Abstract
Aim
The aim of the study was to assess the feasibility of an individualized 18F fluorodeoxyglucose positron emission tomography (FDG-PET)-guided dose escalation boost in non-small cell lung cancer (NSCLC) patients and to assess its impact on local tumor control and toxicity.
Patients and methods
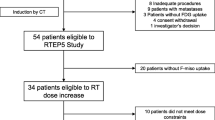
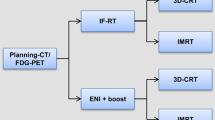
A total of 13 patients with stage II–III NSCLC were enrolled to receive a dose of 62.5 Gy in 25 fractions to the CT-based planning target volume (PTV; primary turmor and affected lymph nodes). The fraction dose was increased within the individual PET-based PTV (PTVPET) using intensity modulated radiotherapy (IMRT) with a simultaneous integrated boost (SIB) until the predefined organ-at-risk (OAR) threshold was reached. Tumor response was assessed during follow-up by means of repeat FDG-PET/computed tomography. Acute and late toxicity were recorded and classified according to the CTCAE criteria (Version 4.0). Local progression-free survival was determined using the Kaplan-Meier method.
Results
The average dose to PTVPET reached 89.17 Gy for peripheral and 75 Gy for central tumors. After a median follow-up period of 29 months, seven patients were still alive, while six had died (four due to distant progression, two due to grade 5 toxicity). Local progression was seen in two patients in association with further recurrences. One and 2-year local progression free survival rates were 76.9% and 52.8%, respectively. Three cases of acute grade 3 esophagitis were seen. Two patients with central tumors developed late toxicity and died due to severe hemoptysis.
Conclusion
These results suggest that a non-uniform and individualized dose escalation based on FDG-PET in IMRT delivery is feasible. The doses reached were higher in patients with peripheral compared to central tumors. This strategy enables good local control to be achieved at acceptable toxicity rates. However, dose escalation in centrally located tumors with direct invasion of mediastinal organs must be performed with great caution in order to avoid severe late toxicity.
Zusammenfassung
Zielsetzung
Ziel der Studie war es, die Anwendbarkeit einer individualisierten Fluordesoxyglukose-Positronenemissionstomographie(FDG-PET)-geführten partiellen Dosissteigerung beim nichtkleinzelligen Lungenkarzinom (NSCLC) zu prüfen und deren Einfluss auf die lokale Tumorkontrolle und Toxizität zu beurteilen.
Patienten und Methoden
Dreizehn Patienten mit NSCLC in Stadium II–III wurden in die Studie einbezogen und erhielten im Rahmen einer Radiochemotherapie eine Dosis von 62,5 Gy in 25 Fraktionen auf das CT-basierte Planungszielvolumen (PTV; primärer Tumor und betroffene Lymphknoten). Dabei wurde die Fraktionsdosis innerhalb des individuellen PET-basierten PTV (PTVPET) unter Anwendung einer intensitätsmodulierten Radiotherapie (IMRT) mit simultan-integriertem Boost (SIB) bis zum Erreichen der vordefinierten Organ-at-risk(OAR)-Grenze erhöht. In der Nachbeobachtungszeit wurde die Tumorantwort mit wiederholter FDG-PET/Computertomographie überprüft. Die frühe und späte Toxizität wurden erfasst und anhand der Common-Terminology-Criteria-for-Adverse-Events(CTCAE)-Kriterien (Version 4.0) klassifiziert. Das lokale progressionsfreie Überleben wurde anhand der Kaplan-Meier-Methode bestimmt.
Ergebnisse
Die Durchschnittsdosis auf das PTVPET erreichte 89,17 Gy für periphere und 75 Gy für zentrale Tumoren. Nach einer medianen Nachbeobachtungszeit von 29 Monaten waren 7 Patienten weiterhin am Leben, 6 waren verstorben (4 davon an Fernrezidiven, 2 an einer Toxizität fünften Grades). Bei 2 Patienten trat eine lokale Progression in Verbindung mit weiteren Rezidiven auf. Die lokalen progressionsfreien 1‑ und 2‑Jahres-Überlebensraten lagen bei 76,9 % bzw. 52,8 %. Es wurden 3 Fälle akuter Ösophagitiden dritten Grades beobachtet. Zwei Patienten mit zentralen Tumoren entwickelten eine späte Toxizität und verstarben infolge einer schwerwiegenden Hämoptyse.
Schlussfolgerung
Gemäß diesen Ergebnissen ist für Patienten mit NSCLC eine im Rahmen der IMRT angewendete, auf der FDG-PET basierende individualisierte Dosiseskalation möglich. Die erreichte Strahlendosis ist bei peripheren Tumoren höher als bei zentralen. Mit dieser Strategie wird eine hohe lokale Kontrolle bei akzeptabler Toxizitätsrate erreicht. Dennoch sollte die Dosissteigerung für zentrale Tumoren mit direktem Eindringen in Mediastinalorgane mit äußerster Vorsicht erfolgen, um eine schwere späte Toxizität zu vermeiden.

Similar content being viewed by others
References
Auperin A, Le Pechoux C, Rolland E, Curran WJ, Furuse K et al (2010) Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 28(13):2181–2190
Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ et al (2010) Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol 28(35):5153–5159
Belderbos JS, Heemsbergen WD, De Jaeger K, Baas P, Lebesque JV (2006) Final results of a Phase I/II dose escalation trial in non-small-cell lung cancer using three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys 66(1):126–134
Bentzen SM, Saunders MI, Dische S (2002) From CHART to CHARTWEL in non-small cell lung cancer: clinical radiobiological modelling of the expected change in outcome. Clin Oncol (R Coll Radiol) 14(5):372–381
Kong FM, Ten Haken RK, Schipper MJ, Sullivan MA, Chen M et al (2005) High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys 63(2):324–333
Machtay M, Bae K, Movsas B, Paulus R, Gore EM et al (2012) Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: an analysis of the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 82(1):425–434
Saunders MI, Rojas A, Lyn BE, Wilson E, Phillips H (2002) Dose-escalation with CHARTWEL (continuous hyperfractionated accelerated radiotherapy week-end less) combined with neo-adjuvant chemotherapy in the treatment of locally advanced non-small cell lung cancer. Clin Oncol (R Coll Radiol) 14(5):352–360
Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G et al (2015) Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 16(2):187–199
Hoffmann AL, Troost EG, Huizenga H, Kaanders JH, Bussink J (2012) Individualized dose prescription for hypofractionation in advanced non-small-cell lung cancer radiotherapy: an in silico trial. Int J Radiat Oncol Biol Phys 83(5):1596–1602
Mehta M, Scrimger R, Mackie R, Paliwal B, Chappell R et al (2001) A new approach to dose escalation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 49(1):23–33
Cox JD (2012) Are the results of RTOG 0617 mysterious? Int J Radiat Oncol Biol Phys 82(3):1042–1044
Wanet M, Lee JA, Weynand B, De Bast M, Poncelet A et al (2011) Gradient-based delineation of the primary GTV on FDG-PET in non-small cell lung cancer: a comparison with threshold-based approaches, CT and surgical specimens. Radiother Oncol 98(1):117–125
Aerts HJ, Bosmans G, van Baardwijk AA, Dekker AL, Oellers MC et al (2008) Stability of 18F-deoxyglucose uptake locations within tumor during radiotherapy for NSCLC: a prospective study. Int J Radiat Oncol Biol Phys 71(5):1402–1407
Aerts HJ, Bussink J, Oyen WJ, van Elmpt W, Folgering AM et al (2012) Identification of residual metabolic-active areas within NSCLC tumours using a pre-radiotherapy FDG-PET-CT scan: a prospective validation. Lung Cancer 75(1):73–76
Borst GR, Belderbos JS, Boellaard R, Comans EF, De Jaeger K et al (2005) Standardised FDG uptake: a prognostic factor for inoperable non-small cell lung cancer. Eur J Cancer 41(11):1533–1541
Petit SF, Aerts HJ, van Loon JG, Offermann C, Houben R et al (2009) Metabolic control probability in tumour subvolumes or how to guide tumour dose redistribution in non-small cell lung cancer (NSCLC): an exploratory clinical study. Radiother Oncol 91(3):393–398
van Baardwijk A, Bosmans G, Dekker A, van Kroonenburgh M, Boersma L et al (2007) Time trends in the maximal uptake of FDG on PET scan during thoracic radiotherapy. A prospective study in locally advanced non-small cell lung cancer (NSCLC) patients. Radiother Oncol 82(2):145–152
Velazquez ER, Aerts HJ, Oberije C, De Ruysscher D, Lambin P (2010) Prediction of residual metabolic activity after treatment in NSCLC patients. Acta Oncol 49(7):1033–1039
Fowler JF, Chappell R (2000) Non-small cell lung tumors repopulate rapidly during radiation therapy. Int J Radiat Oncol Biol Phys 46(2):516–517
Machtay M, Hsu C, Komaki R, Sause WT, Swann RS et al (2005) Effect of overall treatment time on outcomes after concurrent chemoradiation for locally advanced non-small-cell lung carcinoma: analysis of the Radiation Therapy Oncology Group (RTOG) experience. Int J Radiat Oncol Biol Phys 63(3):667–671
van Baardwijk A, Bosmans G, Bentzen SM, Boersma L, Dekker A et al (2008) Radiation dose prescription for non-small-cell lung cancer according to normal tissue dose constraints: an in silico clinical trial. Int J Radiat Oncol Biol Phys 71(4):1103–1110
van Baardwijk A, Reymen B, Wanders S, Borger J, Ollers M et al (2012) Mature results of a phase II trial on individualised accelerated radiotherapy based on normal tissue constraints in concurrent chemo-radiation for stage III non-small cell lung cancer. Eur J Cancer 48(15):2339–2346
Schwarz M, Alber M, Lebesque JV, Mijnheer BJ, Damen EM (2005) Dose heterogeneity in the target volume and intensity-modulated radiotherapy to escalate the dose in the treatment of non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 62(2):561–570
Goossens S, Senny F, Lee JA, Janssens G, Geets X (2014) Assessment of tumor motion reproducibility with audio-visual coaching through successive 4D CT sessions. J Appl Clin Med Phys 15(1):4332
Sterpin E, Janssens G, Orban de Xivry J, Goossens S, Wanet M et al (2012) Helical tomotherapy for SIB and hypo-fractionated treatments in lung carcinomas: a 4D Monte Carlo treatment planning study. Radiother Oncol 104(2):173–180
Geets X, Lee JA, Bol A, Lonneux M, Gregoire V (2007) A gradient-based method for segmenting FDG-PET images: methodology and validation. Eur J Nucl Med Mol Imaging 34(9):1427–1438
Bradley J, Graham MV, Winter K, Purdy JA, Komaki R et al (2005) Toxicity and outcome results of RTOG 9311: a phase I–II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys 61(2):318–328
Bradley JD, Moughan J, Graham MV, Byhardt R, Govindan R et al (2010) A phase I/II radiation dose escalation study with concurrent chemotherapy for patients with inoperable stages I to III non-small-cell lung cancer: phase I results of RTOG 0117. Int J Radiat Oncol Biol Phys 77(2):367–372
Gagliardi G, Constine LS, Moiseenko V, Correa C, Pierce LJ et al (2010) Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys 76(3 Suppl):77–85
Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD et al (2010) Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 76(3 Suppl):S70–6
Shi A, Zhu G, Wu H, Yu R, Li F et al (2010) Analysis of clinical and dosimetric factors associated with severe acute radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with concurrent chemotherapy and intensity-modulated radiotherapy. Radiat Oncol 5:35
Song CH, Pyo H, Moon SH, Kim TH, Kim DW et al (2010) Treatment-related pneumonitis and acute esophagitis in non-small-cell lung cancer patients treated with chemotherapy and helical tomotherapy. Int J Radiat Oncol Biol Phys 78(3):651–658
van Baardwijk A, Wanders S, Boersma L, Borger J, Ollers M et al (2010) Mature results of an individualized radiation dose prescription study based on normal tissue constraints in stages I to III non-small-cell lung cancer. J Clin Oncol 28(8):1380–1386
van Elmpt W, De Ruysscher D, van der Salm A, Lakeman A, van der Stoep J et al (2012) The PET-boost randomised phase II dose-escalation trial in non-small cell lung cancer. Radiother Oncol 104(1):67–71
Werner-Wasik M, Yorke E, Deasy J, Nam J, Marks LB (2010) Radiation dose-volume effects in the esophagus. Int J Radiat Oncol Biol Phys 76(3 Suppl):S86–93
Socinski MA, Morris DE, Halle JS, Moore DT, Hensing TA et al (2004) Induction and concurrent chemotherapy with high-dose thoracic conformal radiation therapy in unresectable stage IIIA and IIIB non-small-cell lung cancer: a dose-escalation phase I trial. J Clin Oncol 22(21):4341–4350
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122S–150S
Skougaard K, Nielsen D, Jensen BV, Hendel HW (2013) Comparison of EORTC criteria and PERCIST for PET/CT response evaluation of patients with metastatic colorectal cancer treated with irinotecan and cetuximab. J Nucl Med 54(7):1026–1031
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O et al (1999) Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 35(13):1773–1782
De Ruysscher D, Wanders S, van Haren E, Hochstenbag M, Geeraedts W et al (2005) Selective mediastinal node irradiation based on FDG-PET scan data in patients with non-small-cell lung cancer: a prospective clinical study. Int J Radiat Oncol Biol Phys 62(4):988–994
Bradley JD, Wahab S, Lockett MA, Perez CA, Purdy JA (2003) Elective nodal failures are uncommon in medically inoperable patients with Stage I non-small-cell lung carcinoma treated with limited radiotherapy fields. Int J Radiat Oncol Biol Phys 56(2):342–347
Emami B, Mirkovic N, Scott C, Byhardt R, Graham MV et al (2003) The impact of regional nodal radiotherapy (dose/volume) on regional progression and survival in unresectable non-small cell lung cancer: an analysis of RTOG data. Lung Cancer 41(2):207–214
Garg S, Gielda BT, Turian JV, Liptay M, Warren WH et al (2013) Patterns of regional failure in stage III non-small cell lung cancer treated with neoadjuvant chemoradiation therapy and resection. Pract Radiat Oncol 3(4):287–293
Rosenzweig KE, Sim SE, Mychalczak B, Braban LE, Schindelheim R et al (2001) Elective nodal irradiation in the treatment of non-small-cell lung cancer with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys 50(3):681–685
Senan S, Burgers S, Samson MJ, van Klaveren RJ, Oei SS et al (2002) Can elective nodal irradiation be omitted in stage III non-small-cell lung cancer? Analysis of recurrences in a phase II study of induction chemotherapy and involved-field radiotherapy. Int J Radiat Oncol Biol Phys 54(4):999–1006
Provencio M, Sanchez A, Garrido P, Valcarcel F (2010) New molecular targeted therapies integrated with radiation therapy in lung cancer. Clin Lung Cancer 11(2):91–97
Zhuang H, Zhao X, Zhao L, Chang JY, Wang P (2014) Progress of clinical research on targeted therapy combined with thoracic radiotherapy for non-small-cell lung cancer. Drug Des Devel Ther 8:667–675
Han CB, Wang WL, Quint L, Xue JX, Matuszak M et al (2014) Pulmonary artery invasion, high-dose radiation, and overall survival in patients with non-small cell lung cancer. Int J Radiat Oncol Biol Phys 89(2):313–321
Cannon DM, Mehta MP, Adkison JB, Khuntia D, Traynor AM et al (2013) Dose-limiting toxicity after hypofractionated dose-escalated radiotherapy in non-small-cell lung cancer. J Clin Oncol 31(34):4343–4348
Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K et al (2006) Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 24(30):4833–4839
Langendijk JA, Tjwa MK, de Jong JM, ten Velde GP, Wouters EF (1998) Massive haemoptysis after radiotherapy in inoperable non-small cell lung carcinoma: is endobronchial brachytherapy really a risk factor? Radiother Oncol 49(2):175–183
Wanet M, Sterpin E, Janssens G, Delor A, Lee JA et al (2014) Validation of the mid-position strategy for lung tumors in helical TomoTherapy. Radiother Oncol. doi:10.1016/j.radonc.2013.10.025
Funding
This research program was supported by a grant from the Belgian national fund for scientific research (FRS-FNRS Télévie, grant number, 7.4537.09). X. Geets is a Postdoctoral Researcher with the FRS-FNRS, partly funded by the Clinical and Experimental Research Institute (IREC). John A. Lee is a Research Associate with the FRS-FNRS (FC 63880).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Wanet, A. Delor, F.-X. Hanin, B. Ghaye, A. Van Maanen, V. Remouchamps, C. Clermont, S. Goossens, J.A. Lee, G. Janssens, A. Bol and X. Geets declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Authors Contributions
M. Wanet participated in the design and coordination of the trial, recruited patients, carried out all imaging sessions and all volume delineations. MW also performed the follow-up during and after the treatment of all patients, tumor assessment on both imaging modalities and drafted the manuscript. A. Delor planned the treatment of 11 patients. F.-X. Hanin supervised the tumor response assessment on FDG-PET imaging (PERCIST and EORTC). B. Ghaye supervised and performed the tumor response assessment on CT imaging (RECIST). A. Van Maanen performed the statistical analysis. V. Remouchamps recruited the patients at the second center (two patients) and performed the follow-up during and after treatment of these patients. C. Clermont planned the treatment of two patients. S. Goossens helped during the imaging session. J.A. Lee participated by providing the method and segmentation algorithm for PET imaging delineation and helped to draft the manuscript. G. Janssens participated by providing the method and non-rigid registration algorithm for ITV generation on CT and PET imaging. A. Bol helped during and after imaging sessions, as well as in tumor response assessment according to PERCIST (SULpeak measures). X. Geets designed the trial and recruited patients, supervised all steps of the trial as well as manuscript drafting. All authors read and approved the final manuscript.
Trial Registration
EU Clinical Trials Register, EudraCT number 2011-003124-12.
Caption Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Wanet, M., Delor, A., Hanin, FX. et al. An individualized radiation dose escalation trial in non-small cell lung cancer based on FDG-PET imaging. Strahlenther Onkol 193, 812–822 (2017). https://doi.org/10.1007/s00066-017-1168-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-017-1168-z