) used adipose stem cells [54, 58–69], six (5%) used synovia [70–75] and three (2%) used periostium-derived MSCs [76–78]. Three studies (3%) used embryonic stem cell-derived MSCs [79–81] whereas 2 studies (2%) used muscle-derived MSCs [82, 83]. One group showed promising results of allogenic MSCs in a rabbit model when compared to autologous cells, although numbers were small [84, 85]. Another used compared autologous chondroprogenitor cells and allogenic chondroprogenitor cells against controls in an equine model and reported that repair tissue quality in the allogenic cell group was not superior to that in the control (fibrin only) group and also showed poorer radiographic changes in the allogenic group [23].
Cell culture, dose and delivery
There was much variation in the number of cells implanted and the number of cell passages from 3–10 or more [79, 86].
The number of cells varied from 4 × 103 – 1 × 1010. The majority of studies used between 106 and 108 cells. Some did not specify the number of cells implanted. Two studies suggested that improved chondrogenesis occurs with a higher implanted cell number [75, 87], although others suggested that the high cell numbers increase the risk of synovitis [75] and synovial proliferation [88].
The cells were transplanted into the defect both as cell therapy (injection directly into the joint) (17 studies, 15%) or by tissue engineering (cell-scaffold combinations) (94 studies, 85%). Fifteen studies [49, 65, 72, 75, 81, 86, 89–97] used a mixture of solutions prepared from hyaluronic acid [65, 92, 94–97], phosphate buffer solution [91], plasma [75], basal medium with chondrogenesis [89], collagen acid [93], sodium alginate [86] or a growth factor medium [90]. Two studies used MSCs only [49, 72].
Scaffold
Ninety-two studies (82%) used a scaffold. The material used was a synthetic polymer either collagen based, fibrinogen glue or a synthetic protein (e.g. rHuBMP-2) in 62 (56%) studies (Table 9).
Growth factors
Thirty-two studies (29%) assessed the effect of growth factors on MSC chondrogenesis. Seventeen out of 38 (44%) used TGF-β1/3 (Table 10), the majority of which show a positive effect on chondrogenesis.
Associated procedures
Ten of the studies compared MSC treatment against other surgical modalities such as debridement [55], microfracture [49, 91, 96, 98, 99] and mosaicplasty [77, 100–102].
Outcome measures
There were a variety of outcome measures used to analyse the results of the studies. The majority of studies (79%) used evidence of hyaline-like cartilage as being a positive outcome (Tables 11 and 12).
Human studies (clinical)
Thirty-one published studies by 15 different groups looked at clinical applications of MSCs. One used allogenic stem cells [103] and the rest autologous stem cells. The types of studies can be seen in Tables 13 and 14.
There were 52 unpublished clinical trials, majority of which are early phase studies (I–II; 63%) and only 5 trials were phase II/III. Table 15 shows a summary of these clinical trials.
Defects
The majority of studies (42%) used MSCs to treat knee osteoarthritis [103–115]. The rest of the studies looked at knee cartilage defects except for two which studied the ankle talar dome [116, 117]. One study used MSCs to treat knee osteoarthritis (OA), knee OA and ankle OA [112].
Of the knee cartilage defects, the patients were heterogeneous with varying defect sizes and locations, including the patellae [118–121], patella-femoral joints [122, 123], femoral condyle [113, 119–121, 123–132], trochlear [119–121] and tibial plateau [121]; and several had multiple defect sites [105, 120, 123, 128].
Previous treatment and associated procedures
The majority of patients who received MSC treatment had undergone previous arthroscopy [103, 104, 118, 119, 122, 124, 130], failed debridement [113, 118, 119, 121–123, 125, 127, 131] or bone marrow stimulation [114, 116, 117, 126].
Cell harvest source
Twenty-one studies (68%) used bone marrow-derived MSCs from the anterior or posterior superior iliac spine [103–105, 109, 111–113, 115–118, 120, 122–128, 130–132]. Five studies (18%) used adipose-derived MSCs [106–108, 110, 114], two studies (7%) used synovium-derived MSCs [129, 133] and two studies (7%) used peripheral blood progenitor cells collected by apheresis [119, 121].
Cell stage
Twenty studies (61%) culture-expanded their cells [103–105, 107–113, 115, 118, 120, 122–126, 129, 133], whereas 11 studies (39%) used fresh concentrated stem cells from bone marrow [116, 117, 127, 128, 130–132], fat tissues [106, 114] or peripheral blood [119, 121] in a one stage-procedure. In studies using bone marrow concentrate, approximately 60 ml of bone marrow aspirate was harvested and concentrated down to a volume of 2–4 ml before use [116, 117, 127, 130–132]. In studies using culture-expanded cells, the majority used cells from early passages, P1–P3 [103, 105, 109, 110, 112, 113, 115, 118, 120, 122–125, 129]. One study reported the use of cells at a late passage (P5) [104] ,and five studies did not specify a passage number [107, 108, 111, 126, 133].
Thirteen studies (42%) confirmed the phenotype of cells before clinical application [105, 108–110, 112, 115, 119, 120, 122–125, 129]. Commonly used surface markers to select MSCs were CD29, CD44, CD73, CD90 and CD105. Also CD14, CD34 and HLA-DR were used to eliminate non-MSCs.
Cell dose and delivery
The number of cells applied (dose) varied from 2–57 million for bone marrow-derived MSCs [103–105, 109, 111–113, 118, 120, 122–125, 129] and from 1.2–100 million for adipose-derived MSCs [107, 108, 110, 114]. For synovial MSCs, 8–77 million cells were used [129, 133], and for peripheral blood progenitor cells, 20 million cells were used [119]. Also, the methods for implantation varied from arthroscopic implantation (35%) [107, 108, 116, 117, 127, 128, 130–133], intra-articular injection [103–106, 109–112, 114, 115, 119, 121, 123] or open surgery (29%) [113, 118, 120, 122–126, 129].
In the cell therapy studies, the cells were suspended with a variety of different co-stimulators, including hydroxyapatite (HA) [106, 119, 121, 123], platelet rich plasma (PRP) [106, 114] and platelet lysate [104]. Some studies also administered multiple injections of stem cells [119, 121] and/or further injection of HA [115, 119, 121, 123], PRP [106, 114] or nucleated cells [104] following a stem cell injection.
The most frequently used scaffolds were type I collagen of porcine or bovine origin [113, 118, 122, 124, 126, 129], followed by ascorbic acid sheet [120, 123] and platelet-rich fibrin glue mixture [108, 125].
Rehabilitation
Early continuous passive motion was employed in 14 studies [113, 117–122, 124–127, 129–131]. Six studies did not report details on post-operation rehabilitation [104–106, 109, 116, 132]. Three studies aimed for full weight bearing very early by week 4 [107, 108, 122] whereas 11 studies (40%) aimed for full weight bearing by the 6th–8th week [113, 117–121, 124, 125, 127, 131, 133]. No study addressed the effect of rehabilitation on the quality of the repair.
Outcomes
Most commonly used outcome measures for treatment efficacy were radiological (77%) [103–106, 109–112, 115–117, 119, 121, 123–125, 127–134] and arthroscopic assessment (61%) [107, 108, 113, 116–122, 124–126, 130–133]. Most commonly used patient-reported outcomes are International Knee Documentation Committee (IKDC) score (36%), followed by a visual analogue scale (VAS) pain (39%) and Tegner activity scale (29%).
Adverse effects
None of the studies reported any severe adverse effects related to the MSC treatment. Two group reported minor adverse events including mild pain and effusion after the injections, which persisted for no more than 7 days [103, 114].
Conclusions
There is a growing fascination with the role of mesenchymal stem cells in cartilage repair.
As early as the 1950s, Pridie showed fibrocartilaginous repair through subchondral drilling [135–137]. Initially, Pridie drilling was reported as a treatment for osteoarthritis [135, 138] and was often associated with many additional procedures such as synovectomy and trimming of osteophytes.
Since Pridie’s initial experiments, the process of marrow stimulation techniques or exposure of mesenchymal stem cells from cancellous bone has changed its guise on several occasions.
Ficat in 1979 described “Spongialization” in which the cancellous bed was exposed in 85 patients with chondral lesions of the patella with encouraging results [139]. Johnson et al. [140] described abrasion arthroplasty and encouraged its use especially in younger patients [141, 142]. Other authors had less positive outcomes [143–146]. Dandy wrote an entertaining article on abrasion arthroplasty where he highlighted that at least in the treatment of osteoarthritis, its effects could relate to the arthroscopic washout, rest or even the placebo effects of the charismatic surgeon [147]. The final evolution of marrow stimulation was the term “Microfracture” enabled by commercially manufactured bone picks used to breach the subchondral bone [8]. Marrow-stimulating technique procedures, in particular microfracture, are now considered the first-line treatment for full-thickness cartilage lesions and have demonstrated good to excellent results in 60–80% of patients [148, 149].
Cartilage repair has evolved from marrow stimulation techniques through to chondrocyte transplant and now stem cells at rapid pace. An ideal translational pipeline would demonstrate how in vitro data was used to inform a pre-clinical model, which would later form a phase I/IIa first-in-man study and subsequently a phase III clinical trial. This would of course be the safe and responsible method by which novel therapies are brought to the market.
This systematic review is the first of its kind to explore the full spectrum of evidence from in vitro studies, through animal studies to human clinical trials, and yet, we found little evidence of connectivity between in vitro, animal and then human work. In fact, we did not find a single group that had carried out and reported studies in all three categories.
Indeed, even from groups, which showed a seemingly hierarchical approach to translation, discrepancies became apparent. For example, Saw et al. from Korea used a pre-clinical goat model to repair cartilage defects using HA plus bone marrow-derived cells [150] and then moved into a first-in-man study, but in doing so, elected to change from bone marrow aspirate to peripheral blood and justified this change because it was easier to harvest peripheral blood than marrow [151].
There are several sources of cells that have been used in cartilage repair including bone marrow, peripheral blood, synovium, adipose tissue and umbilicus (Table 14) without any clear evidence of superiority of one over the other.
One stage vs. two stages
As two stage procedures involving cell culture are expensive and cumbersome, there is an increasing push towards a single stage stem cell treatment. In this situation there is some supportive pre-clinical data [91, 95, 98, 152–154], but there does not appear to be a pre-clinical study that directly compares bone marrow concentrates against cultured MSCs.
Several groups have reported the use of bone marrow concentrates in clinical practice [116, 117, 127, 128, 130–132], in which the buffy coat is used containing the nucleated cells, of which a few will be stem cells.
Briefly, the patient has approximately 60 mL of bone marrow harvested from the iliac crest which is then spun down in a cell centrifuge (SmartPrep, Harvest Technologies Corp., USA, or IOR-G1, Novagenit, Mezzolombardo, TN, Italy) to provide 6 mL of concentrate containing nucleated cells. A small amount of the nucleated cells are then placed onto a hyaluronic acid membrane (Hyalofast, Fidia Advanced Biopolymers, Italy) or collagen membrane (IOR-G1, Novagenit, Mezzolombardo, TN, Italy) as a scaffold, which is then arthroscopically placed into the cartilage defect which had been pre-prepared using a burr or drill. The construct is then held with a platelet gel obtained from a harvest of 120 mL of patient’s venous blood taken the day before surgery (Vivostat system, (Vivolution, Denmark)) [118]. The results of the first 30 patients have been reported as showing improvements in MRI and arthroscopic appearance as well as clinical scores at 3 years follow-up [118].
This new technique is of course an evolution of the autologous matrix-enhanced chondrogenesis (AMIC) which used the stem cells from the adjacent marrow (and not pre-harvested bone marrow concentrates) within either collagen patches [155–157] or polyglycolic acid–hyaluronan-based scaffolds [158, 159].
There has also been a further step taken to avoid bone marrow harvest in which peripheral blood has been used in knee chondral lesions. In an RCT, arthroscopic subchondral drilling was followed by postoperative intra-articular injections of hyaluronic acid (HA) with and without peripheral blood stem cells (PBSC). Fifty patients were studied and randomised 1 week after surgery to receive either 8 injections of HA or 8 injections of HA plus PBSC. Those that underwent PBSC received stimulation with filgrastim, which contains recombinant human granulocyte colony-stimulating factor prior to harvest [106, 151]. At 18 month follow-up, they reported no adverse effects and improved MRI findings in the PBSC group compared to HA alone, took biopsies of 16 of the 25 patients in each group and claimed better tissue morphology in the PBSC group, as graded by the International Cartilage Repair Society Visual Assessment Scale II. Interestingly, however, the same group’s pre-clinical used bone marrow aspirates and not peripheral blood [150].
Autologous vs. allogenic
There is an increasing interest in allogenic cells to avoid donor site morbidity and to reduce cost. The pre-clinical data with regards to allogenic cells is conflicting. One group showed promising results of allogenic MSCs in a rabbit model when compared to autologous cells, although numbers were small [160, 161]. Another group compared autologous chondroprogenitor cells and allogenic chondroprogenitor cells against controls in an equine model and reported inferior repair in the allogenic cell group [23]. Despite conflicting pre-clinical data, human studies using allogenic cells began in Korea in 2009. A phase I/IIa study to assess safety and efficacy of a combination of human umbilical cord blood-derived mesenchymal stem cells and sodium hyaluronate (CARTISTEM® (MEDIPOST Co., Ltd., Korea)) was performed in knee chondral defects (NCT01041001). A parallel phase 3, open-label, multi-centre RCT comparing CARTISTEM® and microfracture in knee chondral defects was carried out in Korea and the USA (NCT01733186). Results are still pending.
Another area of huge controversy is the actual dose of cells that should be used. In vitro between 50,000 cells/mL and 100 billion cells/ml have been studied. In pre-clinical animal studies, this ranged from 1000 to 1 billion cells/mL, and in human studies, the reported range has been 1.2 million cells/mL–24 million cells/mL.
It remains unclear what the most appropriate cell dose should be, with some groups reporting that a higher cell number leads to a better repair [52, 71, 87, 95, 162–164], but Zhao et al. [99] highlighted the limitation to cell saturation and survival, and thus, there may be a top limit to cell number that can be used to aid repair.
A multitude of methods for cell delivery have also been adopted, from direct joint injection or embedded in a plethora of scaffolds, such as type I collagen gels of porcine or bovine origin, ascorbic acid sheets or fibrin glues (Table 14).
In vitro and in pre-clinical studies, a plethora of growth factors have been studied including TGF-β1 and TGF-β2 and BMP-7 but none of these have been included in human clinical trials (Table 5).
It is clear that the relationship between cell passage, cell dose, the use of scaffolds and growth factors and the efficacy of MSC treatment is still to be established.
Future
There is no question that the field of cartilage repair accelerates at rapid pace, and it is clear that the single stage procedures are likely to win over two stage procedures to save costs and reduce the burden on both provider and the patient. The reduction of donor site morbidity is a further driver helping direct progress.
The concept of cell banks of allogenic cells clearly meets all of the above criteria, but the lack of good supporting pre-clinical and long-term safety and efficacy data does little to pacify potential pitfalls of this direction. The fact that the phase 3 RCT of allogenic umbilical stem cells was allowed to be registered (NCT01041001) before the same group registered their phase I/IIa safety study (NCT01733186) intimates that sometimes clinical pace exceeds that of the regulators to lay down new ground.
Tools are likely to be introduced to the operating theatre that might improve the efficacy of treatment, such as fluorescence-activated cell sorting (FACS) machines which can isolate MSCs from the buffy coat of bone marrow aspirate by their cell surface markers. At present, this technology is expensive and complicated and ways to reduce cost and make the process simple are required before they could enter the operating theatre.
Induced pluripotent stem cells (iPSCs) are adult somatic cells that have been genetically reprogrammed to an embryonic stem cell-like state by being forced to express genes and factors important for maintaining the defining properties of embryonic stem cells [165].
These cells show unlimited self-renewal, and some in vitro studies have shown chondrogenic differentiation by iPSCs from human chondrocytes biopsied from osteoarthritic knees [166] and cartilage formation from human neural stem cells [167]. However, this work is at a very early stage, and aside from the ethical considerations, much research into control of cell phenotype and cell fate to alleviate concerns for cancer risk are required before this technology is ready to move into the pre-clinical and clinical realms.
In conclusion, this review is a comprehensive assessment of the evidence base to date behind the translation of basic science to the clinical practice of cartilage repair. We have revealed a lack of connectivity between the in vitro, pre-clinical and human data and a patchwork quilt of synergistic evidence. It appears that the drivers for progress in this space are largely driven by patient demand, surgeon inquisition, and a regulatory framework that is learning at the same pace as new developments take place. We strongly recommend funding body commission studies that have a clear translational purpose in order to drive the science towards patient benefit.
Abbreviations
- ACI:
-
Autologous chondrocyte implantation
- AMIC:
-
Autologous matrix-enhanced chondrogenesis
- AOFAS:
-
American Orthopaedic Foot & Ankle Society
- FACS:
-
Fluorescence-activated cell sorting
- HA:
-
Hydroxyapatite
- IKDC:
-
International Knee Documentation Committee
- iPSCs:
-
Induced pluripotent stem cells
- KOOS:
-
Knee and Osteoarthritis Outcome Score
- MACI:
-
Matrix-induced autologous chondrocyte implantation
- MeSH:
-
Medical Subject Headings
- MSC:
-
Mesenchymal stem cells
- OA:
-
Osteoarthritis
- PBS:
-
Phosphate-buffered saline
- PBSC:
-
Peripheral blood stem cells
- PRP:
-
Platelet rich plasma
- qPCR:
-
Real-time polymerase chain reaction
- RCT:
-
Randomised controlled trial
- VAS:
-
Visual analogue scale
- WOMAC:
-
Western Ontario and McMaster Universities Arthritis Index
References
Oldershaw RA. Cell sources for the regeneration of articular cartilage: the past, the horizon and the future. Int J Exp Pathol. 2012;93(6):389–400. doi:10.1111/j.1365-2613.2012.00837.x.
Dozin B, Malpeli M, Cancedda R, et al. Comparative evaluation of autologous chondrocyte implantation and mosaicplasty: a multicentered randomized clinical trial. Clin J Sport Med. 2005;15(4):220–6. Available at: https://www.ncbi.nlm.nih.gov/pubmed/16003035. Accessed 12 June 2016.
Levy AS, Lohnes J, Sculley S, LeCroy M, Garrett W. Chondral delamination of the knee in soccer players. Am J Sports Med. 1996;24(5):634–9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8883684. Accessed 12 June 2016.
Badri A, Burkhardt J. Arthroscopic debridement of unicompartmental arthritis: fact or fiction? Clin Sports Med. 2014;33(1):23–41. doi:10.1016/j.csm.2013.08.008.
Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347(2):81–8. doi:10.1056/NEJMoa013259.
Bedi A, Feeley BT, Williams RJ. Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92(4):994–1009. doi:10.2106/JBJS.I.00895.
Steinwachs MR, Guggi T, Kreuz PC. Marrow stimulation techniques. Injury. 2008;39 Suppl 1:S26–31. doi:10.1016/j.injury.2008.01.042.
Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 19(5):477-84. doi:10.1053/jars.2003.50112.
Peterson L, Menche D, Grande D PM. Chondrocyte transplantation: an experimental model in the rabbit. Trans Orthop Res Soc. 1984;9:218.
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–95. doi:10.1056/NEJM199410063311401.
Jones DG, Peterson L. Autologous chondrocyte implantation. J Bone Joint Surg Am. 2006;88(11):2502–20. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17115530. Accessed 12 June 2016.
Minas T. Autologous chondrocyte implantation in the arthritic knee. Orthopedics. 2003;26(9):945–7. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14503759. Accessed 12 June 2016.
Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38(6):1259–71. doi:10.1177/0363546509346395.
Matsusue Y, Yamamuro T, Hama H. Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthroscopy. 1993;9(3):318–21. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8323618. Accessed 12 June 2016.
Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RWJ. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. J Bone Joint Surg Br. 2012;94(4):504–9. doi:10.1302/0301-620X.94B4.27495.
De Caro F, Bisicchia S, Amendola A, Ding L. Large fresh osteochondral allografts of the knee: a systematic clinical and basic science review of the literature. Arthroscopy. 2015;31(4):757–65. doi:10.1016/j.arthro.2014.11.025.
Capeci CM, Turchiano M, Strauss EJ, Youm T. Osteochondral allografts: applications in treating articular cartilage defects in the knee. Bull Hosp Jt Dis. 2013;71(1):60–7. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24032585. Accessed 12 June 2016.
Bugbee WD, Khanna G, Cavallo M, McCauley JC, Görtz S, Brage ME. Bipolar fresh osteochondral allografting of the tibiotalar joint. J Bone Joint Surg Am. 2013;95(5):426–32. doi:10.2106/JBJS.L.00165.
Gossec L, Paternotte S, Maillefert JF, et al. The role of pain and functional impairment in the decision to recommend total joint replacement in hip and knee osteoarthritis: an international cross-sectional study of 1909 patients. Report of the OARSI-OMERACT Task Force on total joint replacement. Osteoarthritis Cartilage. 2011;19(2):147–54. doi:10.1016/j.joca.2010.10.025.
Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75(4):532–53. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8478382. Accessed 12 June 2016.
Mithoefer K, Williams RJ, Warren RF, Wickiewicz TL, Marx RG. High-impact athletics after knee articular cartilage repair: a prospective evaluation of the microfracture technique. Am J Sports Med. 2006;34(9):1413–8. doi:10.1177/0363546506288240.
Gobbi A, Nunag P, Malinowski K. Treatment of full thickness chondral lesions of the knee with microfracture in a group of athletes. Knee Surg Sports Traumatol Arthrosc. 2005;13(3):213–21. doi:10.1007/s00167-004-0499-3.
Frisbie DD, Trotter GW, Powers BE, et al. Arthroscopic subchondral bone plate microfracture technique augments healing of large chondral defects in the radial carpal bone and medial femoral condyle of horses. Vet Surg. 28(4):242-55. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10424704. Accessed 12 June 2016.
Bae DK, Yoon KH, Song SJ. Cartilage healing after microfracture in osteoarthritic knees. Arthroscopy. 2006;22(4):367–74. doi:10.1016/j.arthro.2006.01.015.
Brown WE, Potter HG, Marx RG, Wickiewicz TL, Warren RF. Magnetic resonance imaging appearance of cartilage repair in the knee. Clin Orthop Relat Res. 2004;(422):214-23. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15187860. Accessed 12 June 2016.
Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902–8. doi:10.1177/0363546508330137.
Bartha L, Vajda A, Duska Z, Rahmeh H, Hangody L. Autologous osteochondral mosaicplasty grafting. J Orthop Sports Phys Ther. 2006;36(10):739–50. doi:10.2519/jospt.2006.2182.
Feczkó P, Hangody L, Varga J, et al. Experimental results of donor site filling for autologous osteochondral mosaicplasty. Arthroscopy. 2003;19(7):755–61. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12966384. Accessed 12 June 2016.
Bentley G, Bhamra JS, Gikas PD, Skinner JA, Carrington R, Briggs TW. Repair of osteochondral defects in joints—how to achieve success. Injury. 2013;44 Suppl 1:S3–10. doi:10.1016/S0020-1383(13)70003-2.
Wood JJ, Malek MA, Frassica FJ, et al. Autologous cultured chondrocytes: adverse events reported to the United States Food and Drug Administration. J Bone Joint Surg Am. 2006;88(3):503–7. doi:10.2106/JBJS.E.00103.
Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212-34. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10818982. Accessed 12 June 2016.
Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30(1):215–24. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7127471. Accessed 12 June 2016.
Takata N, Furumatsu T, Abe N, Naruse K, Ozaki T. Comparison between loose fragment chondrocytes and condyle fibrochondrocytes in cellular proliferation and redifferentiation. J Orthop Sci. 2011;16(5):589–97. doi:10.1007/s00776-011-0128-1.
Keramaris NC, Kanakaris NK, Tzioupis C, Kontakis G, Giannoudis PV. Translational research: from benchside to bedside. Injury. 2008;39(6):643–50. doi:10.1016/j.injury.2008.01.051.
Woolf SH. The meaning of translational research and why it matters. JAMA. 2008;299(2):211–3. doi:10.1001/jama.2007.26.
Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(5):640–5. doi:10.1302/0301-620X.87B5.15905.
Bhosale AM, Richardson JB. Articular cartilage: structure, injuries and review of management. Br Med Bull. 2008;87:77–95. doi:10.1093/bmb/ldn025.
Koga H, Engebretsen L, Brinchmann JE, Muneta T, Sekiya I. Mesenchymal stem cell-based therapy for cartilage repair: a review. Knee Surg Sports Traumatol Arthrosc. 2009;17(11):1289–97. doi:10.1007/s00167-009-0782-4.
Kon E, Verdonk P, Condello V, et al. Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee: systematic clinical data review and study quality analysis. Am J Sports Med. 2009;37 Suppl 1:156S–66S. doi:10.1177/0363546509351649.
Lubis AM, Lubis VK. Adult bone marrow stem cells in cartilage therapy. Acta Med Indones. 2012;44(1):62–8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22451188. Accessed 12 June 2016.
Magnussen RA, Dunn WR, Carey JL, Spindler KP. Treatment of focal articular cartilage defects in the knee: a systematic review. Clin Orthop Relat Res. 2008;466(4):952–62. doi:10.1007/s11999-007-0097-z.
Matsumoto T, Okabe T, Ikawa T, et al. Articular cartilage repair with autologous bone marrow mesenchymal cells. J Cell Physiol. 2010;225(2):291–5. doi:10.1002/jcp.22223.
Maumus M, Guérit D, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cell-based therapies in regenerative medicine: applications in rheumatology. Stem Cell Res Ther. 2011;2(2):14. doi:10.1186/scrt55.
Pastides P, Chimutengwende-Gordon M, Maffulli N, Khan W. Stem cell therapy for human cartilage defects: a systematic review. Osteoarthritis Cartilage. 2013;21(5):646–54. doi:10.1016/j.joca.2013.02.008.
Ye K, Di Bella C, Myers DE, Choong PFM. The osteochondral dilemma: review of current management and future trends. ANZ J Surg. 2014;84(4):211–7. doi:10.1111/ans.12108.
Zengerink M, Struijs PAA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):238–46. doi:10.1007/s00167-009-0942-6.
Liberati A, Altman DG, Tetzlaff J, et al. PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19622552. Accessed 12 June 2016.
Zhang B, Yang S, Sun Z, et al. Human mesenchymal stem cells induced by growth differentiation factor 5: an improved self-assembly tissue engineering method for cartilage repair. Tissue Eng Part C Methods. 2011;17(12):1189–99. doi:10.1089/ten.tec.2011.0011.
Nam HY, Karunanithi P, Loo WC, et al. The effects of staged intra-articular injection of cultured autologous mesenchymal stromal cells on the repair of damaged cartilage: a pilot study in caprine model. Arthritis Res Ther. 2013;15(5):R129. doi:10.1186/ar4309.
Li Z, Kupcsik L, Yao S-J, Alini M, Stoddart MJ. Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin-polyurethane composites. Tissue Eng Part A. 2009;15(7):1729–37. doi:10.1089/ten.tea.2008.0247.
Erickson IE, Kestle SR, Zellars KH, Dodge GR, Burdick JA, Mauck RL. Improved cartilage repair via in vitro pre-maturation of MSC-seeded hyaluronic acid hydrogels. Biomed Mater. 2012;7(2):24110. doi:10.1088/1748-6041/7/2/024110.
Hui TY, Cheung KMC, Cheung WL, Chan D, Chan BP. In vitro chondrogenic differentiation of human mesenchymal stem cells in collagen microspheres: influence of cell seeding density and collagen concentration. Biomaterials. 2008;29(22):3201–12. doi:10.1016/j.biomaterials.2008.04.001.
Huang C-YC, Reuben PM, D’Ippolito G, Schiller PC, Cheung HS. Chondrogenesis of human bone marrow-derived mesenchymal stem cells in agarose culture. Anat Rec A Discov Mol Cell Evol Biol. 2004;278(1):428–36. doi:10.1002/ar.a.20010.
Wang ZJ, An RZ, Zhao JY, et al. Repair of articular cartilage defects by tissue-engineered cartilage constructed with adipose-derived stem cells and acellular cartilaginous matrix in rabbits. Genet Mol Res. 2014;13(2):4599–606. doi:10.4238/2014.June.18.2.
Raheja LF, Galuppo LD, Bowers-Lepore J, Dowd JP, Tablin F, Yellowley CE. Treatment of bilateral medial femoral condyle articular cartilage fissures in a horse using bone marrow-derived multipotent mesenchymal stromal cells. J Equine Vet Sci. 2011;31(3):147–54. doi:10.1016/j.jevs.2010.12.009.
Wu G, Cui Y, Wang Y, et al. Repair of cartilage defects in BMSCs via CDMP1 gene transfection. Genet Mol Res Mol Res. 2014;13(131):291–301. doi:10.4238/2014.January.17.14.
Mokbel AN, El Tookhy OS, Shamaa AA, Rashed LA, Sabry D, El Sayed AM. Homing and reparative effect of intra-articular injection of autologus mesenchymal stem cells in osteoarthritic animal model. BMC Musculoskelet Disord. 2011;12:259. doi:10.1186/1471-2474-12-259.
Masuoka K, Asazuma T, Hattori H, et al. Tissue engineering of articular cartilage with autologous cultured adipose tissue-derived stromal cells using atelocollagen honeycomb-shaped scaffold with a membrane sealing in rabbits. J Biomed Mater Res B Appl Biomater. 2006;79(1):25–34. doi:10.1002/jbm.b.30507.
Zhang K, Zhang Y, Yan S, et al. Repair of an articular cartilage defect using adipose-derived stem cells loaded on a polyelectrolyte complex scaffold based on poly(l-glutamic acid) and chitosan. Acta Biomater. 2013;9(7):7276–88. doi:10.1016/j.actbio.2013.03.025.
Zhu S, Chen P, Wu Y, et al. Programmed application of transforming growth factor β3 and Rac1 inhibitor NSC23766 committed hyaline cartilage differentiation of adipose-derived stem cells for osteochondral defect repair. Stem Cells Transl Med. 2014;3(10):1242–51. doi:10.5966/sctm.2014-0042.
Murata D, Tokunaga S, Tamura T, et al. A preliminary study of osteochondral regeneration using a scaffold-free three-dimensional construct of porcine adipose tissue-derived mesenchymal stem cells. J Orthop Surg Res. 2015;10:35. doi:10.1186/s13018-015-0173-0.
Cui L, Wu Y, Cen L, et al. Repair of articular cartilage defect in non-weight bearing areas using adipose derived stem cells loaded polyglycolic acid mesh. Biomaterials. 2009;30(14):2683–93. doi:10.1016/j.biomaterials.2009.01.045.
Lu C-H, Yeh T-S, Yeh C-L, et al. Regenerating cartilages by Engineered ASCs: prolonged TGF-β3/BMP-6 expression improved articular cartilage formation and restored zonal structure. Mol Ther. 2014;22(1):186–95. doi:10.1038/mt.2013.165.
Dragoo JL, Carlson G, McCormick F, et al. Healing full-thickness cartilage defects using adipose-derived stem cells. Tissue Eng. 2007;13(7):1615–21. doi:10.1089/ten.2006.0249.
Portron S, Merceron C, Gauthier O, et al. Effects of in vitro low oxygen tension preconditioning of adipose stromal cells on their in vivo chondrogenic potential: application in cartilage tissue repair. PLoS One. 2013;8(4):e62368. doi:10.1371/journal.pone.0062368. Abhay Pandit, ed.
Im G-I, Lee JH. Repair of osteochondral defects with adipose stem cells and a dual growth factor-releasing scaffold in rabbits. J Biomed Mater Res B Appl Biomater. 2010;92(2):552–60. doi:10.1002/jbm.b.31552.
Kang H, Peng J, Lu S, et al. In vivo cartilage repair using adipose-derived stem cell-loaded decellularized cartilage ECM scaffolds. J Tissue Eng Regen Med. 2014;8(6):442–53. doi:10.1002/term.1538.
Gong L, Zhou X, Wu Y, et al. Proteomic analysis profile of engineered articular cartilage with chondrogenic differentiated adipose tissue-derived stem cells loaded polyglycolic acid mesh for weight-bearing area defect repair. Tissue Eng Part A. 2014;20(3-4):575–87. doi:10.1089/ten.TEA.2013.0205.
de Girolamo L, Niada S, Arrigoni E, et al. Repair of osteochondral defects in the minipig model by OPF hydrogel loaded with adipose-derived mesenchymal stem cells. Regen Med. 2015;10(2):135–51. doi:10.2217/rme.14.77.
Pei M, He F, Li J, Tidwell JE, Jones AC, McDonough EB. Repair of large animal partial-thickness cartilage defects through intraarticular injection of matrix-rejuvenated synovium-derived stem cells. Tissue Eng Part A. 2013;19(9-10):1144–54. doi:10.1089/ten.TEA.2012.0351.
Lee J-C, Lee SY, Min HJ, et al. Synovium-derived mesenchymal stem cells encapsulated in a novel injectable gel can repair osteochondral defects in a rabbit model. Tissue Eng Part A. 2012;18(19-20):2173–86. doi:10.1089/ten.TEA.2011.0643.
Nakamura T, Sekiya I, Muneta T, et al. Arthroscopic, histological and MRI analyses of cartilage repair after a minimally invasive method of transplantation of allogeneic synovial mesenchymal stromal cells into cartilage defects in pigs. Cytotherapy. 2012;14(3):327–38. doi:10.3109/14653249.2011.638912.
Koga H, Shimaya M, Muneta T, et al. Local adherent technique for transplanting mesenchymal stem cells as a potential treatment of cartilage defect. Arthritis Res Ther. 2008;10(4):R84. doi:10.1186/ar2460.
Lee J-C, Min HJ, Lee S, Seong SC, Lee MC. Effect of chondroitinase ABC on adhesion and behavior of synovial membrane-derived mesenchymal stem cells in rabbit partial-thickness chondral defects. J Orthop Res. 2013;31(8):1293–301. doi:10.1002/jor.22353.
Lee J-C, Min HJ, Park HJ, Lee S, Seong SC, Lee MC. Synovial membrane-derived mesenchymal stem cells supported by platelet-rich plasma can repair osteochondral defects in a rabbit model. Arthroscopy. 2013;29(6):1034–46. doi:10.1016/j.arthro.2013.02.026.
Perka C, Schultz O, Spitzer R-S, Lindenhayn K. The influence of transforming growth factor β1 on mesenchymal cell repair of full-thickness cartilage defects. J Biomed Mater Res. 2000;52(3):543–52. doi:10.1002/1097-4636(20001205)52:3<543::AID-JBM13>3.0.CO;2-2.
Hui JHP, Chen F, Thambyah A, Lee EH. Treatment of chondral lesions in advanced osteochondritis dissecans: a comparative study of the efficacy of chondrocytes, mesenchymal stem cells, periosteal graft, and mosaicplasty (osteochondral autograft) in animal models. J Pediatr Orthop. 24(4):427-33. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15205626. Accessed 12 June 2016.
Gelse K, von der Mark K, Aigner T, Park J, Schneider H. Articular cartilage repair by gene therapy using growth factor-producing mesenchymal cells. Arthritis Rheum. 2003;48(2):430–41. doi:10.1002/art.10759.
Zhang S, Jiang YZ, Zhang W, et al. Neonatal desensitization supports long-term survival and functional integration of human embryonic stem cell-derived mesenchymal stem cells in rat joint cartilage without immunosuppression. Stem Cells Dev. 2013;22(1):90–101. doi:10.1089/scd.2012.0116.
Wakitani S, Aoki H, Harada Y, et al. Embryonic stem cells form articular cartilage, not teratomas, in osteochondral defects of rat joints. Cell Transplant. 2004;13(4):331–6. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15468674. Accessed 12 June 2016.
Chung J, Song M, Ha C-W, Kim J-A, Lee C-H, Park Y-B. Comparison of articular cartilage repair with different hydrogel-human umbilical cord blood-derived mesenchymal stem cell composites in a rat model. Stem Cell Res Ther. 2014;5(2):39. doi:10.1186/scrt427.
Nawata M, Wakitani S, Nakaya H, et al. Use of bone morphogenetic protein 2 and diffusion chambers to engineer cartilage tissue for the repair of defects in articular cartilage. Arthritis Rheum. 2005;52(1):155–63. doi:10.1002/art.20713.
Grande DA, Southerland SS, Manji R, Pate DW, Schwartz RE, Lucas PA. Repair of articular cartilage defects using mesenchymal stem cells. Tissue Eng. 1995;1(4):345–53. doi:10.1089/ten.1995.1.345.
Tay LX, Ahmad RE, Dashtdar H, et al. Treatment outcomes of alginate-embedded allogenic mesenchymal stem cells versus autologous chondrocytes for the repair of focal articular cartilage defects in a rabbit model. Am J Sports Med. 2012;40(1):83–90. doi:10.1177/0363546511420819.
Dashtdar H, Rothan HA, Tay T, et al. A preliminary study comparing the use of allogenic chondrogenic pre-differentiated and undifferentiated mesenchymal stem cells for the repair of full thickness articular cartilage defects in rabbits. J Orthop Res. 2011;29(9):1336–42. doi:10.1002/jor.21413.
Igarashi T, Iwasaki N, Kawamura D, et al. Repair of articular cartilage defects with a novel injectable in situ forming material in a canine model. J Biomed Mater Res A. 2012;100(1):180–7. doi:10.1002/jbm.a.33248.
Ho STB, Hutmacher DW, Ekaputra AK, Hitendra D, Hui JH. The evaluation of a biphasic osteochondral implant coupled with an electrospun membrane in a large animal model. Tissue Eng Part A. 2010;16(4):1123–41. doi:10.1089/ten.TEA.2009.0471.
Oshima Y, Harwood FL, Coutts RD, Kubo T, Amiel D. Variation of mesenchymal cells in polylactic acid scaffold in an osteochondral repair model. Tissue Eng Part C Methods. 2009;15(4):595–604. doi:10.1089/ten.TEC.2008.0487.
Al Faqeh H, Nor Hamdan BMY, Chen HC, Aminuddin BS, Ruszymah BHI. The potential of intra-articular injection of chondrogenic-induced bone marrow stem cells to retard the progression of osteoarthritis in a sheep model. Exp Gerontol. 2012;47(6):458–64. doi:10.1016/j.exger.2012.03.018.
Iwai R, Fujiwara M, Wakitani S, Takagi M. Ex vivo cartilage defect model for the evaluation of cartilage regeneration using mesenchymal stem cells. J Biosci Bioeng. 2011;111(3):357–64. doi:10.1016/j.jbiosc.2010.11.001.
Nishimori M, Deie M, Kanaya A, Exham H, Adachi N, Ochi M. Repair of chronic osteochondral defects in the rat. A bone marrow-stimulating procedure enhanced by cultured allogenic bone marrow mesenchymal stromal cells. J Bone Joint Surg Br. 2006;88(9):1236–44. doi:10.1302/0301-620X.88B9.17810.
Espinosa M, Vaisman A, Nazal N, Figueroa D, Gallegos M, Conget P. Intraarticular administration of dexamethasone after mesenchymal stem cells implantation does not improve significantly the treatment of preestablished full-thickness chondral defect in a rabbit model. Cartilage. 2013;4(2):144–52. doi:10.1177/1947603512472696.
Deng T, Lv J, Pang J, Liu B, Ke J. Construction of tissue-engineered osteochondral composites and repair of large joint defects in rabbit. J Tissue Eng Regen Med. 2014;8(7):546–56. doi:10.1002/term.1556.
Wan W, Li Q, Gao H, et al. BMSCs laden injectable amino-diethoxypropane modified alginate-chitosan hydrogel for hyaline cartilage reconstruction. J Mater Chem B. 2015;3(9):1990–2005. doi:10.1039/C4TB01394H.
Saw K-Y, Hussin P, Loke S-C, et al. Articular cartilage regeneration with autologous marrow aspirate and hyaluronic acid: an experimental study in a goat model. Arthroscopy. 2009;25(12):1391–400. doi:10.1016/j.arthro.2009.07.011.
McIlwraith CW, Frisbie DD, Rodkey WG, et al. Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy. 2011;27(11):1552–61. doi:10.1016/j.arthro.2011.06.002.
Mokbel A, El-Tookhy O, Shamaa AA, Sabry D, Rashed L, Mostafa A. Homing and efficacy of intra-articular injection of autologous mesenchymal stem cells in experimental chondral defects in dogs. Clin Exp Rheumatol. 29(2):275-84. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21385540. Accessed 12 June 2016.
Fortier LA, Potter HG, Rickey EJ, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92(10):1927–37. doi:10.2106/JBJS.I.01284.
Zhao Q, Wang S, Tian J, et al. Combination of bone marrow concentrate and PGA scaffolds enhance bone marrow stimulation in rabbit articular cartilage repair. J Mater Sci Mater Med. 2013;24(3):793–801. doi:10.1007/s10856-012-4841-x.
Sun J, Hou X-K, Li X, et al. Mosaicplasty associated with gene enhanced tissue engineering for the treatment of acute osteochondral defects in a goat model. Arch Orthop Trauma Surg. 2009;129(6):757–71. doi:10.1007/s00402-008-0761-0.
Leng P, Ding C, Zhang H, Wang Y. Reconstruct large osteochondral defects of the knee with hIGF-1 gene enhanced Mosaicplasty. Knee. 2012;19(6):804–11. doi:10.1016/j.knee.2012.03.009.
Ma X, Sun Y, Cheng X, et al. Repair of osteochondral defects by mosaicplasty and allogeneic BMSCs transplantation. Int J Clin Exp Med. 2015;8(4):6053–9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26131203. Accessed 12 June 2016.
Vega A, Martín-Ferrero MA, Del Canto F, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681–90. doi:10.1097/TP.0000000000000678.
Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 11(3):343-53. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18523506. Accessed 13 June 2016.
Davatchi F, Abdollahi BS, Mohyeddin M, Shahram F, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14(2):211–5. doi:10.1111/j.1756-185X.2011.01599.x.
Pak J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: a case series. J Med Case Rep. 2011;5:296. doi:10.1186/1752-1947-5-296.
Koh YG, Choi YJ, Kwon OR, Kim YS. Second-look arthroscopic evaluation of cartilage lesions after mesenchymal stem cell implantation in osteoarthritic knees. Am J Sports Med. 2014;42(7):1628–37. doi:10.1177/0363546514529641.
Kim YS, Choi YJ, Suh DS, et al. Mesenchymal stem cell implantation in osteoarthritic knees: is fibrin glue effective as a scaffold? Am J Sports Med. 2015;43(1):176–85. doi:10.1177/0363546514554190.
Emadedin M, Aghdami N, Taghiyar L, et al. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch Iran Med. 2012;15(7):422-8. doi:012157/AIM.0010.
Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254–66. doi:10.1002/stem.1634.
Orozco L, Munar A, Soler R, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation. 2013;95(12):1535–41. doi:10.1097/TP.0b013e318291a2da.
Emadedin M, Ghorbani Liastani M, Fazeli R, et al. Long-term follow-up of intra-articular injection of autologous mesenchymal stem cells in patients with knee, ankle, or hip osteoarthritis. Arch Iran Med. 2015;18(6):336-44. doi:015186/AIM.003.
Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10(3):199–206. doi:10.1053/joca.2001.0504.
Koh Y-G, Choi Y-J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19(6):902–7. doi:10.1016/j.knee.2012.04.001.
Wong KL, Lee KBL, Tai BC, Law P, Lee EH, Hui JHP. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy. 2013;29(12):2020–8. doi:10.1016/j.arthro.2013.09.074.
Giannini S, Buda R, Vannini F, Cavallo M, Grigolo B. One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clin Orthop Relat Res. 2009;467(12):3307–20. doi:10.1007/s11999-009-0885-8.
Giannini S, Buda R, Cavallo M, et al. Cartilage repair evolution in post-traumatic osteochondral lesions of the talus: from open field autologous chondrocyte to bone-marrow-derived cells transplantation. Injury. 2010;41(11):1196–203. doi:10.1016/j.injury.2010.09.028.
Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. 2004;13(5):595–600. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15565871. Accessed 13 June 2016.
Saw K-Y, Anz A, Merican S, et al. Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: a report of 5 cases with histology. Arthroscopy. 2011;27(4):493–506. doi:10.1016/j.arthro.2010.11.054.
Nejadnik H, Hui JH, Feng Choong EP, Tai B-C, Lee EH. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38(6):1110–6. doi:10.1177/0363546509359067.
Saw K-Y, Anz A, Siew-Yoke Jee C, et al. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy. 2013;29(4):684–94. doi:10.1016/j.arthro.2012.12.008.
Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 1(1):74-9. doi:10.1002/term.8.
Lee KBL, Wang VTZ, Chan YH, Hui JHP. A novel, minimally-invasive technique of cartilage repair in the human knee using arthroscopic microfracture and injections of mesenchymal stem cells and hyaluronic acid—a prospective comparative study on safety and short-term efficacy. Ann Acad Med Singapore. 2012;41(11):511–7. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23235728. Accessed 16 June 2016.
Kuroda R, Ishida K, Matsumoto T, et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15(2):226–31. doi:10.1016/j.joca.2006.08.008.
Haleem AM, El Singergy AA, Sabry D, et al. The clinical use of human culture-expanded autologous bone marrow mesenchymal stem cells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects: a pilot study and preliminary results. Cartilage. 2010;1(4):253–61. doi:10.1177/1947603510366027.
Kasemkijwattana C, Hongeng S, Kesprayura S, Rungsinaporn V, Chaipinyo K, Chansiri K. Autologous bone marrow mesenchymal stem cells implantation for cartilage defects: two cases report. J Med Assoc Thai. 2011;94(3):395–400. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21560849. Accessed 13 June 2016.
Gigante A, Cecconi S, Calcagno S, Busilacchi A, Enea D. Arthroscopic knee cartilage repair with covered microfracture and bone marrow concentrate. Arthrosc Tech. 2012;1(2):e175–80. doi:10.1016/j.eats.2012.07.001.
Shetty AA, Kim SJ, Shetty V, et al. Autologous bone-marrow mesenchymal cell induced chondrogenesis: single-stage arthroscopic cartilage repair. Tissue Eng Regen Med. 2014;11(3):247–53. doi:10.1007/s13770-014-0061-4.
Akgun I, Unlu MC, Erdal OA, et al. Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: a 2-year randomized study. Arch Orthop Trauma Surg. 2015;135(2):251–63. doi:10.1007/s00402-014-2136-z.
Buda R, Vannini F, Cavallo M, Grigolo B, Cenacchi A, Giannini S. Osteochondral lesions of the knee: a new one-step repair technique with bone-marrow-derived cells. J Bone Joint Surg Am. 2010;92 Suppl 2:2–11. doi:10.2106/JBJS.J.00813.
Enea D, Cecconi S, Calcagno S, et al. Single-stage cartilage repair in the knee with microfracture covered with a resorbable polymer-based matrix and autologous bone marrow concentrate. Knee. 2013;20(6):562–9. doi:10.1016/j.knee.2013.04.003.
Gobbi A, Chaurasia S, Karnatzikos G, Nakamura N. Matrix-induced autologous chondrocyte implantation versus multipotent stem cells for the treatment of large patellofemoral chondral lesions: a nonrandomized prospective trial. Cartilage. 2015;6(2):82–97. doi:10.1177/1947603514563597.
Sekiya I, Muneta T, Horie M, Koga H. Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects. Clin Orthop Relat Res. 2015;473(7):2316–26. doi:10.1007/s11999-015-4324-8.
Katayama R, Wakitani S, Tsumaki N, et al. Repair of articular cartilage defects in rabbits using CDMP1 gene-transfected autologous mesenchymal cells derived from bone marrow. Rheumatology (Oxford). 2004;43(8):980–5. doi:10.1093/rheumatology/keh240.
Insall JN. Intra-articular surgery for degenerative arthritis of the knee. A report of the work of the late K. H. Pridie. J Bone Joint Surg Br. 1967;49(2):211–28. Available at: http://www.ncbi.nlm.nih.gov/pubmed/6026508. Accessed 8 July 2016.
Meachim G, Roberts C. Repair of the joint surface from subarticular tissue in the rabbit knee. J Anat. 1971;109(Pt 2):317–27. Available at: http://www.ncbi.nlm.nih.gov/pubmed/5558237. Accessed 8 July 2016.
Dzioba RB. The classification and treatment of acute articular cartilage lesions. Arthroscopy. 1988;4(2):72–80. Available at: http://www.ncbi.nlm.nih.gov/pubmed/3395420. Accessed 8 July 2016.
Insall J. The Pridie debridement operation for osteoarthritis of the knee. Clin Orthop Relat Res. 1974;(101):61-7. Available at: http://www.ncbi.nlm.nih.gov/pubmed/4837919. Accessed 8 July 2016.
Ficat RP, Ficat C, Gedeon P, Toussaint JB. Spongialization: a new treatment for diseased patellae. Clin Orthop Relat Res. 1979;(144):74-83. Available at: http://www.ncbi.nlm.nih.gov/pubmed/535254. Accessed 8 July 2016.
Johnson LL. Arthroscopic abrasion arthroplasty historical and pathologic perspective: present status. Arthroscopy. 1986;2(1):54–69. Available at: http://www.ncbi.nlm.nih.gov/pubmed/3954840. Accessed 8 July 2016.
Friedman MJ, Berasi CC, Fox JM, Del Pizzo W, Snyder SJ, Ferkel RD. Preliminary results with abrasion arthroplasty in the osteoarthritic knee. Clin Orthop Relat Res. (182):200-5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/6692614. Accessed 8 July 2016.
Ogilvie-Harris DJ, Fitsialos DP. Arthroscopic management of the degenerative knee. Arthrosc J Arthrosc Relat Surg. 1991;7(2):151–7. doi:10.1016/0749-8063(91)90101-3.
Baumgaertner MR, Cannon WD, Vittori JM, Schmidt ES, Maurer RC. Arthroscopic debridement of the arthritic knee. Clin Orthop Relat Res. 1990;(253):197-202. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2317974. Accessed 10 July 2016.
Rand JA. Role of arthroscopy in osteoarthritis of the knee. Arthroscopy. 1991;7(4):358–63. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1755883. Accessed 10 July 2016.
Bert JM. Role of abrasion arthroplasty and debridement in the management of osteoarthritis of the knee. Rheum Dis Clin North Am. 1993;19(3):725–39. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8210584. Accessed 10 July 2016.
Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res. 1999;(365):149-62. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10627699. Accessed 10 July 2016.
Dandy DJ. Abrasion chondroplasty. Arthrosc J Arthrosc Relat Surg. 1986;2(1):51–3. doi:10.1016/S0749-8063(86)80011-1.
Mithoefer K, Williams RJ, Warren RF, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87(9):1911–20. doi:10.2106/JBJS.D.02846.
Steadman JR, Briggs KK, Rodrigo JJ, et al. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthrosc J Arthrosc Relat Surg. 2003;19(5):477–84. doi:10.1053/jars.2003.50112.
Uematsu K, Hattori K, Ishimoto Y, et al. Cartilage regeneration using mesenchymal stem cells and a three-dimensional poly-lactic-glycolic acid (PLGA) scaffold. Biomaterials. 2005;26(20):4273–9. doi:10.1016/j.biomaterials.2004.10.037.
Duan X, Zhu X, Dong X, et al. Repair of large osteochondral defects in a beagle model with a novel type I collagen/glycosaminoglycan-porous titanium biphasic scaffold. Mater Sci Eng C Mater Biol Appl. 2013;33(7):3951–7. doi:10.1016/j.msec.2013.05.040.
Oshima Y, Watanabe N, Matsuda K, Takai S, Kawata M, Kubo T. Fate of transplanted bone-marrow-derived mesenchymal cells during osteochondral repair using transgenic rats to simulate autologous transplantation. Osteoarthritis Cartilage. 2004;12(10):811–7. doi:10.1016/j.joca.2004.06.014.
Betsch M, Schneppendahl J, Thuns S, et al. Bone marrow aspiration concentrate and platelet rich plasma for osteochondral repair in a porcine osteochondral defect model. PLoS One. 2013;8(8):e71602. doi:10.1371/journal.pone.0071602.
Solchaga LA, Gao J, Dennis JE, et al. Treatment of osteochondral defects with autologous bone marrow in a hyaluronan-based delivery vehicle. Tissue Eng. 2002;8(2):333–47. doi:10.1089/107632702753725085.
Gille J, Schuseil E, Wimmer J, Gellissen J, Schulz AP, Behrens P. Mid-term results of autologous matrix-induced chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1456–64. doi:10.1007/s00167-010-1042-3.
Dhollander AAM, De Neve F, Almqvist KF, et al. Autologous matrix-induced chondrogenesis combined with platelet-rich plasma gel: technical description and a five pilot patients report. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):536–42. doi:10.1007/s00167-010-1337-4.
Kusano T, Jakob RP, Gautier E, Magnussen RA, Hoogewoud H, Jacobi M. Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2109–15. doi:10.1007/s00167-011-1840-2.
Zantop T, Petersen W, Murrell GA, et al. Arthroscopic implantation of a matrix to cover large chondral defect during microfracture. Arthrosc J Arthrosc Relat Surg. 2009;25(11):1354–60. doi:10.1016/j.arthro.2009.04.077.
Siclari A, Mascaro G, Gentili C, Cancedda R, Boux E. A cell-free scaffold-based cartilage repair provides improved function hyaline-like repair at one year. Clin Orthop Relat Res. 2012;470(3):910–9. doi:10.1007/s11999-011-2107-4.
Huade Li H, Qiang Zheng Q, Yuxiang Xiao Y, Jie Feng J, Zhongli Shi Z, Zhijun PZ. Rat cartilage repair using nanophase PLGA/HA composite and mesenchymal stem cells. J Bioact Compat Polym. 2009;24(1):83–99. doi:10.1177/0883911508100655.
Yan H, Yu C. Repair of full-thickness cartilage defects with cells of different origin in a rabbit model. Arthroscopy. 2007;23(2):178–87. doi:10.1016/j.arthro.2006.09.005.
Charles Huang C-Y, Reuben PM, D’Ippolito G, Schiller PC, Cheung HS. Chondrogenesis of human bone marrow-derived mesenchymal stem cells in agarose culture. Anat Rec. 2004;278A(1):428–36. doi:10.1002/ar.a.20010.
Koga H, Muneta T, Nagase T, et al. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008;333(2):207–15. doi:10.1007/s00441-008-0633-5.
Erickson IE, Kestle SR, Zellars KH, et al. High mesenchymal stem cell seeding densities in hyaluronic acid hydrogels produce engineered cartilage with native tissue properties. Acta Biomater. 2012;8(8):3027–34. doi:10.1016/j.actbio.2012.04.033.
What are induced pluripotent stem cells? [Stem Cell Information]. Available at: https://stemcells.nih.gov/info/basics.htm. Accessed June 2016.
Wei Y, Zeng W, Wan R, et al. Chondrogenic differentiation of induced pluripotent stem cells from osteoarthritic chondrocytes in alginate matrix. Eur Cell Mater. 2012;23:1–12. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22241609. Accessed 12 Aug 2016.
Medvedev SP, Grigor’eva EV, Shevchenko AI, et al. Human induced pluripotent stem cells derived from fetal neural stem cells successfully undergo directed differentiation into cartilage. Stem Cells Dev. 2011;20(6):1099–112. doi:10.1089/scd.2010.0249.
Millan C, Cavalli E, Groth T, Maniura-Weber K, Zenobi-Wong M. Engineered microtissues formed by schiff base crosslinking restore the chondrogenic potential of aged mesenchymal stem cells. Adv Healthc Mater. 2015;4(9):1348–58. doi:10.1002/adhm.201500102.
Popa EG, Caridade SG, Mano JF, Reis RL, Gomes ME. Chondrogenic potential of injectable κ-carrageenan hydrogel with encapsulated adipose stem cells for cartilage tissue-engineering applications. J Tissue Eng Regen Med. 2015;9(5):550–63. doi:10.1002/term.1683.
Huang Z, Nooeaid P, Kohl B, et al. Chondrogenesis of human bone marrow mesenchymal stromal cells in highly porous alginate-foams supplemented with chondroitin sulfate. Mater Sci Eng C Mater Biol Appl. 2015;50:160–72. doi:10.1016/j.msec.2015.01.082.
Narcisi R, Cleary MA, Brama PAJ, et al. Long-term expansion, enhanced chondrogenic potential, and suppression of endochondral ossification of adult human MSCs via WNT signaling modulation. Stem Cell Reports. 2015;4(3):459–72. doi:10.1016/j.stemcr.2015.01.017.
Castro NJ, O’Brien J, Zhang LG. Integrating biologically inspired nanomaterials and table-top stereolithography for 3D printed biomimetic osteochondral scaffolds. Nanoscale. 2015;7(33):14010–22. doi:10.1039/c5nr03425f.
Rey-Rico A, Venkatesan JK, Sohier J, Moroni L, Cucchiarini M, Madry H. Adapted chondrogenic differentiation of human mesenchymal stem cells via controlled release of TGF-β1 from poly(ethylene oxide)-terephtalate/poly(butylene terepthalate) multiblock scaffolds. J Biomed Mater Res A. 2015;103(1):371–83. doi:10.1002/jbm.a.35181.
Kim J, Lin B, Kim S, Choi B, Evseenko D, Lee M. TGF-β1 conjugated chitosan collagen hydrogels induce chondrogenic differentiation of human synovium-derived stem cells. J Biol Eng. 2015;9:1. doi:10.1186/1754-1611-9-1.
Dang PN, Solorio LD, Alsberg E. Driving cartilage formation in high-density human adipose-derived stem cell aggregate and sheet constructs without exogenous growth factor delivery. Tissue Eng Part A. 2014;20(23-24):3163–75. doi:10.1089/ten.tea.2012.0551.
Focaroli S, Teti G, Salvatore V, et al. Chondrogenic differentiation of human adipose mesenchimal stem cells: influence of a biomimetic gelatin genipin crosslinked porous scaffold. Microsc Res Tech. 2014;77(11):928–34. doi:10.1002/jemt.22417.
Leijten J, Georgi N, Moreira Teixeira L, van Blitterswijk CA, Post JN, Karperien M. Metabolic programming of mesenchymal stromal cells by oxygen tension directs chondrogenic cell fate. Proc Natl Acad Sci U S A. 2014;111(38):13954–9. doi:10.1073/pnas.1410977111.
Jagielski M, Wolf J, Marzahn U, et al. The influence of IL-10 and TNFα on chondrogenesis of human mesenchymal stromal cells in three-dimensional cultures. Int J Mol Sci. 2014;15(9):15821–44. doi:10.3390/ijms150915821.
Frisch J, Venkatesan J, Rey-Rico A, et al. Influence of insulin-like growth factor I overexpression via recombinant adeno-associated vector gene transfer upon the biological activities and differentiation potential of human bone marrow-derived mesenchymal stem cells. Stem Cell Res Ther. 2014;5(4):103. doi:10.1186/scrt491.
Meng F, He A, Zhang Z, et al. Chondrogenic differentiation of ATDC5 and hMSCs could be induced by a novel scaffold-tricalcium phosphate-collagen-hyaluronan without any exogenous growth factors in vitro. J Biomed Mater Res Part A. 2014;102(8):2725–35. doi:10.1002/jbm.a.34948.
Ye K, Felimban R, Traianedes K, et al. Chondrogenesis of infrapatellar fat pad derived adipose stem cells in 3D printed chitosan scaffold. PLoS One. 2014;9(6):e99410. doi:10.1371/journal.pone.0099410. Reilly G, ed.
Bhumiratana S, Eton RE, Oungoulian SR, Wan LQ, Ateshian GA, Vunjak-Novakovic G. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proc Natl Acad Sci. 2014;111(19):6940–5. doi:10.1073/pnas.1324050111.
Mhanna R, Öztürk E, Vallmajo-Martin Q, Millan C, Müller M, Zenobi-Wong M. GFOGER-modified MMP-sensitive polyethylene glycol hydrogels induce chondrogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2014;20(7-8):1165–74. doi:10.1089/ten.TEA.2013.0519.
Sato Y, Wakitani S, Takagi M. Xeno-free and shrinkage-free preparation of scaffold-free cartilage-like disc-shaped cell sheet using human bone marrow mesenchymal stem cells. J Biosci Bioeng. 2013;116(6):734–9. doi:10.1016/j.jbiosc.2013.05.019.
Guenther D, Oks A, Ettinger M, et al. Enhanced migration of human bone marrow stromal cells in modified collagen hydrogels. Int Orthop. 2013;37(8):1605–11. doi:10.1007/s00264-013-1894-5.
Yang X, Shang H, Katz A, Li X. A modified aggregate culture for chondrogenesis of human adipose-derived stem cells genetically modified with growth and differentiation factor 5. Biores Open Access. 2013;2(4):258–65. doi:10.1089/biores.2013.0014.
Saha S, Kirkham J, Wood D, Curran S, Yang XB. Informing future cartilage repair strategies: a comparative study of three different human cell types for cartilage tissue engineering. Cell Tissue Res. 2013;352(3):495–507. doi:10.1007/s00441-013-1586-x.
Neumann AJ, Alini M, Archer CW, Stoddart MJ. Chondrogenesis of human bone marrow-derived mesenchymal stem cells is modulated by complex mechanical stimulation and adenoviral-mediated overexpression of bone morphogenetic protein 2. Tissue Eng Part A. 2013;19(11-12):1285–94. doi:10.1089/ten.TEA.2012.0411.
Kim D-H, Kim D-D, Yoon I-S. Proliferation and chondrogenic differentiation of human adipose-derived mesenchymal stem cells in sodium alginate beads with or without hyaluronic acid. J Pharm Investig. 2013;43(2):145–51. doi:10.1007/s40005-013-0059-2.
Chen X, Zhang F, He X, et al. Chondrogenic differentiation of umbilical cord-derived mesenchymal stem cells in type I collagen-hydrogel for cartilage engineering. Injury. 2013;44(4):540–9. doi:10.1016/j.injury.2012.09.024.
Pei M, Zhang Y, Li J, Chen D. Antioxidation of decellularized stem cell matrix promotes human synovium-derived stem cell-based chondrogenesis. Stem Cells Dev. 2013;22(6):889–900. doi:10.1089/scd.2012.0495.
Petrou M, Niemeyer P, Stoddart MJ, et al. Mesenchymal stem cell chondrogenesis: composite growth factor-bioreactor synergism for human stem cell chondrogenesis. Regen Med. 2013;8(2):157–70. doi:10.2217/rme.13.3.
Cheng N-C, Estes BT, Young T-H, Guilak F. Genipin-crosslinked cartilage-derived matrix as a scaffold for human adipose-derived stem cell chondrogenesis. Tissue Eng Part A. 2013;19(3-4):484–96. doi:10.1089/ten.tea.2012.0384.
López-Ruiz E, Perán M, Cobo-Molinos J, et al. Chondrocytes extract from patients with osteoarthritis induces chondrogenesis in infrapatellar fat pad-derived stem cells. Osteoarthr Cartil. 2013;21(1):246–58. doi:10.1016/j.joca.2012.10.007.
Spoliti M, Iudicone P, Leone R, De Rosa A, Rossetti FR, Pierelli L. In vitro release and expansion of mesenchymal stem cells by a hyaluronic acid scaffold used in combination with bone marrow. Muscles Ligaments Tendons J. 2012;2(4):289–94. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23738312. Accessed June 22, 2016.
Mifune Y, Matsumoto T, Murasawa S, et al. Therapeutic superiority for cartilage repair by CD271-positive marrow stromal cell transplantation. Cell Transplant. 2013;22(7):1201–11. doi:10.3727/096368912X657378.
Ousema PH, Moutos FT, Estes BT, et al. The inhibition by interleukin 1 of MSC chondrogenesis and the development of biomechanical properties in biomimetic 3D woven PCL scaffolds. Biomaterials. 2012;33(35):8967–74. doi:10.1016/j.biomaterials.2012.08.045.
Li F, Chen Y-Z, Miao Z-N, Zheng S, Jin J. Human placenta-derived mesenchymal stem cells with silk fibroin biomaterial in the repair of articular cartilage defects. 2012.
Popa E, Reis R, Gomes M. Chondrogenic phenotype of different cells encapsulated in κ-carrageenan hydrogels for cartilage regeneration strategies. Biotechnol Appl Biochem. 59(2):132-41. doi:10.1002/bab.1007.
Suzuki S, Muneta T, Tsuji K, et al. Properties and usefulness of aggregates of synovial mesenchymal stem cells as a source for cartilage regeneration. Arthritis Res Ther. 2012;14(3):R136. doi:10.1186/ar3869.
Musumeci G, Lo Furno D, Loreto C, et al. Mesenchymal stem cells from adipose tissue which have been differentiated into chondrocytes in three-dimensional culture express lubricin. Exp Biol Med. 2011;236(11):1333–41. doi:10.1258/ebm.2011.011183.
Yoon I-S, Chung CW, Sung J-H, et al. Proliferation and chondrogenic differentiation of human adipose-derived mesenchymal stem cells in porous hyaluronic acid scaffold. J Biosci Bioeng. 2011;112(4):402–8. doi:10.1016/j.jbiosc.2011.06.018.
Liu TM, Guo XM, Tan HS, Hui JH, Lim B, Lee EH. Zinc-finger protein 145, acting as an upstream regulator of SOX9, improves the differentiation potential of human mesenchymal stem cells for cartilage regeneration and repair. Arthritis Rheum. 2011;63(9):2711–20. doi:10.1002/art.30430.
Im G-I, Kim H-J, Lee JH. Chondrogenesis of adipose stem cells in a porous PLGA scaffold impregnated with plasmid DNA containing SOX trio (SOX-5,-6 and -9) genes. Biomaterials. 2011;32(19):4385–92. doi:10.1016/j.biomaterials.2011.02.054.
Chen W-C, Yao C-L, Chu I-M, Wei Y-H. Compare the effects of chondrogenesis by culture of human mesenchymal stem cells with various type of the chondroitin sulfate C. J Biosci Bioeng. 2011;111(2):226–31. doi:10.1016/j.jbiosc.2010.10.002.
García-Álvarez F, Alegre-Aguarón E, Desportes P, et al. Chondrogenic differentiation in femoral bone marrow-derived mesenchymal cells (MSC) from elderly patients suffering osteoarthritis or femoral fracture. Arch Gerontol Geriatr. 52(2):239-42. doi:10.1016/j.archger.2010.03.026.
Abrahamsson CK, Yang F, Park H, et al. Chondrogenesis and mineralization during in vitro culture of human mesenchymal stem cells on three-dimensional woven scaffolds. Tissue Eng Part A. 2010;16(12):3709–18. doi:10.1089/ten.TEA.2010.0190.
Baumgartner L, Arnhold S, Brixius K, Addicks K, Bloch W. Human mesenchymal stem cells: influence of oxygen pressure on proliferation and chondrogenic differentiation in fibrin glue in vitro. J Biomed Mater Res Part A. 2009;9999A(3):NA-NA. doi:10.1002/jbm.a.32577.
Kim H-J, Lee J-H, Im G-I. Chondrogenesis using mesenchymal stem cells and PCL scaffolds. J Biomed Mater Res Part A. 2009;9999A(2):NA-NA. doi:10.1002/jbm.a.32414.
Kobayashi T, Ochi M, Yanada S, et al. Augmentation of degenerated human cartilage in vitro using magnetically labeled mesenchymal stem cells and an external magnetic device. Arthroscopy. 2009;25(12):1435–41. doi:10.1016/j.arthro.2009.06.009.
Hildner F, Concaro S, Peterbauer A, et al. Human adipose-derived stem cells contribute to chondrogenesis in coculture with human articular chondrocytes. Tissue Eng Part A. 2009;15(12):3961–9. doi:10.1089/ten.TEA.2009.0002.
Angele P, Müller R, Schumann D, et al. Characterization of esterified hyaluronan-gelatin polymer composites suitable for chondrogenic differentiation of mesenchymal stem cells. J Biomed Mater Res Part A. 2009;91A(2):416–27. doi:10.1002/jbm.a.32236.
Lee S, Kim JH, Jo CH, Seong SC, Lee JC, Lee MC. Effect of serum and growth factors on chondrogenic differentiation of synovium-derived stromal cells. Tissue Eng Part A. 2009;15(11):3401–15. doi:10.1089/ten.TEA.2008.0466.
Jung Y, Chung Y-I, Kim SH, et al. In situ chondrogenic differentiation of human adipose tissue-derived stem cells in a TGF-beta1 loaded fibrin-poly(lactide-caprolactone) nanoparticulate complex. Biomaterials. 2009;30(27):4657–64. doi:10.1016/j.biomaterials.2009.05.034.
Spadaccio C, Rainer A, Trombetta M, et al. Poly-L-lactic acid/hydroxyapatite electrospun nanocomposites induce chondrogenic differentiation of human MSC. Ann Biomed Eng. 2009;37(7):1376–89. doi:10.1007/s10439-009-9704-3.
Seda Tigli R, Ghosh S, Laha MM, et al. Comparative chondrogenesis of human cell sources in 3D scaffolds. J Tissue Eng Regen Med. 2009;3(5):348–60. doi:10.1002/term.169.
Heymer A, Bradica G, Eulert J, Nöth U. Multiphasic collagen fibre-PLA composites seeded with human mesenchymal stem cells for osteochondral defect repair: an in vitro study. J Tissue Eng Regen Med. 2009;3(5):389–97. doi:10.1002/term.175.
Pilgaard L, Lund P, Duroux M, et al. Effect of oxygen concentration, culture format and donor variability on in vitro chondrogenesis of human adipose tissue-derived stem cells. Regen Med. 2009;4(4):539–48. doi:10.2217/rme.09.28.
Kim H-J, Im G-I. Combination of transforming growth factor-beta2 and bone morphogenetic protein 7 enhances chondrogenesis from adipose tissue-derived mesenchymal stem cells. Tissue Eng Part A. 2009;15(7):1543–51. doi:10.1089/ten.tea.2008.0368.
Cheng N-C, Estes BT, Awad HA, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng Part A. 2009;15(2):231–41. doi:10.1089/ten.tea.2008.0253.
Babister JC, Tare RS, Green DW, Inglis S, Mann S, Oreffo ROC. Genetic manipulation of human mesenchymal progenitors to promote chondrogenesis using “bead-in-bead” polysaccharide capsules. Biomaterials. 2008;29(1):58–65. doi:10.1016/j.biomaterials.2007.09.006.
Miyamoto C, Matsumoto T, Sakimura K, Shindo H. Osteogenic protein-1 with transforming growth factor-β1: potent inducer of chondrogenesis of synovial mesenchymal stem cells in vitro. J Orthop Sci. 2007;12(6):555–61. doi:10.1007/s00776-007-1176-4.
Mehlhorn AT, Schmal H, Kaiser S, et al. Mesenchymal stem cells maintain TGF-beta-mediated chondrogenic phenotype in alginate bead culture. Tissue Eng. 2006;12(6):1393–403. doi:10.1089/ten.2006.12.1393.
Estes BT, Wu AW, Guilak F. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum. 2006;54(4):1222–32. doi:10.1002/art.21779.
Zhang X, Mitsuru A, Igura K, et al. Mesenchymal progenitor cells derived from chorionic villi of human placenta for cartilage tissue engineering. Biochem Biophys Res Commun. 2006;340(3):944–52. doi:10.1016/j.bbrc.2005.12.091.
Yokoyama A, Sekiya I, Miyazaki K, Ichinose S, Hata Y, Muneta T. In vitro cartilage formation of composites of synovium-derived mesenchymal stem cells with collagen gel. Cell Tissue Res. 2005;322(2):289–98. doi:10.1007/s00441-005-0010-6.
Sekiya I, Larson BL, Vuoristo JT, Reger RL, Prockop DJ. Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 2005;320(2):269–76. doi:10.1007/s00441-004-1075-3.
Wickham MQ, Erickson GR, Gimble JM, Vail TP, Guilak F. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res. 2003;412:196–212. doi:10.1097/01.blo.0000072467.53786.ca.
Dragoo JL, Samimi B, Zhu M, et al. Tissue-engineered cartilage and bone using stem cells from human infrapatellar fat pads. J Bone Joint Surg Br. 2003;85(5):740–7. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12892203. Accessed 22 June 2016.
Nöth U, Tuli R, Osyczka AM, Danielson KG, Tuan RS. In vitro engineered cartilage constructs produced by press-coating biodegradable polymer with human mesenchymal stem cells. 2004. http://online.liebertpub.com/doi/abs/10.1089/107632702753503126. Accessed June 2016.
Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;290(2):763–9. doi:10.1006/bbrc.2001.6270.
Caterson EJ, Nesti LJ, Li WJ, et al. Three-dimensional cartilage formation by bone marrow-derived cells seeded in polylactide/alginate amalgam. J Biomed Mater Res. 2001;57(3):394–403. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11523034. Accessed 22 June 2016.
Ponticiello MS, Schinagl RM, Kadiyala S, Barry FP. Gelatin-based resorbable sponge as a carrier matrix for human mesenchymal stem cells in cartilage regeneration therapy. J Biomed Mater Res. 2000;52(2):246–55. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10951362. Accessed 22 June 2016.
Yoo JU, Barthel TS, Nishimura K, et al. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80(12):1745–57. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9875932. Accessed 22 June 2016.
Huang JI, Kazmi N, Durbhakula MM, Hering TM, Yoo JU, Johnstone B. Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: a patient-matched comparison. J Orthop Res. 2005;23(6):1383–9. doi:10.1016/j.orthres.2005.03.018.
Chen W-C, Wei Y-H, Chu I-M, Yao C-L. Effect of chondroitin sulphate C on the in vitro and in vivo chondrogenesis of mesenchymal stem cells in crosslinked type II collagen scaffolds. J Tissue Eng Regen Med. 2013;7(8):665–72. doi:10.1002/term.1463.
Bornes TD, Jomha NM, Mulet-Sierra A, et al. Hypoxic culture of bone marrow-derived mesenchymal stromal stem cells differentially enhances in vitro chondrogenesis within cell-seeded collagen and hyaluronic acid porous scaffolds. Stem Cell Res Ther. 2015;6(1):84. doi:10.1186/s13287-015-0075-4.
Benders KEM, Boot W, Cokelaere SM, et al. Multipotent stromal cells outperform chondrocytes on cartilage-derived matrix scaffolds. Cartilage. 2014;5(4):221–30. doi:10.1177/1947603514535245.
Huang H, Zhang X, Hu X, et al. Directing chondrogenic differentiation of mesenchymal stem cells with a solid-supported chitosan thermogel for cartilage tissue engineering. Biomed Mater. 2014;9(3):35008. doi:10.1088/1748-6041/9/3/035008.
Lam J, Lu S, Meretoja VV, Tabata Y, Mikos AG, Kasper FK. Generation of osteochondral tissue constructs with chondrogenically and osteogenically predifferentiated mesenchymal stem cells encapsulated in bilayered hydrogels. Acta Biomater. 2014;10(3):1112–23. doi:10.1016/j.actbio.2013.11.020.
Zhang L, Yuan T, Guo L, Zhang X. An in vitro study of collagen hydrogel to induce the chondrogenic differentiation of mesenchymal stem cells. J Biomed Mater Res Part A. 2012;100A(10):2717–25. doi:10.1002/jbm.a.34194.
Xie X, Wang Y, Zhao C, et al. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials. 2012;33(29):7008–18. doi:10.1016/j.biomaterials.2012.06.058.
Giannoni P, Lazzarini E, Ceseracciu L, Barone AC, Quarto R, Scaglione S. Design and characterization of a tissue-engineered bilayer scaffold for osteochondral tissue repair. J Tissue Eng Regen Med. 2015;9(10):1182–92. doi:10.1002/term.1651.
Coburn JM, Gibson M, Monagle S, Patterson Z, Elisseeff JH. Bioinspired nanofibers support chondrogenesis for articular cartilage repair. Proc Natl Acad Sci U S A. 2012;109(25):10012–7. doi:10.1073/pnas.1121605109.
Kim M, Erickson IE, Choudhury M, Pleshko N, Mauck RL. Transient exposure to TGF-β3 improves the functional chondrogenesis of MSC-laden hyaluronic acid hydrogels. J Mech Behav Biomed Mater. 2012;11:92–101. doi:10.1016/j.jmbbm.2012.03.006.
Shafiee A, Soleimani M, Chamheidari GA, et al. Electrospun nanofiber-based regeneration of cartilage enhanced by mesenchymal stem cells. J Biomed Mater Res A. 2011;99(3):467–78. doi:10.1002/jbm.a.33206.
Meyer EG, Buckley CT, Thorpe SD, Kelly DJ. Low oxygen tension is a more potent promoter of chondrogenic differentiation than dynamic compression. J Biomech. 2010;43(13):2516–23. doi:10.1016/j.jbiomech.2010.05.020.
Vinardell T, Thorpe SD, Buckley CT, Kelly DJ. Chondrogenesis and integration of mesenchymal stem cells within an in vitro cartilage defect repair model. Ann Biomed Eng. 2009;37(12):2556–65. doi:10.1007/s10439-009-9791-1.
Diao H, Wang J, Shen C, et al. Improved cartilage regeneration utilizing mesenchymal stem cells in TGF-beta1 gene-activated scaffolds. Tissue Eng Part A. 2009;15(9):2687–98. doi:10.1089/ten.TEA.2008.0621.
Eslaminejad MB, Taghiyar L, Falahi F. Co-culture of mesenchymal stem cells with mature chondrocytes: producing cartilage construct for application in cartilage regeneration. Iran J Med Sci. 2015;34(4):251–8.
Schulz RM, Zscharnack M, Hanisch I, Geiling M, Hepp P, Bader A. Cartilage tissue engineering by collagen matrix associated bone marrow derived mesenchymal stem cells. Biomed Mater Eng. 2008;18(1 Suppl):S55–70. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18334724. Accessed 16 June 2016.
Hannouche D, Terai H, Fuchs JR, et al. Engineering of implantable cartilaginous structures from bone marrow-derived mesenchymal stem cells. Tissue Eng. 2007;13(1):87–99. doi:10.1089/ten.2006.0067.
Hegewald AA, Ringe J, Bartel J, et al. Hyaluronic acid and autologous synovial fluid induce chondrogenic differentiation of equine mesenchymal stem cells: a preliminary study. Tissue Cell. 2004;36(6):431–8. doi:10.1016/j.tice.2004.07.003.
Worster AA, Brower-Toland BD, Fortier LA, Bent SJ, Williams J, Nixon AJ. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-beta1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J Orthop Res. 2001;19(4):738–49. doi:10.1016/S0736-0266(00)00054-1.
Angele P, Kujat R, Nerlich M, Yoo J, Goldberg V, Johnstone B. Engineering of osteochondral tissue with bone marrow mesenchymal progenitor cells in a derivatized hyaluronan-gelatin composite sponge. 2007. http://dx.doi.org/101089/ten19995545. Accessed June 2016.
Ding X, Zhu M, Xu B, et al. Integrated trilayered silk fibroin scaffold for osteochondral differentiation of adipose-derived stem cells. ACS Appl Mater Interfaces. 2014;6(19):16696–705. doi:10.1021/am5036708.
Lu C-H, Lin K-J, Chiu H-Y, et al. Improved chondrogenesis and engineered cartilage formation from TGF-β3-expressing adipose-derived stem cells cultured in the rotating-shaft bioreactor. Tissue Eng Part A. 2012;18(19-20):2114–24. doi:10.1089/ten.TEA.2012.0010.
Gong Z, Xiong H, Long X, et al. Use of synovium-derived stromal cells and chitosan/collagen type I scaffolds for cartilage tissue engineering. Biomed Mater. 2010;5(5):55005. doi:10.1088/1748-6041/5/5/055005.
Varshney RR, Zhou R, Hao J, et al. Chondrogenesis of synovium-derived mesenchymal stem cells in gene-transferred co-culture system. Biomaterials. 2010;31(26):6876–91. doi:10.1016/j.biomaterials.2010.05.038.
Han Y, Wei Y, Wang S, Song Y. Cartilage regeneration using adipose-derived stem cells and the controlled-released hybrid microspheres. Joint Bone Spine. 2010;77(1):27–31. doi:10.1016/j.jbspin.2009.05.013.
Wei Y, Hu H, Wang H, Wu Y, Deng L, Qi J. Cartilage regeneration of adipose-derived stem cells in a hybrid scaffold from fibrin-modified PLGA. Cell Transplant. 2009;18(2):159–70. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19499704. Accessed 22 June 2016.
Han Y, Wei Y, Wang S, Song Y. Regenerative and technological section enhanced chondrogenesis of adipose-derived stem cells by the controlled release of transforming growth factor-NL 1 from hybrid microspheres. Gerontology. 2009;55:592–9. doi:10.1159/000235547.
Wei Y, Hu Y, Hao W, et al. A novel injectable scaffold for cartilage tissue engineering using adipose-derived adult stem cells. J Orthop Res. 2008;26(1):27–33. doi:10.1002/jor.20468.
Wei Y, Hu Y, Lv R, Li D. Regulation of adipose-derived adult stem cells differentiating into chondrocytes with the use of rhBMP-2. Cytotherapy. 2006;8(6):570–9. doi:10.1080/14653240600987690.
An C, Cheng Y, Yuan Q, Li J. IGF-1 and BMP-2 induces differentiation of adipose-derived mesenchymal stem cells into chondrocytes-like cells. Ann Biomed Eng. 2010;38(4):1647–54. doi:10.1007/s10439-009-9892-x.
Lee CSD, Watkins E, Burnsed OA, Schwartz Z, Boyan BD. Tailoring adipose stem cell trophic factor production with differentiation medium components to regenerate chondral defects. Tissue Eng Part A. 2013;19(11-12):1451–64. doi:10.1089/ten.TEA.2012.0233.
Froelich K, Setiawan LE, Technau A, et al. Influence of different growth factors on chondrogenic differentiation of adipose-derived stem cells in polyurethane-fibrin composites. Int J Artif Organs. 2012;35(12):1047–60. doi:10.5301/ijao.5000132.
Buckley CT, Vinardell T, Thorpe SD, et al. Functional properties of cartilaginous tissues engineered from infrapatellar fat pad-derived mesenchymal stem cells. J Biomech. 2010;43(5):920–6. doi:10.1016/j.jbiomech.2009.11.005.
Feng G, Wan Y, Balian G, Laurencin CT, Li X. Adenovirus-mediated expression of growth and differentiation factor-5 promotes chondrogenesis of adipose stem cells. Growth Factors. 2008;26(3):132–42. doi:10.1080/08977190802105917.
Park Y, Sugimoto M, Watrin A, Chiquet M, Hunziker EB. BMP-2 induces the expression of chondrocyte-specific genes in bovine synovium-derived progenitor cells cultured in three-dimensional alginate hydrogel. Osteoarthritis Cartilage. 2005;13(6):527–36. doi:10.1016/j.joca.2005.02.006.
Steinert A, Weber M, Dimmler A, et al. Chondrogenic differentiation of mesenchymal progenitor cells encapsulated in ultrahigh-viscosity alginate. J Orthop Res. 2003;21(6):1090–7. doi:10.1016/S0736-0266(03)00100-1.
Marquass B, Somerson JS, Hepp P, et al. A novel MSC-seeded triphasic construct for the repair of osteochondral defects. J Orthop Res. 2010;28(12):1586–99. doi:10.1002/jor.21173.
Lee KBL, Hui JHP, Song IC, Ardany L, Lee EH. Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model. Stem Cells. 2007;25(11):2964–71. doi:10.1634/stemcells.2006-0311.
Zhou XZ, Leung VY, Dong QR, Cheung KM, Chan D, Lu WW. Mesenchymal stem cell-based repair of articular cartilage with polyglycolic acid-hydroxyapatite biphasic scaffold. Int J Artif Organs. 2008;31(6):480–9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18609500. Accessed 12 June 2016.
Shao X, Goh JCH, Hutmacher DW, Lee EH, Zigang G. Repair of large articular osteochondral defects using hybrid scaffolds and bone marrow-derived mesenchymal stem cells in a rabbit model. Tissue Eng. 2006;12(6):1539–51. doi:10.1089/ten.2006.12.1539.
Zhang Y, Wang F, Chen J, Ning Z, Yang L. Bone marrow-derived mesenchymal stem cells versus bone marrow nucleated cells in the treatment of chondral defects. Int Orthop. 2012;36(5):1079–86. doi:10.1007/s00264-011-1362-z.
Zhu S, Zhang B, Man C, Ma Y, Liu X, Hu J. Combined effects of connective tissue growth factor-modified bone marrow-derived mesenchymal stem cells and NaOH-treated PLGA scaffolds on repair of articular cartilage defect in rabbits. Cell Transplant. 2013. doi:10.3727/096368913X6697790.
Oshima Y, Watanabe N, Matsuda K, Takai S, Kawata M, Kubo T. Behavior of transplanted bone marrow-derived GFP mesenchymal cells in osteochondral defect as a simulation of autologous transplantation. J Histochem Cytochem. 2005;53(2):207–16. doi:10.1369/jhc.4A6280.2005.
Lim CT, Ren X, Afizah MH, et al. Repair of osteochondral defects with rehydrated freeze-dried oligo[poly(ethylene glycol) fumarate] hydrogels seeded with bone marrow mesenchymal stem cells in a porcine model. Tissue Eng Part A. 2013;19(15-16):1852–61. doi:10.1089/ten.TEA.2012.0621.
Li T, Nina F, Xiaozuo T, Xiaopeng L, Zhuo W, Na L. Chondrogenic differentiation of mesenchymal stem cells for repairing articular cartilage. J Clin Rehabil Tissue Eng Res. 2009;13(46):9041–4.
Sato M, Uchida K, Nakajima H, et al. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res Ther. 2012;14(1):R31. doi:10.1186/ar3735.
Ivkovic A, Pascher A, Hudetz D, et al. Articular cartilage repair by genetically modified bone marrow aspirate in sheep. Gene Ther. 2010;17(6):779–89. doi:10.1038/gt.2010.16.
Song F, Tang J, Geng R, et al. Comparison of the efficacy of bone marrow mononuclear cells and bone mesenchymal stem cells in the treatment of osteoarthritis in a sheep model. Int J Clin Exp Pathol. 2014;7(4):1415–26. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24817937. Accessed 12 June 2016.
Ishihara K, Nakayama K, Akieda S, Matsuda S, Iwamoto Y. Simultaneous regeneration of full-thickness cartilage and subchondral bone defects in vivo using a three-dimensional scaffold-free autologous construct derived from high-density bone marrow-derived mesenchymal stem cells. J Orthop Surg Res. 2014;9:98. doi:10.1186/s13018-014-0098-z.
Wang W, Li B, Li Y, Jiang Y, Ouyang H, Gao C. In vivo restoration of full-thickness cartilage defects by poly(lactide-co-glycolide) sponges filled with fibrin gel, bone marrow mesenchymal stem cells and DNA complexes. Biomaterials. 2010;31(23):5953–65. doi:10.1016/j.biomaterials.2010.04.029.
Xue D, Zheng Q, Zong C, et al. Osteochondral repair using porous poly(lactide-co-glycolide)/nano-hydroxyapatite hybrid scaffolds with undifferentiated mesenchymal stem cells in a rat model. J Biomed Mater Res A. 2010;94(1):259–70. doi:10.1002/jbm.a.32691.
Wayne JS, McDowell CL, Shields KJ, Tuan RS. In vivo response of polylactic acid-alginate scaffolds and bone marrow-derived cells for cartilage tissue engineering. Tissue Eng. 11(5-6):953-63. doi:10.1089/ten.2005.11.953.
Bal BS, Rahaman MN, Jayabalan P, et al. In vivo outcomes of tissue-engineered osteochondral grafts. J Biomed Mater Res B Appl Biomater. 2010;93(1):164–74. doi:10.1002/jbm.b.31571.
Qi Y, Du Y, Li W, Dai X, Zhao T, Yan W. Cartilage repair using mesenchymal stem cell (MSC) sheet and MSCs-loaded bilayer PLGA scaffold in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1424–33. doi:10.1007/s00167-012-2256-3.
Zhou G, Liu W, Cui L, Wang X, Liu T, Cao Y. Repair of porcine articular osteochondral defects in non-weightbearing areas with autologous bone marrow stromal cells. Tissue Eng. 2006;12(11):3209–21. doi:10.1089/ten.2006.12.3209.
Fan H, Hu Y, Qin L, Li X, Wu H, Lv R. Porous gelatin-chondroitin-hyaluronate tri-copolymer scaffold containing microspheres loaded with TGF-beta1 induces differentiation of mesenchymal stem cells in vivo for enhancing cartilage repair. J Biomed Mater Res A. 2006;77(4):785–94. doi:10.1002/jbm.a.30647.
Fan H, Hu Y, Zhang C, et al. Cartilage regeneration using mesenchymal stem cells and a PLGA-gelatin/chondroitin/hyaluronate hybrid scaffold. Biomaterials. 2006;27(26):4573–80. doi:10.1016/j.biomaterials.2006.04.013.
Nathan S, De Das S, Thambyah A, Fen C, Goh J, Lee EH. Cell-based therapy in the repair of osteochondral defects: a novel use for adipose tissue. Tissue Eng. 2003;9(4):733–44. doi:10.1089/107632703768247412.
Diduch DR, Jordan LC, Mierisch CM, Balian G. Marrow stromal cells embedded in alginate for repair of osteochondral defects. Arthroscopy. 2000;16(6):571–7. doi:10.1053/jars.2000.4827.
Grigolo B, Lisignoli G, Desando G, et al. Osteoarthritis treated with mesenchymal stem cells on hyaluronan-based scaffold in rabbit. Tissue Eng Part C Methods. 2009;15(4):647–58. doi:10.1089/ten.TEC.2008.0569.
Cui JH, Park SR, Park K, Choi BH, Min B-H. Preconditioning of mesenchymal stem cells with low-intensity ultrasound for cartilage formation in vivo. Tissue Eng. 2007;13(2):351–60. doi:10.1089/ten.2006.0080.
Løken S, Jakobsen RB, Arøen A, et al. Bone marrow mesenchymal stem cells in a hyaluronan scaffold for treatment of an osteochondral defect in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2008;16(10):896–903. doi:10.1007/s00167-008-0566-2.
Tan W, Zha Z, Zhang J, Zheng L, Liang Y XJ. Animal-origin osteochondral scaffold combined with bone marrow mesenchymal stem cells/chondrocytes for repair of composite osteochondral defects in rabbit knee joints. J Clin Rehabil Tissue Eng Res. 2011;15(12). doi:10.3969/j.issn.1673-8225.2011.12.043.
Hu B, Ren J-L, Zhang J-R, Ma Q, Liu Y-P, Mao T-Q. Enhanced treatment of articular cartilage defect of the knee by intra-articular injection of Bcl-xL-engineered mesenchymal stem cells in rabbit model. J Tissue Eng Regen Med. 2010;4(2):105–14. doi:10.1002/term.212.
Xie J, Han Z, Naito M, et al. Articular cartilage tissue engineering based on a mechano-active scaffold made of poly(L-lactide-co-epsilon-caprolactone): in vivo performance in adult rabbits. J Biomed Mater Res B Appl Biomater. 2010;94(1):80–8. doi:10.1002/jbm.b.31627.
Wakitani S, Goto T, Pineda SJ, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76(4):579–92. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8150826. Accessed 12 June 2016.
Jeong W-K, Oh S-H, Lee J-H, Im G-I. Repair of osteochondral defects with a construct of mesenchymal stem cells and a polydioxanone/poly(vinyl alcohol) scaffold. Biotechnol Appl Biochem. 2008;49(Pt 2):155–64. doi:10.1042/BA20070149.
Im GI, Kim DY, Shin JH, Hyun CW, Cho WH. Repair of cartilage defect in the rabbit with cultured mesenchymal stem cells from bone marrow. J Bone Joint Surg Br. 2001;83(2):289–94. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11284583. Accessed 12 June 2016.
Kayakabe M, Tsutsumi S, Watanabe H, Kato Y, Takagishi K. Transplantation of autologous rabbit BM-derived mesenchymal stromal cells embedded in hyaluronic acid gel sponge into osteochondral defects of the knee. Cytotherapy. 2006;8(4):343–53. doi:10.1080/14653240600845070.
Tatebe M, Nakamura R, Kagami H, Okada K, Ueda M. Differentiation of transplanted mesenchymal stem cells in a large osteochondral defect in rabbit. Cytotherapy. 2005;7(6):520–30. doi:10.1080/14653240500361350.
Yoshioka T, Mishima H, Sakai S, Uemura T. Long-term results of cartilage repair after allogeneic transplantation of cartilaginous aggregates formed from bone marrow-derived cells for large osteochondral defects in rabbit knees. Cartilage. 2013;4(4):339–44. doi:10.1177/1947603513494003.
Liu P-F, Guo L, Zhao D-W, et al. Study of human acellular amniotic membrane loading bone marrow mesenchymal stem cells in repair of articular cartilage defect in rabbits. Genet Mol Res. 2014;13(3):7992–8001. doi:10.4238/2014.September.29.12.
Jung M, Kaszap B, Redöhl A, et al. Enhanced early tissue regeneration after matrix-assisted autologous mesenchymal stem cell transplantation in full thickness chondral defects in a minipig model. Cell Transplant. 2009;18(8):923–32. doi:10.3727/096368909X471297.
Chang C-H, Kuo T-F, Lin F-H, et al. Tissue engineering-based cartilage repair with mesenchymal stem cells in a porcine model. J Orthop Res. 2011;29(12):1874–80. doi:10.1002/jor.21461.
Kamei G, Kobayashi T, Ohkawa S, et al. Articular cartilage repair with magnetic mesenchymal stem cells. Am J Sports Med. 2013;41(6):1255–64. doi:10.1177/0363546513483270.
Coleman RM, Schwartz Z, Boyan BD, Guldberg RE. The therapeutic effect of bone marrow-derived stem cell implantation after epiphyseal plate injury is abrogated by chondrogenic predifferentiation. Tissue Eng Part A. 2013;19(3-4):475–83. doi:10.1089/ten.TEA.2012.0125.
Dahlin RL, Kinard LA, Lam J, et al. Articular chondrocytes and mesenchymal stem cells seeded on biodegradable scaffolds for the repair of cartilage in a rat osteochondral defect model. Biomaterials. 2014;35(26):7460–9. doi:10.1016/j.biomaterials.2014.05.055.
Marquass B, Schulz R, Hepp P, et al. Matrix-associated implantation of predifferentiated mesenchymal stem cells versus articular chondrocytes: in vivo results of cartilage repair after 1 year. Am J Sports Med. 2011;39(7):1401–12. doi:10.1177/0363546511398646.
Zscharnack M, Hepp P, Richter R, et al. Repair of chronic osteochondral defects using predifferentiated mesenchymal stem cells in an ovine model. Am J Sports Med. 2010;38(9):1857–69. doi:10.1177/0363546510365296.
Guo X, Wang C, Zhang Y, et al. Repair of large articular cartilage defects with implants of autologous mesenchymal stem cells seeded into beta-tricalcium phosphate in a sheep model. Tissue Eng. 10(11-12):1818-29. doi:10.1089/ten.2004.10.1818.
Caminal M, Moll X, Codina D, et al. Transitory improvement of articular cartilage characteristics after implantation of polylactide:polyglycolic acid (PLGA) scaffolds seeded with autologous mesenchymal stromal cells in a sheep model of critical-sized chondral defect. Biotechnol Lett. 2014;36(10):2143–53. doi:10.1007/s10529-014-1585-3.
Wilke MM, Nydam DV, Nixon AJ. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J Orthop Res. 2007;25(7):913–25. doi:10.1002/jor.20382.
Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48(12):3464–74. doi:10.1002/art.11365.
Ma A, Jiang L, Song L, et al. Reconstruction of cartilage with clonal mesenchymal stem cell-acellular dermal matrix in cartilage defect model in nonhuman primates. Int Immunopharmacol. 2013;16(3):399–408. doi:10.1016/j.intimp.2013.02.005.
Araki S, Imai S, Ishigaki H, et al. Improved quality of cartilage repair by bone marrow mesenchymal stem cells for treatment of an osteochondral defect in a cynomolgus macaque model. Acta Orthop. 2015;86(1):119–26. doi:10.3109/17453674.2014.958807.
Qi B, Yu A, Zhu S, Zhou M, Wu G. Chitosan/poly(vinyl alcohol) hydrogel combined with Ad-hTGF-β1 transfected mesenchymal stem cells to repair rabbit articular cartilage defects. Exp Biol Med (Maywood). 2013;238(1):23–30. doi:10.1258/ebm.2012.012223.
Qi Y, Zhao T, Xu K, Dai T, Yan W. The restoration of full-thickness cartilage defects with mesenchymal stem cells (MSCs) loaded and cross-linked bilayer collagen scaffolds on rabbit model. Mol Biol Rep. 2012;39(2):1231–7. doi:10.1007/s11033-011-0853-8.
Park JS, Woo DG, Yang HN, et al. Chondrogenesis of human mesenchymal stem cells encapsulated in a hydrogel construct: neocartilage formation in animal models as both mice and rabbits. J Biomed Mater Res A. 2010;92(3):988–96. doi:10.1002/jbm.a.32341.
Liu Y, Shu XZ, Prestwich GD. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng. 2006;12(12):3405–16. doi:10.1089/ten.2006.12.3405.
Acknowledgements
None
Funding
There was no external funding for this work.
Availability of data and materials
Not applicable
Authors’ contributions
All authors were involved in the conception and design of the study or acquisition of the data or analysis and interpretation of the data and contributed to drafting the article or revising it critically for important intellectual content. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable
Ethics approval and consent to participate
Not applicable
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Goldberg, A., Mitchell, K., Soans, J. et al. The use of mesenchymal stem cells for cartilage repair and regeneration: a systematic review. J Orthop Surg Res 12, 39 (2017). https://doi.org/10.1186/s13018-017-0534-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-017-0534-y