Abstract
Background
It is hypothesized that Affordable Care Act (ACA) Medicaid expansions could substantially improve access to health insurance and healthcare services for patients at risk for diabetes mellitus (DM), with pre-DM, or already diagnosed with DM. The ACA called for every state to expand Medicaid coverage by 2014. In a 2012 legal challenge, the US Supreme Court ruled that states were not required to implement Medicaid expansions. This 'natural experiment' presents a unique opportunity to learn whether and to what extent Medicaid expansion can affect healthcare access and services for patients with DM risk, pre-DM, or DM.
Methods/design
Data from electronic health records (EHRs) from the Accelerating Data Value Across a National Community Health Center Network (ADVANCE) clinical data research network, which has data from >700 community health centers (CHCs), was included in the study. EHR data will be linked to Oregon Medicaid claims data. Data collection will include information on changes in health insurance, service receipt, and health outcomes, spanning 9 years (pre- and post-expansion), comparing states that expanded Medicaid, and those that did not. Patients included in this study will be diagnosed with DM, be at risk for DM, or have pre-DM, between the ages of 19 and 64, with ≥1 ambulatory visit. Sample size is estimated to be roughly 275,000 patients. Biostatistical analyses will include the difference-in-differences (DID) methodology and a generalized linear mixed model. Econometric analyses will include a DID two-part method to calculate the difference in Medicaid expenditures in Oregon among newly insured CHC patients.
Discussion
Findings will have national relevance on DM health services and outcomes and will be shared through national conferences and publications. The findings will provide information needed to impact the policy as it is related to access to health insurance and receipt of healthcare among a vulnerable population.
Trial registration
This project is registered with ClinicalTrials.gov (NCT02685384). Registered 18 May 2016.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Diabetes mellitus (DM) is one of the nation’s leading causes of morbidity and mortality [1, 2]. In 2012, >29 million people in the USA had DM, of which 1.7 million were newly diagnosed [3]. Uninsured patients are more likely to have undiagnosed DM, and the longer they lack insurance, the higher this likelihood [4]. Uninsured patients with DM are also less likely to receive recommended DM care and have poorer DM control than insured patients [5–8]; lack of health insurance can greatly exacerbate the challenges of successful DM care and management [9–13]. Previous research showed uninsured patients had lower odds of receiving DM-related services, even when they came in for a clinic visit, compared to insured patients at a similar visit [5]. Thus, health insurance and continued access to healthcare services are essential for optimal DM detection, care, and management.
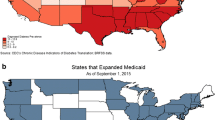
It is hypothesized that Affordable Care Act (ACA) Medicaid expansions could substantially improve access to health insurance and healthcare services for patients at risk for DM, with pre-DM, or DM. The ACA is the largest healthcare-related legislation in the USA since Medicare’s establishment in 1966. With the goal of covering all low-income citizens and legal residents [14], the ACA called for Medicaid expansions to all individuals earning ≤138% of the federal poverty level (FPL). However, in 2012, the US Supreme Court ruled that states were not legally required to implement the Medicaid expansions [15]. As of April 2016, 32 states and the District of Columbia implemented expansions while 18 states did not [16]; current estimates show that Medicaid enrollment grew by 18% in expansion states and by 5% in non-expansion states [17]. This ‘natural experiment’ presents a unique opportunity to learn whether and to what extent Medicaid expansion can affect healthcare access and services for patients at risk for DM, with pre-DM, or DM. Here, we present the project aims, methods, and planned analyses for the project.
Methods
Study aims
To assess this natural policy experiment, we will use electronic health record (EHR) data from community health centers (CHCs) in states that expanded and did not expand Medicaid. The study has the following specific aims.
Aim 1
Compare pre-post ACA insurance status, overall visits, and chronic disease management visits among patients with DM risk, pre-DM, or DM, among CHC patients in expansion versus non-expansion states.
Aim 2
Compare pre-post ACA receipt of primary and secondary DM preventive services [e.g., screening for obesity, lipid levels, glycosylated hemoglobin (HbA1c)] among patients with DM risk, pre-DM, or DM, among CHC patients in expansion versus non-expansion states.
Aim 3
Compare pre-post ACA changes in DM-related biomarkers (e.g., body mass index, blood pressure, lipid levels) in CHC patients with DM risk, pre-DM, or DM among newly insured (gained Medicaid in post-period), already insured (had Medicaid coverage in pre- and post-periods), and continuously uninsured (pre- and post-periods) patients in states that expanded Medicaid.
Aim 4
Measure pre-post ACA changes in Oregon Medicaid expenditures among newly insured compared to already insured CHC patients with DM risk, pre-DM, or DM.
Data sources
We will use EHR data from the Accelerating Data Value Across a National Community Health Center Network (ADVANCE) clinical data research network (CDRN) of PCORNnet (National Patient-Centered Clinical Research Network) [18]. The ADVANCE CDRN is a unique ‘community laboratory’ for research with underrepresented populations receiving care in CHCs—our nation’s safety net [18]. Led by the OCHIN (not an acronym) community health information network, the ADVANCE CDRN’s research-ready data warehouse integrates longitudinal outpatient EHR data from OCHIN, Health Choice Network (HCN), and Fenway Health. These three CHC networks primarily serve vulnerable populations and, as of June 2016, include 2,195 CHCs with >3.1 million active patients in 23 states (1194 CHCs in 12 states that implemented Medicaid expansion in January 2014, and 1001 CHCs in 11 states that did not). Of note, the project will use a more restricted database due to eligibility criteria as described below.
OCHIN is a collaborative that includes >450 CHCs and other community-based clinics [19]. OCHIN is the nation’s largest CHC network utilizing a single instance of one EHR system (and the only one using Epic©). Pioneering the implementation of a single, hosted instance of Epic© Systems EHR across hundreds of clinics, OCHIN maintains one enterprise-wide master patient index. Thus, OCHIN patients have a single medical record across all clinics in the network, and all data are managed centrally.
HCN has a history and organizational structure similar to OCHIN’s. In 1994, HCN was founded in Florida by a group of CHCs collaborating to recover from the impact of Hurricane Andrew; membership now spans 9 states. HCN members are hosted on a centralized EHR platform (Intergy™ by Vitera™) and supported by network-wide clinical informatics and analytic tools. In 2011, HCN partnered with OCHIN to develop the ADVANCE data and aggregation system. In addition to the ADVANCE research-ready data warehouse, this partnership has helped CHCs aggregate data for quality reporting, EHR “meaningful use,” and patient-centered medical home recognition.
Fenway Health, a free-standing CHC, was founded in 1971. In its early response to the AIDS epidemic, Fenway Health developed the capacity to support clinical research and has received significant federal funding [20]. Fenway has been a partner with OCHIN since 2010 [21]. Fenway has received national recognition for reducing healthcare disparities for sexual and gender minority populations, and is the home of the National Center for Lesbian, Gay, Bisexual and Transgender Health Education [22]. Fenway Health has had an EHR for >15 years and has participated in several national research consortia using EHR-based data.
We will also link OCHIN EHR data to Oregon Medicaid claims data in order to measure changes in Medicaid expenditures. Oregon’s Medicaid recipients are assigned unique individual identification (ID) numbers, facilitating data linkages across multiple databases, including the ADVANCE data warehouse. As we have done previously [23–25], we will use claims data from Oregon’s Medicaid Management Information System, recognized for exemplary data validation protocols by Centers for Medicare and Medicaid Services.
Eligibility criteria
We will include patients with DM risk, pre-DM, or diagnosed DM, between the ages of 19 and 64, with one or more ambulatory visit. Data will derive from >700 CHCs in 20 states for which their EHR were ‘live’ as of 1/1/2013. We set these age criteria because the Medicaid expansion was aimed at adults aged 19 and older, many states’ Medicaid programs cover children through age 18, and nationally, individuals aged 65 and older are eligible for Medicare. We will exclude pregnant women to eliminate the possibility of having patients with gestational DM in the dataset.
Definitions of patients with pre-DM, at-risk for DM, and DM
Patients at-risk for DM:
Patients aged 45 and older and with a BMI ≥ 25, following criteria from the Centers for Disease Control and Prevention [26].
Patients with pre-DM:
Patients with a single HbA1c between 5.7 and 6.4% and/or a fasting glucose between 100 and125 mg/deciliter.
Patients with DM:
The ADVANCE CDRN has developed a computable phenotype with criteria for identifying potential DM cohort members. This phenotype includes relevant information for identifying type 1 and type 2 DM. Both types of DM will be included in the study, although most patients have type 2 DM. Previous studies have validated the use of a similar method [27–29]. Patients with DM are identified as those with any combination of two “events” from outpatient diagnoses, diagnostic level laboratory results, or order of anti-hyperglycemic agents no more than 730 days apart. Examples:
-
(1)
At least two visits with a DM-related International Classification of Disease (ICD)-9 or 10 code,
-
(2)
One ICD-9/10-coded visit and one HbA1c or glucose test positive for DM, according to American Diabetes Association thresholds [30],
-
(3)
One ICD-9/10 coded visit and a diabetes-related medication order, or
-
(4)
A diabetes-related medication order and a positive HbA1c or glucose test.
Because some patients may not have the opportunity to have two events in the post-period (or pre-period), we risk an underestimation of patients with DM. Thus, we will conduct sensitivity analyses using one event to define DM patients and ensure robust findings. Table 1 shows a breakdown of ADVANCE patients with DM risk, pre-DM, or diagnosed with DM.
Measures
This project has two main independent variables: Medicaid expansion status (states that expanded versus not) or insurance status.
Medicaid expansion status
We will define the pre- and post-Medicaid expansion periods based on if and when a state expanded Medicaid. As illustrated in Fig. 1, each row below the time arrow represents the pre-post periods depending on when ACA-sponsored Medicaid expansion was/will be implemented in states. Our study period will range from January 1, 2012 to December 31, 2020. We will have data spanning 9 years, which will allow us to examine the short-term (1–2 years) and medium term (4–5 years) impact of the Medicaid expansion on reducing disparities in access to and receipt of DM screening, treatment, and health outcomes as well as changes in expenditures. When the ACA-Medicaid expansion took effect on January 1, 2014, 14 states in the ADVANCE CDRN adopted Medicaid expansion. Their pre-period (first row) is thus 24 months prior to 2014 (i.e., 2012 through 2014) and their post-period is 2014 through 2020 (see first row in Fig. 1). Other states adopted the expansion later, such as Indiana and Alaska (pre/post period in row 2), Montana (pre/post period in row 3), and others (i.e., Oklahoma) may change their policies during the remainder of the study period.
Insurance status
Since EHR health insurance data is primarily based on information collected at each visit [31], we propose to define newly insured, already insured, and continuously uninsured patients as follows:
-
(1)
Newly insured patients will have been uninsured at all visits in the pre-period and had all visits in the post-period paid by Medicaid;
-
(2)
Already insured patients will have all visits paid by Medicaid in both the pre- and post-periods;
-
(3)
Uninsured patients will have no coverage for all visits in both the pre- and post-periods.
Covariates will include sociodemographic variables (age, gender, race, ethnicity, poverty level, language preference, and urbanicity) and the frequency of healthcare visits.
Outcome measures
Healthcare coverage refers to patient’s health insurance status including coverage status, type of health insurance (e.g., Medicaid, private, Medicare) and percent of insured visits.
Healthcare delivery includes rates of all billed encounters (all, primary care visits, and mental and behavioral health encounters) and receipt of recommended preventive services [32, 33] (e.g., tobacco assessment, vaccinations, cholesterol screening, diabetic preventive care, blood pressure measurement, obesity screening, foot and retina exams, appropriate prescriptions).
We will also evaluate change in DM-related biomarkers, by identifying patients with elevated HbA1c (HbA1c >7% [34]), low-density lipoprotein (LDL ≥ 100 mg/dl [32–34]), blood pressure (last measure >140/90 mmHg), body mass index (BMI ≥ 30) and diabetes complications (e.g., retinopathy, nephropathy, neuropathy). We will examine absolute changes in these biomarker values, as well as the proportion achieving control and rates of change.
Medicaid expenditures for services internal and external (Medicaid recipients only) to the patients’ clinic will be determined. Evaluating expenditures requires two steps.
-
(1)
For the subset of our cohort residing in Oregon, link patients with DM risk, pre-DM, or DM to Oregon’s Medicaid claims data.
-
(2)
Attach expenditures to uninsured individuals for services. Although these individuals do not have paid Oregon Medicaid claims, they do have encounter data that are captured by the ADVANCE data. These encounter data include the Current Procedural Terminology (CPT) codes that identify the services rendered to those patients. We will attach an expenditure for each service based on its average Medicaid fee-for-service reimbursement in the first year of the sample. In other words, a standardized price will be defined for each service provided by the clinic. In order to be consistent, we will attach these prices to all patients, including those with insurance. These repriced claims reflect differences in utilization only (and not payment or capitation rates). The investigators have used this approach previously [35].
Additional outcome variables may be added if more data become available.
Analytic procedures
Biostatistical analyses
Our primary methodological approach to address study aims will utilize difference-in-differences (DID) methodology [36–38]. To address aims 1–3, we will use a generalized linear mixed model (GLMM) approach to adjust for serial correlation and other potential confounders. An interaction term for Medicaid expansion (or insurance status) and a post-expansion indicator variable will be included in the model to determine the DID in the outcomes. Additionally, this model is flexible enough to construct models stratified by patients with DM risk, pre-DM, or DM. Further, we will stratify the models by types of insurance (i.e., Medicaid vs. private/employer-sponsored) because healthcare delivery may vary by type.
Moreover, we will test three-way interaction terms of demographic indicators (i.e., race/ethnicity, gender, age), time, and Medicaid expansion indicator. We will compare the potential effect of gaining insurance on DM, pre-DM, or DM risk patients’ care, outcomes, and expenditures by demographic characteristics because of the differential prevalence of DM between these sociodemographic groups.
We will use propensity score weighting methods to reduce the observed bias, help minimize external threats to the validity of the results, and adjust for imbalances between expansion and non-expansion groups [39]. Clinic and patient panel characteristics that remain unbalanced between the intervention and control groups after propensity score adjustment will be included as covariates in the GLMM models to control for residual confounding. Longitudinal GLMM models will account for correlation within matched clinic site pairs and within CHCs through random effects.
Econometric analyses
To address study aim 4, using DID methods, we will calculate the average pre-post difference in Medicaid expenditures at the CHC attributable to the subpopulation of Oregon newly insured DM risk, pre-DM, or DM patients in ADVANCE data, subtracted by the average difference among the already insured patients. We will use a well-validated approach for modeling this phenomenon: the 2-part model [40]. Part 1 will use logistic regression to estimate the probability of any expenditure. Part 2 will focus on individuals with non-zero expenditures. We will use recent literature to guide the appropriate estimation approach, taking into account the potentially skewed distribution of the dependent variable [41, 42].
This study was reviewed and approved by the Oregon Health & Science University Institutional Review Board. It is registered with ClinicalTrials.gov (NCT02685384).
Discussion
By making the Medicaid expansion optional for states, the US Supreme Court created a natural policy experiment to analyze the impact of a large-scale, national expansion of Medicaid on DM prevention and treatment. Our study capitalizes on this natural experiment by including data from 20 states; analyses will uniquely inform national and state policy decisions as states grapple with how to equitably distribute healthcare resources after the passage of federal health reform. To be useful, health policy reform evaluation must be timely, yet data from most currently available national sources have several years’ delay between data collection and analysis. EHR data overcome these limitations as they are current and can provide information about the immediate, real-time impacts of the ACA’s policies on clinic populations.
Fortunately, the rapid growth of EHR use in CHCs serving vulnerable populations yields unprecedented opportunities for real-time evaluation of how health policy changes impact access to care, and utilization and delivery of CHC services. Further, because CHC patients are primarily low-income, racial/ethnic minorities, and/or from rural populations, this study will monitor and evaluate whether the ACA Medicaid expansion mitigated health disparities, especially among patients with DM.
This study has some limitations. First, EHR data are not originally developed for research; however, we have conducted multiple data validation studies, built many EHR research datasets, and successfully conducted policy-relevant research using EHR datasets in the past [24, 43, 44]. Second, we anticipate missing data, either from services documented inaccessibly in the EHR (likely random) or from patients who went outside the ADVANCE CDRN to receive services (perhaps not random). Our analyses can accommodate missing data resulting from patient attrition. We will model missingness by including related variables in the analysis as covariates [45] or using a method such as multiple imputation to include these patients in analyses [46]. Third, as with any ‘real-world’ study, unobserved changes may occur over time, making it difficult to isolate the effect of the ACA.
In conclusion, this project assesses the natural policy experiment created by ACA Medicaid expansions when some US states expanded Medicaid while others did not. Findings will have national relevance on DM prevention, diagnosis, treatment, expenditures, and health outcomes. It investigates how Medicaid expansions impact access to and changes in receipt of healthcare services among a vulnerable population of patients with DM risk, pre-DM, or DM and creates validated data sources for studying vulnerable populations.
Abbreviations
- ACA:
-
Affordable Care Act
- ADVANCE:
-
Accelerating Data Value Across a National Community Health Center Network
- BMI:
-
Body mass index
- CDRN:
-
Clinical data research network
- CHC:
-
Community health centers
- CPT:
-
Current Procedural Terminology
- DID:
-
Difference-in-differences
- DM:
-
Diabetes mellitus
- EHR:
-
Electronic health record
- FPL:
-
Federal poverty level
- GLMM:
-
Generalized linear mixed model
- HbA1c:
-
Glycosylated hemoglobin
- HCN:
-
Health Choice Network
- ICD:
-
International Classification of Disease
- LDL:
-
Low-density lipoprotein
References
American DA. Economic costs of diabetes in the U.S. In 2007. Diabetes Care. 2008;31(3):596–615. doi:10.2337/dc08-9017.
Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011.
National Center for Chronic Disease Prevention and Health Promotion. National diabetes statistics report, 2014. Atlanta, GA: Centers for Disease Control and Prevention; 2014.
Zhang X, Geiss LS, Cheng YJ, Beckles GL, Gregg EW, Kahn HS. The missed patient with diabetes: how access to health care affects the detection of diabetes. Diabetes Care. 2008;31(9):1748–53. doi:10.2337/dc08-0572.
Bailey SR, O'Malley JP, Gold R, Heintzman J, Marino M, DeVoe JE. Receipt of diabetes preventive services differs by insurance status at visit. Am J Prev Med. 2015;48(2):229–33. doi:10.1016/j.amepre.2014.08.035.
Gold R, DeVoe J, Shah A, Chauvie S. Insurance continuity and receipt of diabetes preventive care in a network of federally qualified health centers. Med Care. 2009;47(4):431–9.
Nelson KM, Chapko MK, Reiber G, Boyko EJ. The association between health insurance coverage and diabetes care; data from the 2000 Behavioral Risk Factor Surveillance System. Health Serv Res. 2005;40(2):361–72. doi:10.1111/j.1475-6773.2005.00361.x.
Zhang JX, Huang ES, Drum ML, Kirchhoff AC, Schlichting JA, Schaefer CT, et al. Insurance status and quality of diabetes care in community health centers. Am J Public Health. 2009;99(4):742–7. doi:10.2105/AJPH.2007.125534.
Levine DA, Allison JJ, Cherrington A, Richman J, Scarinci IC, Houston TK. Disparities in self-monitoring of blood glucose among low-income ethnic minority populations with diabetes. United States Ethn Dis. 2009;19(2):97–103.
Robbins JM, Thatcher GE, Webb DA, Valdmanis VG. Nutritionist visits, diabetes classes, and hospitalization rates and charges: the Urban Diabetes Study. Diabetes Care. 2008;31(4):655–60. doi:10.2337/dc07-1871.
Rhee MK, Cook CB, El-Kebbi I, Lyles RH, Dunbar VG, Panayioto RM, et al. Barriers to diabetes education in urban patients: perceptions, patterns, and associated factors. Diabetes Educ. 2005;31(3):410–7. doi:10.1177/0145721705277022.
Vest BM, Kahn LS, Danzo A, Tumiel-Berhalter L, Schuster RC, Karl R, et al. Diabetes self-management in a low-income population: impacts of social support and relationships with the health care system. Chronic Illn. 2013;9(2):145–55. doi:10.1177/1742395313475674.
Wallia A, Molitch ME. Insulin therapy for type 2 diabetes mellitus. JAMA. 2014;311(22):2315–25. doi:10.1001/jama.2014.5951.
The Henry J. Kaiser Family Foundation. Menlo Park, CA: Summary of the Affordable Care Act; 2013. Contract No.: 8061-02.
Supreme Court of the United States. National Federation of Independent Business v Sebelius. 2012. http://www.supremecourt.gov/opinions/11pdf/11-393c3a2.pdf. Accessed September 27 2016.
The Henry J Kaiser Family Foundation. Status of state action on the Medicaid expansion decision. 2015. http://kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/. Accessed September 27 2016.
Wachino V, Artiga S, Rudowitz R. How is the ACA Impacting Medicaid Enrollment? 2014. http://kff.org/medicaid/issue-brief/how-is-the-aca-impacting-medicaid-enrollment/. Accessed January 15 2015.
DeVoe JE, Gold R, Cottrell E, Bauer V, Brickman A, Puro J, et al. The ADVANCE network: accelerating data value across a national community health center network. J Am Med Inform Assoc. 2014;21(4):591–5. doi:10.1136/amiajnl-2014-002744.
Davis MM, Keller S, DeVoe JE, Cohen J. Characteristics and lessons learned from practice-based research networks (PBRNs) in the United States. J Health Care Leadership. 2012;4:107–16.
Mayer K, Appelbaum J, Rogers T, Lo W, Bradford J, Boswell S. The evolution of the Fenway Community Health model. Am J Public Health. 2001;91(6):892–4.
Likumahuwa S, Song H, Singal R, Weir RC, Crane H, Muench J, et al. Building research infrastructure in community health centers: a Community Health Applied Research Network (CHARN) report. J Am Board Fam Med. 2013;26(5):579–87. doi:10.3122/jabfm.2013.05.130025.
The Fenway Institute. The National LGBT Health Education Center. Boston, MA. 2013. www.LGBThealtheducation.org. Accessed September 27 2016.
Angier H, Gold R, Gallia C, Casciato A, Tillotson CJ, Marino M, et al. Variation in outcomes of quality measurement by data source. Pediatrics. 2014;133(6):e1676–82. doi:10.1542/peds.2013-4277.
DeVoe JE, Gold R, McIntire P, Puro J, Chauvie S, Gallia CA. Electronic health records vs Medicaid claims: completeness of diabetes preventive care data in community health centers. Ann Fam Med. 2011;9(4):351–8. doi:10.1370/afm.1279.
DeVoe JE, Marino M, Angier H, O’Malley J, Crawford C, Nelson C, et al. Effect of expanding Medicaid for parents on children’s health insurance coverage: lessons from the Oregon experiment. JAMA Peds. 2015;169(1):e143145.
Centers for Disease Control and Prevention. Preventing diabetes. n.d. http://www.cdc.gov/diabetes/basics/prevention.html. Accessed February 16 2015.
Mooy JM, Grootenhuis PA, de Vries H, Kostense PJ, Popp-Snijders C, Bouter LM, et al. Intra-individual variation of glucose, specific insulin and proinsulin concentrations measured by two oral glucose tolerance tests in a general Caucasian population: the Hoorn Study. Diabetologia. 1996;39(3):298–305.
Klompas M, Eggleston E, McVetta J, Lazarus R, Li L, Platt R. Automated detection and classification of type 1 versus type 2 diabetes using electronic health record data. Diabetes Care. 2013;36(4):914–21. doi:10.2337/dc12-0964.
Wilke RA, Berg RL, Peissig P, Kitchner T, Sijercic B, McCarty CA, et al. Use of an electronic medical record for the identification of research subjects with diabetes mellitus. Clin Med Res. 2007;5(1):1–7. doi:10.3121/cmr.2007.726.
American Diabetes Assocation. Standards of Medical Care-2013. Diabetes Care. 2013;36 Suppl 1:S11–66.
Heintzman J, Marino M, Hoopes M, Bailey SR, Gold R, O'Malley J, et al. Supporting health insurance expansion: do electronic health records have valid insurance verification and enrollment data? J Am Med Inform Assoc. 2015;22(4):909-13. doi:10.1093/jamia/ocv033.
Expert Panel on Detection E, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol in ddults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–97.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo Jr JL, et al. The seventh report of the joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72. doi:10.1001/jama.289.19.2560.
American Diabetes Association. (8) Cardiovascular disease and risk management. Diabetes Care. 2015;38(Suppl):S49–57. doi:10.2337/dc15-S011.
McConnell KJ, Wallace NT, Gallia CA, Smith JA. Effect of eliminating behavioral health benefits for selected medicaid enrollees. Health Serv Res. 2008;43(4):1348–65. doi:10.1111/j.1475-6773.2008.00844.x.
Bertrand M, Duflo E, Mullainathan S. How much should we trust differences-in-differences estimates? Q J Econ. 2004;119(1):249–75.
Friedberg MW, Schneider EC, Rosenthal MB, Volpp KG, Werner RM. Association between participation in a multipayer medical home intervention and changes in quality, utilization, and costs of care. JAMA. 2014;311(8):815–25. doi:10.1001/jama.2014.353.
Higgins S, Chawla R, Colombo C, Snyder R, Nigam S. Medical homes and cost and utilization among high-risk patients. Am J Manag Care. 2014;20(3):e61–71.
Guo S, Fraser MW. Propensity score analysis: statistical methods and applications advanced quantitative techniques in the social sciences series. Thousand Oaks, CA: Sage Publications, Inc; 2014.
Duan NH, Manning WG, Morris CN, Newhouse JP. A comparison of alternative models for demand for medical care. J Bus Econ Stat. 1983;1(2):115–26.
Manning WG. The logged dependent variable, heteroscedasticity, and the retransformation problem. J Health Econ. 1998;17(3):283–95.
Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20(4):461–94.
Gold R, Muench J, Hill C, Turner A, Mital M, Milano C, et al. Collaborative development of a randomized study to adapt a diabetes quality improvement initiative for federally qualified health centers. J Health Care Poor Underserved. 2012;23 Suppl 3:236–46. doi:10.1353/hpu.2012.0132.
Cowburn S, Carlson MJ, Lapidus JA, DeVoe JE. The association between insurance status and cervical cancer screening in community health centers: exploring the potential of electronic health records for population-level surveillance, 2008-2010. Prev Chronic Dis. 2013;10:E173. doi:10.5888/pcd10.130034.
Little TD, Schnabel KU, Baumert J. Modeling longitudinal and multilevel data 2000. New Jersey: Lawrence Earlbaum Associates; 2000.
Little RJA, Rubin DB. Statistical analysis with missing data. Hoboken, NJ: John Wiley & Sons; 2002.
Acknowledgements
This work was supported by the Agency for Healthcare Research and Quality (AHRQ), grant number R01HS024270, and by the National Cancer Institute (NCI), grant numbers R01CA204267 and R01CA181452. The authors acknowledge the significant contributions to this study that were provided by collaborating investigators in the NEXT-D2 (Natural Experiments in Translation for Diabetes) Study Two. ADVANCE (Accelerating Data Value Across a National Community Health Center Network) is led by the OCHIN Community Health Information Network in partnership with the Health Choice Network (HCN), Fenway Health, CareOregon, Kaiser Permanente Center for Health Research, Legacy Health, Oregon Health & Science University (OHSU), and the Robert Graham Center. The authors also acknowledge the participation of Dr. Greg Nichols at Northwest Kaiser Permanente Center for Health Research as well as our partnering health systems. The views presented in this article are solely the responsibility of the authors and do not necessarily represent the views of the funding agencies.
Funding
This study was made possible by Cooperative Agreement Number U18DP006116 jointly funded by the US Centers for Disease Control and Prevention, the National Institute of Diabetes and Digestive and Kidney Diseases, and the Patient-Centered Outcomes Research Institute.
Availability of data and materials
Please contact the authors for data requests.
Authors’ contributions
JD is PI and lead the writing of the grant application with contribution from NH, HA, MM, and KJMcC. NH lead the writing of the protocol manuscript with significant contributions from MH, JO, LR, S LA, and HH. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable
Ethics approval and consent to participate
This study uses secondary data analysis and therefore approval for consent to participate was waived. The Institutional Review Board for Oregon Health & Science University approved this study. Approval reference number is IRB00011858.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Huguet, N., Angier, H., Marino, M. et al. Protocol for the analysis of a natural experiment on the impact of the Affordable Care Act on diabetes care in community health centers. Implementation Sci 12, 14 (2017). https://doi.org/10.1186/s13012-017-0543-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13012-017-0543-6