Abstract
Background
Concomitant chemo-radiotherapy is the reference treatment for non-resectable locally-advanced Non-Small Cell Lung Cancer (NSCLC). Increasing radiotherapy total dose in the whole tumour volume has been shown to be deleterious. Functional imaging with positron emission tomography (PET/CT) offers the potential to identify smaller and biologically meaningful target volumes that could be irradiated with larger doses without compromising Organs At Risk (OAR) tolerance. This study investigated four scenarios, based on 18FDG and 18F-miso PET/CT, to delineate the target volumes and derive radiotherapy plans delivering up to 74Gy.
Method
Twenty-one NSCLC patients, selected from a prospective phase II trial, had 18FDG- and 18F-miso PET/CT before the start of radiotherapy and 18FDG PET/CT during the radiotherapy (42Gy). The plans were based planned on a standard plan delivering 66 Gy (plan 1) and on three different boost strategies to deliver 74Gy total dose in pre-treatment 18FDG hotspot (70% of SUVmax) (plan 2), pre-treatment 18F-miso target (SUVmax > 1.4) (plan 3) and per-treatment 18FDG residual (40% of SUVmax). (plan 4).
Results
The mean target volumes were 4.8 cc (± 1.1) for 18FDG hotspot, 38.9 cc (± 14.5) for 18F-miso and 36.0 cc (± 10.1) for per-treatment 18FDG. In standard plan (66 Gy), the mean dose covering 95% of the PTV (D95%) were 66.5 (± 0.33), 66.1 (± 0.32) and 66.1 (± 0.32) Gy for 18FDG hotspot, 18F-miso and per-treatment 18FDG. In scenario 2, the mean D95% was 72.5 (± 0.25) Gy in 18FDG hotspot versus 67.9 (± 0.49) and 67.9 Gy (± 0.52) in 18F-miso and per-treatment 18FDG, respectively. In scenario 3, the mean D95% was 72.2 (± 0.27) Gy to 18F-miso versus 70.4 (± 0.74) and 69.5Gy (± 0.74) for 18FDG hotspot and per-treatment 18FDG, respectively. In scenario 4, the mean D95% was 73.1 (± 0.3) Gy to 18FDG per-treatment versus 71.9 (± 0.61) and 69.8 (± 0.61) Gy for 18FDG hotspot and 18F-miso, respectively. The dose/volume constraints to OARs were matched in all scenarios.
Conclusion
Escalated doses can be selectively planned in NSCLC target volumes delineated on 18FDG and 18F-miso PET/CT functional images. The most relevant strategy should be investigated in clinical trials.
Trial registration
(RTEP5, NCT01576796, registered 15 june 2012)
Similar content being viewed by others
Background
Non-small cell lung cancer (NSCLC) is a deadly disease. The majority of non-metastatic NSCLC cannot undergo surgical resection with curative-intent, either due to the patient’s medical condition or to cancer local extension. Concomitant Radiotherapy - Chemotherapy (RTCT) is the standard curative-intent treatment for non-operable patients/non resectable cancers [1]. Large efforts in radiotherapy techniques are made to improve tumour control and survival [1, 2]. A total dose > 60 Gy to the entire tumour volume defined on CT or PET was deleterious in the RTOG 0617 randomized trial [3]. Many relapses occur within the radiotherapy target volume, suggesting insufficient total dose [4, 5]. Reducing the target volume to high recurrence risk areas is assumed to allow isotoxic dose escalation. Experimental and clinical data have shown that tumour subvolumes defined by high 18F-FDG (metabolic hotspot) or 18F-misonidazole (18F-miso) uptake are associated to recurrence and cancer death [6].
In a phase II study (NCT01576796, RTEP5 study), we used 18F-miso to identify and delineate hypoxic areas within the 18FDG-defined Gross Target Volume (GTV) [7]. The total radiotherapy dose was safely increased in 24 out of 34 patients with 18F-miso uptake. Doses up to 86 Gy could not reverse the poor prognosis features of 18F-miso positive tumours. In this study, hypoxic tumours with boost had the same local control despite twice as large volumes. The patients’ data were used to test in silico three strategies for selective increase in total dose, based on functional imaging. For the strategies with boost, we tested at a dose of 74 Gy to be comparable to the study of Bradley et al. [3] and our current phase II/III (RTEP7; NCT02473133). In 21 patients, a standard plan (66 Gy to whole Planning Target Volume PTV plan 1) to 74 Gy was compared to the pre-RT 18FDG metabolic hotspot (70% of SUVmax) (plan 2), 2/ the pre-RT 18F-MISO-affine (SUV > 1.4) volume (plan 3) or the per-treatment 18FDG uptake (40% of SUVmax) (plan 4).
Methods
Study design and patients
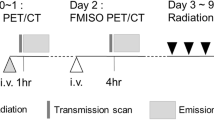
The details of the study can be found elsewhere [7]. Fifty-four patients with NSCLC, eligible for curative-intent RTCT, and with significant FDG uptake on pre-RT PET/CT were prospectively selected. The 21 patients with significant per-RT F-miso uptake and meeting the dose/volume constraints for the organs at risk (OAR) form the basis of the present study (Fig. 1). All the patients had signed a written consent to participate to RTEP5.
PET/CT imaging
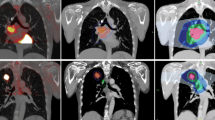
Two 18FDG PET/CT (FDG1 and FDG2) and two 18F-miso PET/CT (F-miso1 and F-miso1) acquisitions were performed before and during RT using the same machine and under the identical operational conditions, a centrally supervised quality control securing homogeneity in the image quality in all centres [7]. FDG1 was acquired in treatment position (arms over the head, free breathing), at least 15 days after the last administration of chemotherapy. F-miso1 was scheduled 48 h after the FDG1. The F-miso PET/CT acquisitions were reviewed by 3 independent experts (out of the 9 experts) who decided upon the presence or absence of uptake [8]. The CT scan images were used to register all PET/CT acquisitions, delineate target volumes and plan radiotherapy. Respiratory-gated 4D acquisitions were not performed. No further chemotherapy was allowed between FDG1/F-miso1 and the start of radiotherapy. The F-miso2 was not associated with a planification due to the very low contrast in per-treatment [7].
Target volume definition
For each patient, the CTs of FDG1, FDG2 and F-miso1 were co-registered to the planning CT scanner (Oncoplanet, DosiSoft, France, v 1.4), focusing on the tumour. The GTV for FDG1 was defined as the sum of the voxels with uptake > 40% of the SUVmax inside primary tumour or nodes and corrected from CT data. The 18FDG hotspot was defined as all voxels with uptake > 70% of the SUVmax inside the primary tumour or nodes (BTVm1). The hypoxic volume (BTVh) was defined as the sum of voxels with SUV ≥1.4 on F-miso1 [7, 8]. The residual volume on FDG PET/CT2 (BTVm2) was defined as the sum of the voxels with uptake > 40% of the SUVmax inside the primary tumour or nodes. The co-registered volumes were then transferred to an Eclipse planning platform (V13.6, Varian Inc.). The CTV66 were obtained either by isotropic expansion around the primary tumour (6 mm for squamous cell carcinoma, 8 mm for adenocarcinoma), then manually edited to exclude the bones, the large vessels and heart, the muscle and the trachea, or by anatomical delineation of the involved nodal stations [9, 10]. The margins around the BTVs or CTVs to delineate the Planning Target Volume (PTV) were 10 to 15 mm. All delineation were performed by an experienced radiation oncologist (ST).
Planning scenarios
Four IMRT by step and shoot scenarios were applied in each patient. All the dose calculations were corrected for heterogeneity (V13.6, AAA v10.0.28 Varian Inc.) and for the optimization (DVO v10.0.28, Varian Inc). The total dose was prescribed at ICRU point, the dose delivered in the PTV having to be within 95% and 107% of the prescribed dose. Absolute priority constraints were a maximum dose to the spinal cord < 45 Gy and no more than 30% of the total lung volume (excluding the PTV) receiving ≥20 Gy (V20Gy). The secondary dose/volume constraints were no more than 30% of the oesophagus or the heart receiving ≥50 or ≥ 35 Gy, respectively.
The first scenario (standard or reference plan; plan 1) was to deliver 66 Gy in the PTV based on FDG1. The experimental scenarios had to deliver 66 Gy in the FDG1 PTV (PTV66) and an additional dose up to 74 Gy in three different smaller target volumes. The boost target volumes were the metabolic hotspot on pre-treatment FDG PET/CT (BTVm1, scenario 2), the hypoxic volume on pre-treatment F-miso PET/CT (BTVh, scenario 3), and the residual uptake on the FDG PET/CT at 42 Gy (BTVm2, scenario 4). The treatment was planned with a simultaneous boost from the start of the radiotherapy for plan 2 and 3 and from 50 Gy for the plan 4.
Statistical analyses
Descriptive statistics (n, mean, SE minimum and maximum) were calculated for the quantitative variables. Frequency and percentages with 95% confidence intervals (CI) were computed for the qualitative variables. Levene’s test was used to assess variances equality when comparing the quantitative variables means between two or more groups (ANOVA). All statistical calculations were performed with MedCalc Software (version16.2.0, Ostend, Belgium).
Results
Population
The present study was based on 21 patients (4 women and 20 men, mean age (±SE) = 59 ± 8 years). There were 7 adenocarcinomas, 12 squamous cell carcinomas and 2 undifferentiated carcinomas. The stages distribution was 1 IIB, 12 IIIA and 8 IIIB (Table 1).
Target volumes and dose distribution
The mean CTV66Gy was 244 ± 50 cc, larger than all BTVs. The 18FDG hotspot (BTV FDG 70%) smaller than the BTV FDG per-RT (4.8 ± 1.1 versus 36 ± 10 cc, p = 0.03) and the BTV hypoxic (39 ± 15 cc, p = 0.13). Similarly, the mean PTV66Gy was 473 ± 69 cc versus 35 ± 5.8 cc for PTV FDG 70%, 111 ± 24 cc for PTV FDG per-RT, and 105 ± 26 cc for PET F-miso (Table 1).
In scenarios 2, 3 and 4, the mean doses to the specific target volume was higher than the doses given to the other biological volumes or PTV 66 Gy. In scenario 2 (boost to 18FDG hotspot), the mean dose to 95% of the PTV (D95%) was 72.5 ((± 0.25) Gy versus 67.9 (± 0.49) (p < 0.0001) in F-miso PTV and 67.9 Gy (± 0.52) (p < 0.0001) in per-treatment 18FDG PTV. In scenario 3 (boost to 18F-miso), the mean D95% was 72.2 (± 0.27) Gy versus 70.4 (± 0.33) (p = 0.74) in 18FDG hotspot and 69.5 (± 0.74) Gy (p = 0.009) in per-treatment 18FDG PTV. In scenario 4 (boost to per-treatment 18FDG), the mean D95% was 73.1 (± 0.3) Gy versus 71.9 (± 0.61) (p = 0.2) in 18FDG hotspot and 69.8 (± 0.61) Gy (p = 0.0001) to FMISO PTV (Fig. 2). The results are presented for CTV (Table 2), PTV (Table 3) and Organs at Risk (Table 4).
The dose/volume constraints to the organs at risk were matched without significant differences between scenarios except to the heart between the plan to 66 Gy and FDG per-treatment plan with V35 at respectively 5% (± 1.7) and 5.4% (± 1.8) (p = 0.05) (Table 4).
Discussion
The present planning study confirms that selecting various sub-volumes to increase radiotherapy total dose results in different dose distributions. The data of 21 patients were retrieved from a prospective phase II study investigating the clinical feasibility of boosting the radiotherapy dose in tumour hypoxic areas delineated on 18F-miso PET/CT. Pre- and per-radiotherapy 18FDG allowed us to compare three different biologically-oriented strategies.
RTCT is the reference treatment for locally advanced non operable NSCLC. The high incidence of relapse within the target volume suggest insufficient total doses of radiotherapy to achieve local control. The RTOG 0617 randomized trial [3] demonstrated that an indiscriminate dose increase in all patients and to the entire 18FDG PET/CT volume was deleterious. The combination of functional information (metabolism or hypoxia) and improved radiotherapy delivery (IMRT) opens the way to selectively increase total dose in biologically-relevant parts of the tumour. In a previous study, we showed that areas of high 18FDG uptake (SUV > 70% SUVmax) on pre-treatment PET/CT scans were associated to tumour areas at greater risk of relapse [11]. Similar results have been reported in NSCLC [12] and in oesophageal cancer patients [13]. A European randomized phase II is currently investigating an integrated boost up to 72 Gy in the > 50% SUVmax volume delineated on pre-treatment 18FDG PET/CT (PET Boost; NCT01024829). We are presently conducting a phase II study (RTEP7; NCT02473133) where the radiotherapy dose is escalated up to 74 Gy in the metabolic residual as assessed on FDG-PET/CT performed at 42 Gy. Targeting the hypoxic volume as identified by 18F-miso PET/CT before or during radiotherapy was investigated in RTEP5 [7] and RTOG-1106 (NCT01507428) trials, respectively, as well as in head and neck cancer patients [14, 15].
The three scenarios investigated here yielded different target volumes for radiotherapy dose escalation. Our data contradict the idea that 18FDG uptake is associated to the presence of hypoxia via the upregulation of glucose transporter 1 by hypoxia-inducible factor 1 [16, 17]. 18FDG and 18F-miso provide different and possibly complementary information. Given the impact on dose distribution as observed here, the selection of the most relevant strategy will rely on clinical trials. In our RTEP5 phase II [7], the patients with significant 18F-miso uptake had worse disease-free and overall survival probabilities. This observation questions the assumption that hypoxia-related radio-resistance could be overcome by moderate additional doses targeted to the hypoxic volume. On the other hand, most of the dose escalation trials (including our RTEP5 phase II) achieved higher doses by adding several 2-Gy fractions to the reference irradiation schedule. The protraction of radiotherapy (up to 7.5 weeks in RTOG 0617) is known to favour tumour cell proliferation and to reduce the probability of tumour control. In our ongoing RTEP7 phase II, the boost is given by fractions of 3 Gy to avoid longer treatment times. In the present scenarios, IMRT allows to delivered simultaneous integrated boost (2 Gy per fraction in PTV66 and 2.24 Gy per fraction in the boost defined on pre-treatment PET/CT. Note that scenario 4 requires a 2-step planning (up to 50 Gy, then up to 74 Gy with 8 fractions of 3 Gy. Other approaches could be proton therapy [18] or stereotaxic radiotherapy [19].
Non respiratory-gated images for planning CT and PET/CT could have hampered the delineation precision and strategy for reducing the PTV margins. When the RTEP5 study was initiated, 4D acquisitions (and IMRT) were not routinely performed in the participating centres. The decision was made to require ungated acquisitions (and 3D conformal radiotherapy) in order to secure our recruitment objectives and the exportability of our results. The majority of our patients had large stage III tumours involving the mediastinum, limiting breathing movements. In addition, our delineation criteria on 18F-miso PET/CT was validated in free-breathing patients [8]. Precise radiotherapy (4D, IMRT) is indispensable if you want to target tumoral sub-volumes.
Patient selection is another issue. Positive 18F-miso uptake was associated to worse prognosis in our previous RTEP5 study [7]. Ten patients, out of 34 with 18F-miso uptake, were not eligible to the present planning study, mostly because of too large target volumes precluding dose escalation without compromising OAR tolerance. Beyond feasibility studies, randomised trials are warranted to demonstrate the value of radiotherapy assisted by functional imaging.
Conclusion
Pre−/per-treatment 18FDG and pre-treatment 18F-miso PET/CT yield different candidate target volumes for selective increase in radiotherapy dose in patients with NSCLC. Our in-silico study shows that IMRT provides radiotherapy plans matching the pre-defined dose/volume objectives and constraints. Clinical trials are required to select the relevant strategies to improve outcome after concomitant chemo-radiotherapy.
Abbreviations
- BTV:
-
Biologic Target Volume
- GTV:
-
Gross Target Volume
- IMRT:
-
Intensity modulated Radiation Therapy
- NSCLC:
-
Non-small cell lung cancer
- OAR:
-
Organs at risk
- PTV:
-
Planning Target Volume
- RTCT:
-
Radiotherapy - Chemotherapy
References
Arriagada R, Le Chevalier T, Quoix E, Ruffie P, de Cremoux H, Douillard JY, et al. ASTRO (American Society for Therapeutic Radiology and Oncology) plenary: effect of chemotherapy on locally advanced non-small cell lung carcinoma: a randomized study of 353 patients. GETCB (Groupe d'Etude et Traitement des cancers Bronchiques), FNCLCC (Féderation Nationale des Centres de Lutte contre le Cancer) and the CEBI trialists. Int J Radiat Oncol Biol Phys. 1991;20(6):1183–90.
Wang YC, Tseng HL, Lin YH, Kao CH, Huang WC, Huang TC. Improvement of internal tumor volumes of non-small cell lung cancer patients for radiation treatment planning using interpolated average CT in PET/CT. PLoS One. 2013;8(5):e64665.
Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–99.
Garg S, Gielda BT, Kiel K, Turian JV, Fidler MJ, Batus M, et al. Patterns of locoregional failure in stage III non-small cell lung cancer treated with definitive chemoradiation therapy. Pract Radiat Oncol. 2014;4(5):342–8.
Machtay M, Paulus R, Moughan J, Komaki R, Bradley JE, Choy H, et al. Defining local-regional control and its importance in locally advanced non-small cell lung carcinoma. J Thorac Oncol. 2012;7(4):716–22.
Eschmann SM, Paulsen F, Reimold M, Dittmann H, Welz S, Reischl G, Machulla HJ, Bares R. Prognostic impact of hypoxia imaging with 18F-misonidazole PET in non-small cell lung cancer and head and neck cancer before radiotherapy. J Nucl Med. 2005;46(2):253–60.
Vera P, Thureau S, Chaumet-Riffaud P, Modzelewski R, Bohn P, Vermandel M, et al. Phase II Study of a Radiotherapy Total Dose Increase in Hypoxic Lesions Identified by 18F-Misonidazole PET/CT in Patients with Non-Small Cell Lung Carcinoma (RTEP5 Study). J Nucl Med. 2017;58(7):1045–53.
Thureau S, Chaumet-Riffaud P, Modzelewski R, Fernandez P, Tessonnier L, Vervueren L, et al. Interobserver agreement of qualitative analysis and tumor delineation of 18F-fluoromisonidazole and 3'-deoxy-3'-18F-fluorothymidine PET images in lung cancer. J Nucl Med. 2013;54(9):1543–50.
Giraud P, Antoine M, Larrouy A, Milleron B, Callard P, De Rycke Y, et al. Evaluation of microscopic tumor extension in non-small-cell lung cancer for three-dimensional conformal radiotherapy planning. Int J Radiat Oncol Biol Phys. 2000;48(4):1015–24.
Chapet O, Kong FM, Quint LE, Chang AC, Ten Haken RK, Eisbruch A, et al. CT-based definition of thoracic lymph node stations: an atlas from the University of Michigan. Int J Radiat Oncol Biol Phys. 2005;63(1):170–8.
Calais J, Thureau S, Dubray B, Modzelewski R, Thiberville L, Gardin I, et al. Areas of high 18F-FDG uptake on preradiotherapy PET/CT identify preferential sites of local relapse after chemoradiotherapy for non-small cell lung cancer. J Nucl Med. 2015;56(2):196–203.
Aerts HJ, Bussink J, Oyen WJ, van Elmpt W, Folgering AM, Emans D, et al. Identification of residual metabolic-active areas within NSCLC tumours using a pre-radiotherapy FDG-PET-CT scan: a prospective validation. Lung Cancer. 2012;75(1):73–6.
Calais J, Dubray B, Nkhali L, Thureau S, Lemarignier C, Modzelewski R, et al. High FDG uptake areas on pre-radiotherapy PET/CT identify preferential sites of local relapse after chemoradiotherapy for locally advanced oesophageal cancer. Eur J Nucl Med Mol Imaging. 2015;42(6):858–67.
Lee NY, Mechalakos JG, Nehmeh S, Lin Z, Squire OD, Cai S, Chan K, Zanzonico PB, Greco C, Ling CC, Humm JL, Schöder H. Fluorine-18-labeled Fluoromisonidazole positron emission and computed tomography-guided intensity-modulated radiotherapy for head and neck Cancer: a feasibility study. Int J Radiat Oncol Biol Phys. 2010. https://doi.org/10.1016/j.ijrobp.2007.06.039 21.
Lee N, Nehmeh S, Schoder H, Fury M, Chan K, Ling CC, Humm J. Prospective trial incorporating pre-/mid-treatment [18 F]-misonidazole positron emission tomography for head-and-neck cancer patients undergoing concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2009;75(1):101–8.
Mees G, Dierckx R, Vangestel C, Laukens D, Van Damme N, Van de Wiele C. Pharmacologic activation of tumor hypoxia: a means to increase tumor 2-deoxy-2-[18F]fluoro-D-glucose uptake? Mol Imaging. 2013;12(1):49–58.
van Baardwijk A, Dooms C, van Suylen RJ, Verbeken E, Hochstenbag M, Dehing-Oberije C, et al. The maximum uptake of (18)F-deoxyglucose on positron emission tomography scan correlates with survival, hypoxia inducible factor-1alpha and GLUT-1 in non-small cell lung cancer. Eur J Cancer. 2007;43(9):1392–8.
Liao ZX, Lee JJ, Komaki R, Gomez DR, O'Reilly M, Allen P, et al. Bayesian randomized trial comparing intensity modulated radiation therapy versus passively scattered proton therapy for locally advanced non-small cell lung cancer. J Clin Oncol. 2016;34(15 suppl):8500.
J F, Arnold SM, Shelton BJ, Sinha P, Conrad G, Chen L, Rinehart J, McGarry RC. Stereotactic body radiation therapy can be used safely to boost residual disease in locally advanced non-small cell lung cancer: a prospective study. Int J Radiat Oncol Biol Phys. 2013;85(5):1325–31.
Acknowledgements
This study was supported by a grant from the French National Cancer Institute (PHRC 2011). The authors thank the technologists (E. Auger and S. Vincent) from the Department of Radiotherapy and Medical Physics (Centre Henri Becquerel) for their help in planning.
Funding
This study was supported by a grant from the French National Cancer Institute (PHRC 2011).
Availability of data and materials
All the data are available in our center at the unit of clinical research.
Author information
Authors and Affiliations
Contributions
ST, PV, BMD wrote the article. ST, SV, DG and EA collected the data. ST, PG, SH and NP have been working on data. PB, SH and PG has been working on PET data quality. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All data were extracted from one prospective study (NCT01576796). For all patients, we are a consent.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Thureau, S., Dubray, B., Modzelewski, R. et al. FDG and FMISO PET-guided dose escalation with intensity-modulated radiotherapy in lung cancer. Radiat Oncol 13, 208 (2018). https://doi.org/10.1186/s13014-018-1147-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-018-1147-2