Abstract
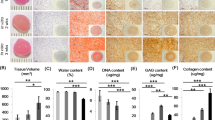
Integration of repair tissue is a key indicator of the long-term success of cell-based therapies for cartilage repair. The objective of this study was to compare the in vitro chondrogenic differentiation and integration of agarose hydrogels seeded with either chondrocytes or bone marrow-derived mesenchymal stem cells (MSCs) in defects created in cartilage explants. Chondrocytes and MSCs were isolated from porcine donors, suspended in 2% agarose and then injected into cylindrical defects within the explants. These constructs were maintained in a chemically defined medium supplemented with 10 ng/mL of TGF-β3. Cartilage integration was assessed by histology and mechanical push-out tests. After 6 weeks in culture, chondrocyte-seeded constructs demonstrated a higher integration strength (64.4 ± 8.3 kPa) compared to MSC-seeded constructs (22.7 ± 5.9 kPa). Glycosaminoglycan (GAG) (1.27 ± 0.3 vs. 0.19 ± 0.03 kPa) and collagen (0.31 ± 0.08 vs. 0.09 ± 0.01 kPa) accumulation in chondrocyte-seeded constructs was greater than that measured in the MSC-seeded group. The GAG, collagen, and DNA content of both chondrocyte- and MSC-seeded hydrogels cultured in cartilage explants was significantly lower than control constructs cultured in free swelling conditions. The results of this study suggest that the explant model may constitute a more rigorous in vitro test to assess MSC therapies for cartilage defect repair.






Similar content being viewed by others
References
Ahsan, T., and R. L. Sah. Biomechanics of integrative cartilage repair. Osteoarthr. Cartil. 7(1):29–40, 1999.
Ait Si Selmi, T., P. Neyret, P. C. M. Verdonk, and L. Barnouin. Autologous chondrocyte transplantation in combination with an alginate-agarose based hydrogel (Cartipatch). Tech. Knee Surg. 6(4):253–258, 2007.
Albro, M. B., N. O. Chahine, R. Li, K. Yeager, C. T. Hung, and G. A. Ateshian. Dynamic loading of deformable porous media can induce active solute transport. J. Biomech. 41(15):3152–3157, 2008.
Awad, H. A., M. Q. Wickham, H. A. Leddy, J. M. Gimble, and F. Guilak. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials 25(16):3211–3222, 2004.
Barry, F., R. E. Boynton, B. Liu, and J. M. Murphy. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp. Cell Res. 268(2):189–200, 2001.
Boon, C. H., T. Cao, and H. L. Eng. Directing stem cell differentiation into the chondrogenic lineage in vitro. Stem Cells 22(7):1152–1167, 2004.
Bos, P. K., J. DeGroot, M. Budde, J. A. Verhaar, and G. J. van Osch. Specific enzymatic treatment of bovine and human articular cartilage: implications for integrative cartilage repair. Arthritis Rheum. 46(4):976–985, 2002.
Brittberg, M., A. Lindahl, A. Nilsson, C. Ohlsson, O. Isaksson, and L. Peterson. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 331(14):889–895, 1994.
Bruder, S. P., N. Jaiswal, and S. E. Haynesworth. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J. Cell. Biochem. 64(2):278–294, 1997.
Campbell, J. J., D. A. Lee, and D. L. Bader. Dynamic compressive strain influences chondrogenic gene expression in human mesenchymal stem cells. Biorheology 43(3–4):455–470, 2006.
Caplan, A. I. Mesenchymal stem cells. J. Orthop. Res. 9(5):641–650, 1991.
Dhert, W. J., C. C. Verheyen, L. H. Braak, J. R. de Wijn, C. P. Klein, K. de Groot, and P. M. Rozing. A finite element analysis of the push-out test: influence of test conditions. J. Biomed. Mater. Res. 26(1):119–130, 1992.
Elder, B. D., and K. A. Athanasiou. Effects of confinement on the mechanical properties of self-assembled articular cartilage constructs in the direction orthogonal to the confinement surface. J. Orthop. Res. 26(2):238–246, 2008.
Erickson, I. E., A. H. Huang, C. Chung, R. T. Li, J. A. Burdick, and R. L. Mauck. Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Eng. Part A 15(5):1041–1052, 2009.
Gratz, K. R., V. W. Wong, A. C. Chen, L. A. Fortier, A. J. Nixon, and R. L. Sah. Biomechanical assessment of tissue retrieved after in vivo cartilage defect repair: tensile modulus of repair tissue and integration with host cartilage. J. Biomech. 39(1):138–146, 2006.
Horas, U., D. Pelinkovic, G. Herr, T. Aigner, and R. Schnettler. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J. Bone Joint Surg. Am. 85(2):185–192, 2003.
Huang, C. Y. C., K. L. Hagar, L. E. Frost, Y. Sun, and H. S. Cheung. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells 22(3):313–323, 2004.
Huang, C. Y. C., P. M. Reuben, G. D’Ppolito, P. C. Schiller, and H. S. Cheung. Chondrogenesis of human bone marrow-derived mesenchymal stem cells in agarose culture. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 278(1):428–436, 2004.
Hunter, C. J., and M. E. Levenston. The influence of repair tissue maturation on the response to oscillatory compression in a cartilage defect repair model. Biorheology 39(1–2):79–88, 2002.
Hunter, C. J., and M. E. Levenston. Maturation and integration of tissue-engineered cartilages within an in vitro defect repair model. Tissue Eng. 10(5–6):736–746, 2004.
Ignat’eva, N. Y., N. A. Danilov, S. V. Averkiev, M. V. Obrezkova, V. V. Lunin, and E. N. Sobol. Determination of hydroxyproline in tissues and the evaluation of the collagen content of the tissues. J. Anal. Chem. 62(1):51–57, 2007.
Johnson, T. S., J. W. Xu, V. V. Zaporojan, J. M. Mesa, C. Weinand, M. A. Randolph, L. J. Bonassar, J. M. Winograd, and M. J. Yaremchuk. Integrative repair of cartilage with articular and nonarticular chondrocytes. Tissue Eng. 10(9–10):1308–1315, 2004.
Johnstone, B., T. M. Hering, A. I. Caplan, V. M. Goldberg, and J. U. Yoo. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 238(1):265–272, 1998.
Kadiyala, S., R. G. Young, M. A. Thiede, and S. P. Bruder. Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro. Cell Transplant. 6(2):125–134, 1997.
Kafienah, W., and T. J. Sims. Biochemical methods for the analysis of tissue-engineered cartilage. Methods Mol. Biol. 238:217–230, 2004.
Kim, Y. J., R. L. Sah, J. Y. Doong, and A. J. Grodzinsky. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal. Biochem. 174(1):168–176, 1988.
Kuroda, R., K. Ishida, T. Matsumoto, T. Akisue, H. Fujioka, K. Mizuno, H. Ohgushi, S. Wakitani, and M. Kurosaka. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthr. Cartil. 15(2):226–231, 2007.
Leddy, H. A., and F. Guilak. Site-specific molecular diffusion in articular cartilage measured using fluorescence recovery after photobleaching. Ann. Biomed. Eng. 31(7):753–760, 2003.
Lennon, D. P., and A. I. Caplan. Isolation of human marrow-derived mesenchymal stem cells. Exp. Hematol. 34(11):1604–1605, 2006.
Li, W. J., R. Tuli, C. Okafor, A. Derfoul, K. G. Danielson, D. J. Hall, and R. S. Tuan. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 26(6):599–609, 2005.
Lu, Z. F., B. Zandieh Doulabi, P. I. Wuisman, R. A. Bank, and M. N. Helder. Differentiation of adipose stem cells by nucleus pulposus cells: Configuration effect. Biochem. Biophys. Res. Commun. 359(4):991–996, 2007.
Maniatopoulos, C., J. Sodek, and A. H. Melcher. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 254(2):317–330, 1988.
Marcacci, M., E. Kon, S. Zaffagnini, G. Filardo, M. Delcogliano, M. P. Neri, F. Iacono, and A. P. Hollander. Arthroscopic second generation autologous chondrocyte implantation. Knee Surg. Sports Traumatol. Arthrosc. 15(5):610–619, 2007.
Maroudas, A. Distribution and diffusion of solutes in articular cartilage. Biophys. J. 10(5):365–379, 1970.
Mauck, R. L., C. T. Hung, and G. A. Ateshian. Modeling of neutral solute transport in a dynamically loaded porous permeable gel: implications for articular cartilage biosynthesis and tissue engineering. J. Biomech. Eng. 125(5):602–614, 2003.
Mauck, R. L., X. Yuan, and R. S. Tuan. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthr. Cartil. 14(2):179–189, 2006.
Moretti, M., D. Wendt, D. Schaefer, M. Jakob, E. B. Hunziker, M. Heberer, and I. Martin. Structural characterization and reliable biomechanical assessment of integrative cartilage repair. J. Biomech. 38(9):1846–1854, 2005.
Mouw, J. K., J. T. Connelly, C. G. Wilson, K. E. Michael, and M. E. Levenston. Dynamic compression regulates the expression and synthesis of chondrocyte-specific matrix molecules in bone marrow stromal cells. Stem Cells 25(3):655–663, 2007.
Ni, Y. F., X. F. Li, Y. Liu, Z. J. Lei, and Q. Lu. In vivo chondrogenesis by co-culture of rabbit bone marrow-derived mesenchymal stem cells and chondrocytes. J. Clin. Rehabil. Tissue Eng. Res. 12(16):3185–3188, 2008.
Obradovic, B., I. Martin, R. F. Padera, S. Treppo, L. E. Freed, and G. Vunjak-Novakovic. Integration of engineered cartilage. J. Orthop. Res. 19(6):1089–1097, 2001.
Pedrozo, H. A., Z. Schwartz, R. Gomez, A. Ornoy, W. Xin-Sheng, S. L. Dallas, L. F. Bonewald, D. D. Dean, and B. D. Boyan. Growth plate chondrocytes store latent transforming growth factor (TGF)-β1 in their matrix through latent TGF-β1 binding protein-1. J. Cell. Physiol. 177(2):343–354, 1998.
Pelttari, K., E. Steck, and W. Richter. The use of mesenchymal stem cells for chondrogenesis. Injury 39(Suppl. 1):S58–65, 2008.
Peretti, G. M., L. J. Bonassar, E. M. Caruso, M. A. Randolph, C. A. Trahan, and D. J. Zaleske. Biomechanical analysis of a chondrocyte-based repair model of articular cartilage. Tissue Eng. 5(4):317–326, 1999.
Peterson, L., T. Minas, M. Brittberg, A. Nilsson, E. Sjögren-Jansson, and A. Lindahl. Two-to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin. Orthop. Relat. Res. 374:212–234, 2000.
Pittenger, M. F., A. M. Mackay, S. C. Beck, R. K. Jaiswal, R. Douglas, J. D. Mosca, M. A. Moorman, D. W. Simonetti, S. Craig, and D. R. Marshak. Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147, 1999.
Richardson, S. M., R. V. Walker, S. Parker, N. P. Rhodes, J. A. Hunt, A. J. Freemont, and J. A. Hoyland. Intervertebral disc cell-mediated mesenchymal stem cell differentiation. Stem Cells 24(3):707–716, 2006.
Ruoslahti, E., and Y. Yamaguchi. Proteoglycans as modulators of growth factor activities. Cell 64(5):867–869, 1991.
Schneiderman, R., E. Snir, O. Popper, J. Hiss, H. Stein, and A. Maroudas. Insulin-like growth factor-I and its complexes in normal human articular cartilage: studies of partition and diffusion. Arch. Biochem. Biophys. 324(1):159–172, 1995.
Selmi, T. A. S., P. Verdonk, P. Chambat, F. Dubrana, J. F. Potel, L. Barnouin, and P. Neyret. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J. Bone Joint Surg. Br. 90(5):597–604, 2008.
Silverman, R. P., L. Bonasser, D. Passaretti, M. A. Randolph, and M. J. Yaremchuk. Adhesion of tissue-engineered cartilage to native cartilage. Plast. Reconstr. Surg. 105(4):1393–1398, 2000.
Tam, H. K., A. Srivastava, C. W. Colwell, Jr., and D. D. D’Lima. In vitro model of full-thickness cartilage defect healing. J. Orthop. Res. 25(9):1136–1144, 2007.
Thorpe, S. D., C. T. Buckley, T. Vinardell, F. J. O’Brien, V. A. Campbell, and D. J. Kelly. Dynamic compression can inhibit chondrogenesis of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 377(2):458–462, 2008.
Tognana, E., F. Chen, R. F. Padera, H. A. Leddy, S. E. Christensen, F. Guilak, G. Vunjak-Novakovic, and L. E. Freed. Adjacent tissues (cartilage, bone) affect the functional integration of engineered calf cartilage in vitro. Osteoarthr. Cartil. 13(2):129–138, 2005.
Torzilli, P. A., T. C. Adams, and R. J. Mis. Transient solute diffusion in articular cartilage. J. Biomech. 20(2):203–214, 1987.
Torzilli, P. A., J. M. Arduino, J. D. Gregory, and M. Bansal. Effect of proteoglycan removal on solute mobility in articular cartilage. J. Biomech. 30(9):895–902, 1997.
Tsuchiya, K., G. Chen, T. Ushida, T. Matsuno, and T. Tateishi. The effect of coculture of chondrocytes with mesenchymal stem cells on their cartilaginous phenotype in vitro. Mater. Sci. Eng. C 24(3):391–396, 2004.
Wakitani, S., M. Nawata, K. Tensho, T. Okabe, H. Machida, and H. Ohgushi. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J. Tissue Eng. Regen. Med. 1(1):74–79, 2007.
Williams, C. G., T. K. Kim, A. Taboas, A. Malik, P. Manson, and J. Elisseeff. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 9(4):679–688, 2003.
Worster, A. A., B. D. Brower-Toland, L. A. Fortier, S. J. Bent, J. Williams, and A. J. Nixon. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-β1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J. Orthop. Res. 19(4):738–749, 2001.
Yamaguchi, Y., D. M. Mann, and E. Ruoslahti. Negative regulation of transforming growth factor-β by the proteoglycan decorin. Nature 346(6281):281–284, 1990.
Acknowledgment
Funding was provided by Science Foundation Ireland (President of Ireland Young Researcher Award—08/YI5/B1336).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vinardell, T., Thorpe, S.D., Buckley, C.T. et al. Chondrogenesis and Integration of Mesenchymal Stem Cells Within an In Vitro Cartilage Defect Repair Model. Ann Biomed Eng 37, 2556–2565 (2009). https://doi.org/10.1007/s10439-009-9791-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-009-9791-1