Abstract
Background
Genetically modified cotton accounts for 64% of the world’s cotton growing area (22.3 million hectares). The genome sequencing of the diploid cotton progenitors Gossypium raimondii and Gossypium arboreum as well as the cultivated Gossypium hirsutum has provided a wealth of genetic information that could be exploited for crop improvement. Unfortunately, gene functional characterization in cotton is lagging behind other economically important crops due to the low efficiency, lengthiness and technical complexity of the available stable transformation methods. We present here a simple, fast and efficient method for the transient transformation of G. hirsutum that can be used for gene characterization studies.
Results
We developed a transient transformation system for gene characterization in upland cotton. Using β-glucuronidase as a reporter for Agrobacterium-mediated transformation assays, we evaluated multiple transformation parameters such as Agrobacterium strain, bacterial density, length of co-cultivation, chemicals and surfactants, which can affect transformation efficiency. After the initial characterization, the Agrobacterium EHA105 strain was selected and a number of binary constructs used to perform gene characterization studies. 7-days-old cotton seedlings were co-cultivated with Agrobacterium and transient gene expression was observed 5 days after infection of the plants. Transcript levels of two different transgenes under the control of the cauliflower mosaic virus (CaMV) 35S promoter were quantified by real-time reverse transcription PCR (qRT-PCR) showing a 3–10 times increase over the levels observed in non-infected controls. The expression patterns driven by the promoters of two G. hirsutum genes as well as the subcellular localization of their corresponding proteins were studied using the new transient expression system and our observations were consistent with previously published results using Arabidopsis as a heterologous system.
Conclusions
The Agrobacterium-mediated transient transformation method is a fast and easy transient expression system enabling high transient expression and transformation efficiency in upland cotton seedlings. Our method can be used for gene functional studies such as promoter characterization and protein subcellular localization in cotton, obviating the need to perform such studies in a heterologous system such as Arabidopsis.
Similar content being viewed by others
Background
Cotton (Gossypium spp.) is a very important economic crop, providing the basic resource for thousands of consumer and industrial products worldwide and its contribution to the fiber and food industries continues to grow in importance. However, cotton growers face a number of important challenges such as insect pests, weeds, and viruses that result in substantial economic losses [1, 2]. Therefore, it is important to improve the agronomic performance of cotton, and especially to enhance the insect and disease resistance of cotton plants as well as cotton fiber quality and yield.
The development of transgenic techniques for cotton in the last decade has contributed to improvements in insect resistance, fiber quality and yield [3, 4]. Despite the fact that transgenic cotton lines were first produced 30 years ago [5, 6], cotton transformation is still very difficult, inefficient and time consuming compared to other crops (e.g., rice, wheat and tomato) [3, 7]. Agrobacterium-mediated transformation is the preferred method to introduce transgenes into cotton and new transformation vectors, culture methodologies and techniques have had a noticeable but limited impact in the original transformation efficiency [8, 9]. Stable transformation is crucial for the implementation of the recently developed CRISPR (clustered regularly interspaced short palindromic repeats)/Cas9 genome editing technology and has been used in many animal and plant species, including cotton [10,11,12,13,14]. Although transient expression systems have inherent limitations, they can have important applications for gene functional studies as well as the rapid and scalable production of recombinant proteins in plants.
Most of the world’s cotton fiber is derived from G. hirsutum, the most widely cultivated cotton species [15]. This species is an allotetraploid with a complex genome, and most of its genes are present as multiple copies located in the At and Dt subgenomes. In recent years, two draft genome maps of G. hirsutum have been produced with reference genome information from two diploid progenitors, Gossypium arboreum and Gossypium raimondii [15, 16]. The new genomic information has provided a treasure trove of genes with potential applications in cotton crop improvement, however the difficult, lengthy and inefficient transformation method has left most of those genes unexplored. Transient techniques such as virus-induced gene silencing (VIGS) can be powerful tools for functional genomic studies, and it has been used in cotton [17,18,19,20]. In addition to silencing approaches, fast transient expression methods can prove valuable for functional studies in cotton but have not been reported so far.
In this study, we describe the development of a transient expression system for cotton, and used it to analyze the localization and expression patterns of two previously characterized members of the glutathione peroxidase (GPX) gene family, GhGPX1 and GhGPX8 [21]. The Agrobacterium-mediated transient transformation method is a fast and easy transient expression system for systematic gene function analysis in upland cotton.
Methods
Plant material
Seeds of G. hirsutum (TM-1 variety) were wrapped in moist absorbent cotton, placed in Petri dishes and kept in an incubator under a 14/10 h light/dark photoperiod with light intensity of 150–200 μmol m−2 s−1 at 25 °C for 3 days to germinate. The seedlings were grown in sterile culture vessels with Hoagland’s nutrient solution [22] under long-day conditions (16/8 h light/dark photoperiod) with 26/20 °C day/night temperatures. After 4 days, the seedlings (before the first true leaf appeared) were used for Agrobacterium-mediated transformation.
Plasmid construction
To study the subcellular localization of GhGPX1 and GhGPX8, their coding sequences (CDS) were ligated in frame with the green fluorescent protein (GFP) coding region in the p35S-GFP vector, to construct the recombinant plasmid p35S-GhGPX1/8-GFP. Approximately 2 kb of the promoter regions of GhGPX1 and GhGPX8 were cloned (Fast Pfu DNA polymerase, Novoprotein) into the pCAMBIA1381 vector upstream of the GUS reporter gene as described previously [23], primers used in all PCRs were listed in Additional file 1.
Transient expression procedure
Recombinant binary vectors containing the different gene constructs were transformed into Agrobacterium EHA105. Agrobacterium liquid cultures were grown at 28 °C overnight in YEP medium containing 50 μg/mL kanamycin and 50 μg/mL rifampicin until the OD600 reached between 1.2 and 1.5. Cells were collected by centrifugation (2100 g, 15 min) and re-suspended to an OD600 0.8–1.0 in transformation solution: 1/2 Murashige and Skoog (MS) medium [24] (pH 5.8; Life Technologies), 165 μM acetosyringone (AS), 3% (w/v) sucrose, and 0.01% (v/v) Tween 20. Before use, the transformation solution (without Tween 20) was degassed using a diaphragm-type dry vacuum pump (Ulvac Kiko Inc., Kanagawa, Japan) under 0.7 kg/cm2 pressure for 3 min.
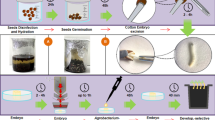
Seven-day-old seedlings of G. hirsutum were washed in double-distilled water (ddH2O), and sprayed with 70% ethanol. The seedlings were then immersed in 0.1% (w/v) HgCl for 2 min (If the sterilizing time is more than 2 min, the seedling would be harmed or stressed) and washed with sterile ddH2O five times, for 2 min each time. The aseptic seedlings were placed in sterile wide-necked bottles filled with vacuum treated transformation solution (without Tween 20) for 2 h, and seedlings were put in a sterile petri dish for vacuum infiltration using a vacuum pump under 0.7 kg/cm2 pressure for 2 min, then the seedlings were placed in the same sterile wide-necked bottles filled with vacuum treated transformation solution (with Tween 20) containing Agrobacterium and shaken at 25 °C for 5 h (120 rpm). After the co-cultivation, the seedlings were washed twice with sterile ddH2O, for 2 min each time and transferred onto 1/2 MS medium, pH 5.8, 0.8% (w/v) agarose that contained kanamycin 50 μg/mL, and rifampicin 50 μg/mL and kept under long-day conditions (16 h/8 h light/dark photoperiod) with light intensity of 80–90 μmol m−2 s−1 and 26/20 °C day/night temperatures. After 5 days, plants were used to analyze gene transcript levels and protein localization. Figure 1 summarizes the procedure to generate transient transgenic cotton lines, and photos of the experimental process are shown in Additional file 2.
Gene expression analyses
Total RNA was extracted from tissues of G. hirsutum using an RNAprep Pure Plant Kit (Polysaccharides & Polyphenolics-rich, Tiangen, Beijing, China). The cDNA was synthesized using M-MLV Reverse Transcriptase (Promega, Madison, WI, USA) and diluted 30-fold with deionized water before use as template for qRT-PCR. The PCR reaction was performed on a 7500 Fast Real-Time PCR System (Applied Biosystems, Palo Alto, CA, USA) using GoTaq® qPCR Master Mix kit (Promega). Transcript levels of GhGPX1 and GhGPX8 were normalized against an internal reference genes, GhUBQ1 (ubiquitin 1, accession number: EU304080) and GhUBQ7 (ubiquitin 7, accession number: DQ116441). The delta-delta Ct method was used for relative quantification data analysis in qRT-PCR experiments. At least three different sections of a single cotyledon from one seedling were processed separately as three technical replicates, and three different seedlings were used to make three biological replicates. All experiments for gene expression analysis were repeated at least for three independent biological and technical replicates were analyzed by qRT-PCR. All primers used in this study are listed in Additional file 1.
Histochemical detection of GUS activity
For GUS assays, excised tissues from cotton plants transiently expressing the GhGPX1 and GhGPX8 promoter-GUS construct were stained as previously described [25]. The GUS activity of the transformed cotton seedlings was determined according to Jefferson et al. [26]. For GUS activity assays, at least three different sections of a single cotyledon from one seedling were processed separately as three technical replicates, and three different seedlings were used to make three biological replicates.
Subcellular localization analyses
Transient expression of the recombinant vectors p35S-GhGPXs-GFP and control vector p35S-GFP was performed in G. hirsutum seedlings. GFP fluorescence was observed under a laser scanning confocal microscope (LSCM 710, Zeiss) with excitation at 488 nm and emission at 500–560 nm. Chloroplast autofluorescence was detected at 650–750 nm.
Results
Development of the transient expression method in G. hirsutum seedlings
Various factors can influence Agrobacterium-mediated transformation efficiency, including bacterial strain, Agrobacterium cell density, co-cultivation time, protein expression kinetics, chemicals and surfactants [8, 27]. To test the efficiency of the method we selected two previously characterized genes, GhGPX1 and GhGPX8 [21]. The promoter regions (~ 2 kb) of each gene were cloned upstream of GUS in the pCAMBIA1381 vector to make the recombinant plasmids, GhGPX1pro-GUS and GhGPX8pro-GUS. We first evaluated the transient expression efficiency of different Agrobacterium strains. GhGPX1pro-GUS was introduced into three commonly used Agrobacterium strains GV3101, EHA105 and LBA4404. GUS staining of inoculated cotton cotyledons showed the strongest GhGPX1pro-GUS expression in LBA4404-inoculated plants, and the lowest in GV3101-inoculated plants (Fig. 2a). Consistent with this result, cotyledons of seedlings transiently transformed with LBA4404 showed the highest GUS activity, ~ 4-fold higher than EHA105, and 10-fold higher than GV3101-inoculated plants (Fig. 2b). It is well known that Agrobacterium infection can cause wound-associated responses [28]. To study the extent of the wound-response caused by each of the three Agrobacterium strains tested, we measured the expression levels of the wound-inducible gene GhWRKY40 [29]. As shown in Fig. 2c, LBA4404 infection caused a ~ 7-fold increase in the GhWRKY40 expression levels compared to WT, while EHA105- and GV3101-mediated transformation produced lower levels of GhWRKY40 expression with 4-fold and 2-fold increases over WT respectively. Therefore, we chose to use EHA105 for the development of the transient transformation system as a compromise to obtain meaningful expression levels while minimizing wound-induced gene expression.
Expression patterns of GhGPX1 promoter-GUS constructs and quantification of wound-inducible GhWRKY40 expression levels in G. hirsutum plants inoculated with different Agrobacterium strains. GUS staining (a) and GUS activity in cotyledons (b) of plants transiently expressing GhGPX1 promoter-GUS constructs using Agrobacterium EHA105, GV3101 and LBA4404 strains; at least three independent seedlings were used for GUS staining; GUS activity values are the means of approximately 5 seedlings. c qRT-PCR analysis of wound-inducible GhWRKY40 gene transcript levels in cotyledons of WT and transiently expressing seedlings. The reference gene GhUBQ7 was used for normalization purposes in the qRT-PCR assays. For each sample of GUS activity assay, at least three different sections of a single cotyledon from one seedling were processed separately as three technical replicates, and three different seedlings were used to make three biological replicates. Asterisks indicate significant differences from WT at **p < 0.01 (two tailed Students t test)
To rule out that the observed GUS signal was not due to GUS expression from residual bacteria within the plant tissues, microscopic observations were performed on GUS stained tissues. Our microscopy analysis shows GUS staining confined to plant cells in stem, root and leaf samples (Additional file 3a–c). Moreover, GUS staining of a EHA105 culture containing the GhGPX1pro-GUS vector did not show any blue color (Additional file 3d).
To determine the optimal parameters for transient expression, the effect of Agrobacterium density was studied using EHA105 carrying the GhGPX1pro-GUS plasmid in cotton seedlings. Three Agrobacterium densities were tested, OD600 = 0.5, 0.9 and 1.3, with GUS signal being weak at OD600 = 0.5, and progressively increasing with higher bacterial densities (Fig. 3a, b). However, the expression levels of GhWRKY40 increased nearly 2-, 4-, and 6-fold with Agrobacterium densities of OD600 = 0.5, 0.9 and 1.3, respectively (Additional file 4a). Although strong GUS activity was detected in seedlings inoculated with an OD600 = 1.3, these seedlings were often susceptible to bacterial overgrowth and showed high wound-induced gene expression.
Expression patterns of GhGPX1 promoter-GUS constructs in EHA105-inoculated G. hirsutum plants under different transformation condition. GUS staining (a, c, e, g) and relative GUS activity (b, d, f, h) of plants transiently expressing GhGPX1 promoter constructs using Agrobacterium EHA105; a, b, effect of different Agrobacterium concentrations; c, d, GhGPX1 expression levels at different times after transformation; e, f, effect of different acetosyringone (AS) concentrations; g, h, effect of different types and concentrations of surfactants. At least three independent seedlings were used for GUS staining. GUS activity values are the means of approximately 5 seedlings. The GUS activity of plants transformed with an Agrobacterium density of OD600 = 0.5 (a), infected for 3 days (b), using an AS concentration of 80 μM (c) and no surfactants (d) were given an arbitrary value of 1. For each sample of GUS activity assay, at least three different sections of a single cotyledon from one seedling were processed separately as three technical replicates, and three different seedlings were used to make three biological replicates. Asterisks indicate significant differences from WT at *p < 0.05 and **p < 0.01 (two tailed Students t-test)
To determine the kinetics of protein production in our method, we performed GUS staining and measured GUS enzymatic activity at different times after inoculation of cotton seedlings using GhGPX1pro-GUS. GUS staining was visible 3 days after transformation and steadily increased until day 7 (Fig. 3c) and GUS activity assays confirmed the staining results with day 7 showing double the amount of activity than at day 3 (Fig. 3d). The expression levels of GhWRKY40 increased nearly 2-, 3-, and 7-fold at days 3, 5, and 7 after Agrobacterium transformation respectively (Additional file 4b). Thus, we considered 5 days after infection as the optimal time for functional studies.
We also optimized the concentrations of different transformation components such as AS and surfactants using GhGPX1pro-GUS. Three different AS concentrations, 80, 165 and 300 μM, were used in transient assays with GUS staining and activity being highest at 165 μM (Fig. 3e, f), meanwhile the GhWRKY40 transcript levels did not show any significant differences among the three AS concentrations (Additional file 4c). The effect of different concentrations of two surfactants, Tween 20 and silwet L77, on GUS expression was tested. Our results showed that the presence of either 0.03 or 0.05% (v/v) silwet L77 in the transformation buffer produced a dramatic increase in GUS expression (Fig. 3g, h), but it also resulted in a strong increase in GhWRKY40 expression levels (Additional file 4d). The addition of 0.01% (v/v) Tween 20 to the transformation buffer increased GUS activity significantly (Fig. 3g, h) and had no effect in the GhWRKY40 expression levels compared with the use of no surfactants (Additional file 4d). Given these results, we chose the use of 165 μM AS and Tween 20 as the optimal parameters for our transient transformation system.
Vacuum pre-infiltration of seedlings with transformation solution before Agrobacterium inoculation is important to maximize Agrobacterium growth. We tested whether inclusion of Agrobacterium in the pre-infiltration solution improved efficiency but seedlings pre-infiltrated with buffer containing Agrobacterium died within 2 days of inoculation (Additional file 5). We also investigated whether the use of the antibiotic cefotaxime in the media to control Agrobacterium overgrowth could improve efficiency. We tested three different experimental conditions using (a) cefotaxime-containing media; (b) growth in rifampicin and kanamycin containing media for 5 days; and (c) initial growth in rifampicin and kanamycin containing media for 3 days followed by transfer to cefotaxime-containing media for 2 days (Additional file 6). The strongest GUS staining intensity was observed when seedlings were continuously grown on rifampicin and kanamycin containing media, while no staining was observed when seedlings were grown on cefotaxime-containing media (Additional file 6).
To determine the universality of the method, we performed transient assays in seedlings of three different cotton varieties, TM-1, CCRI36 and Coker201 using Agrobacterium, strain EHA105 containing the GhGPX1pro-GUS vector. GUS staining was visible in all three cultivars (Additional file 7) with the strongest staining observed on the TM1 variety which was chosen for all subsequent experiments.
Generation of G. hirsutum seedlings with strong transgene expression
Agrobacterium tumefaciens, EHA105 strain, carrying the GhGPX1pro-GUS or GhGPX8pro-GUS plasmids were used for the transient transformation of G. hirsutum seedlings by the procedure developed in this study and plants were grown on antibiotic-containing (kanamycin and rifampicin) MS medium. As shown in Additional file 8, the overall transformation efficiency was 57.6% (number of seedlings harboring GhGPX1/GhGPX8pro-GUS after 8 days/total number of seedlings infected with Agrobacterium).
In order to study the expression levels achieved by our transient transformation method, the coding regions of GhGPX1 and GhGPX8 were fused in frame with GFP under the control of the CaMV 35S promoter generating the p35S-GhGPX1-GFP and p35S-GhGPX8-GFP plasmids. Seedlings subjected to transient transformation were analyzed by qRT-PCR using the endogenous reference gene GhUBQ7 as control (accession number: DQ116441), showing high expression levels of the transgenes (Fig. 4a, b). The GhGPX1 transcript levels were 5–11 times higher than those observed for untransformed controls (Fig. 4a) while GhGPX8 showed 3–5 times higher levels than controls (Fig. 4b). To confirm our results a second endogenous reference gene, GhUBQ1 (accession number: EU304080), was used in the qRT-PCR assays with similar results (Additional file 9). These results confirmed the success of our method in generating cotton plants transiently overexpressing target genes.
Quantification of transgene expression levels in un-inoculated controls and EHA105-inoculated G. hirsutum plants. GhGPX1 (a) and GhGPX8 (b) transcript levels in cotyledons of control and transiently expressing seedlings was determined by qRT-PCR. Three independent sets of seedlings (35S:GhGPX1-1/2/3 and 35S:GhGPX8-1/2/3) were inoculated and a minimum of six different cotyledon sections from each seedling were used for the qRT-PCR assay. Endogenous WT level for each gene was given the arbitrary value of 1. Asterisks indicate significant differences from WT at **p < 0.01 (two tailed Students t-test)
Characterization of the GhGPX1 and GhGPX8 promoters
Seedlings transiently transformed with the GhGPX1pro-GUS and GhGPX8pro-GUS constructs were studied to characterize the expression patterns driven by the promoters of the two genes. Histochemical staining revealed that GhGPX1pro-GUS was expressed throughout the plant, including cotyledons (Fig. 5a. i), stems (Fig. 5a. ii) and roots (Fig. 5a. iii), with the strongest staining detected in the cotyledons. GUS activity assays confirmed the histochemical observations with cotyledon and stem GUS activity values being 3- and 2.5-fold higher than root respectively (Fig. 5b). Whole seedlings transformed with GhGPX8pro-GUS showed strong staining in stems (Fig. 5c ii) and roots (Fig. 5c iii), while staining in cotyledons (Fig. 5c i) was weaker. Again, GUS activity assays confirmed the histochemical observations (Fig. 5d). These results are consistent with the GhGPX1 and GhGPX8 endogenous transcript levels quantified by qRT-PCR in untransformed G. hirsutum plants (Fig. 5e, f), indicating that the Agrobacterium-mediated transient transformation method was suitable for promoter characterization in cotton.
Expression patterns of GhGPX1 and GhGPX8 promoter-GUS constructs in G. hirsutum plants. GUS staining of plants transiently expressing GhGPX1 and GhGPX8 promoter constructs (a) and (c); expression patterns in whole seedlings (i), stems (ii), and roots (iii) were visualized by histochemical GUS staining. Relative GUS activity in root, stem and cotyledon of plants transiently expressing GhGPX1 (b) or GhGPX8 (d) promoter constructs. e, f Quantification of endogenous GhGPX1 and GhGPX8 transcript levels by qRT-PCR in WT non-inoculated plants. For each experiment, tissue from three different 12-d-old G. hirsutum seedlings was collected. For all experiments on gene expression analysis, at least three different sections of each tissue from one seedling were processed separately as three technical replicates, and three different seedlings were used to make three biological replicates were performed. Asterisks indicate significant differences from root tip at **p < 0.01 (two tailed Students t-test)
GhGPX1 and GhGPX8 are localized in the chloroplast and cytoplasm, respectively
We also determined whether our method is suitable for intracellular localization studies by analyzing the GFP fluorescence patterns in the epidermis of seedlings transiently expressing either GhGPX1-GFP or GhGPX8-GFP fusion proteins. Compared with the control transformed with the empty p35S-GFP vector (Fig. 6a), transient transformation with the p35S-GhGPX1-GFP construct produced a strong fluorescence signal confined to chloroplasts and especially strong in guard cells (Fig. 6b), consistent with our previous finding that GhGPX1 was located in the chloroplasts of Arabidopsis protoplasts [21]. Seedlings expressing GhGPX8-GFP showed strong fluorescence in the cytoplasm of cotyledon and root cells (Fig. 6c), also consistent with our previous results showing that GhGPX8 was located in the cytoplasm of Arabidopsis protoplasts [21]. These results confirmed that the transient transformation method was suitable for subcellular localization studies in cotton.
Subcellular localization of GFP-tagged GhGPX1 and GhGPX8 in G. hirsutum. a Transient expression of control vector pro35S-GFP. b Transient expression of the Pro35S-GhGPX1-GFP construct resulted in strong GFP fluorescence in the chloroplasts of epidermal and guard cells detected by confocal microscopy. Pictures in the bottom line show a close up of a guard cell. c Confocal microscopy of plants transiently expressing the Pro35S-GhGPX8-GFP construct shows fluorescence in the cytosol of epidermal and root cells. White bars, 5 μm; red bars, 10 μm; yellow bars, 100 μm. At least 7 different seedlings from three independent biological and technical replicates were used for each experiment with similar results
Discussion
We have developed a transient expression system to perform rapid and technically easy gene characterization studies in G. hirsutum. Plant transformation is a core research tool for cotton improvement as well as gene functional studies. Although, several transformation methods have been developed and refined to increase transformation efficiency and stably express transgenes in cotton, they are technically complex and time-consuming [30].
Agrobacterium-mediated transient transformation is a complex process with many environmental and biological factors affecting its efficiency such as the composition of the culture medium, the binary plasmid vector, the host plant and the Agrobacterium strain [9, 29,30,31,32,33]. We established the optimal parameters to obtain high efficiency as well as high expression levels of the target gene in G. hirsutum. The choice of optimal conditions for the assay needs to take into account the purpose of the experiment. A number of factors have a dramatic effect on the efficiency of the transformation system as well as the levels of expression achieved but can also result in secondary and unwanted effects. For purposes, such as promoter studies and overexpression of transgenes, the Agrobacterium strain EHA105 was selected because it produced good GUS activity levels, although not the highest, and a moderate wound response (Fig. 2). Similar consideration was given to the Agrobacterium concentration, with OD600 0.8–1.0 resulting in good efficiency, while higher concentrations led to seedling death and low concentrations resulted in few or no plants showing transient expression; the optimal co-cultivation period was 6 h, with shorter or longer incubation time negatively affecting efficiency and/or leading to seedling browning or death (Additional file 10). Previous studies have shown that stable expression requires T-DNA integration into the host genome, and that the use of selection results in an increase in transgene expression levels 10–14 days post infection of plant tissues [34]. Indirect evidence indicates that transient expression predominantly occurs from T-DNA copies that are not integrated into the host genome [35, 36]. Transient expression usually peaks 2–4 days post infection before declining in the number of expressing cells as well as the expression level per transformed cell but the kinetics ultimately depends on the host plant genotype [35]. In this study, we observed that gene transcript levels varied over time and selected materials at 5 days post infection for the analyses of gene expression studies (Fig. 3c, d; Additional file 4b). For subcellular localization characterization, Agrobacterium GV3101 gave similar results to EHA105 (Additional file 11). Moreover, for localization studies, where secondary wounding effects do not influence the localization of most cellular proteins, different optimal parameters should be chosen such as the use of the LBA4404 strain, higher Agrobacterium density and silwet L77 as surfactant. We observed that wounding enhanced the transformation efficiency as well as the transcript levels of the transgenes (Fig. 3; Additional file 4). However, wounding significantly affects the steady state expression levels of many genes and therefore the wound-related results have not been included in this manuscript.
Our Agrobacterium-mediated transient transformation method for upland cotton is fast and easy to scale up to produce relatively large amounts of recombinant protein. Aside from the applications shown in this work, the method could also be used for protein–protein interaction studies and other research requiring the expression of genes in cotton. In future work, we will focus on further enhancing the transformation efficiency and performing gene functional studies in cotton.
Conclusion
We have developed a fast and easy Agrobacterium-mediated transient transformation method for upland cotton. Our method can be used for gene functional studies such as promoter characterization and protein subcellular localization in cotton, obviating the need to perform such studies in a heterologous system such as Arabidopsis.
Abbreviations
- G. hirsutum :
-
gossypium hirsutum
- CGP:
-
cotton genome project
- qRT-PCR:
-
quantitative real-time reverse transcriptase polymerase chain reaction
- T-DNA:
-
transferred DNA
- PCR:
-
polymerase chain reaction
- GFP:
-
green fluorescent protein
- GUS:
-
β-glucuronidase
- GPX:
-
glutathione peroxidase
- AS:
-
acetosyringone
References
Sattar MN, Kvarnheden A, Saeed M, Briddon RW. Cotton leaf curl disease - an emerging threat to cotton production worldwide. J Gen Virol. 2013;94:695–710.
Zhang Z, Zhao J, Ding L, Zou L, Li Y, Chen G, Zhang T. Constitutive expression of a novel antimicrobial protein, Hcm1, confers resistance to both Verticillium and Fusarium wilts in cotton. Sci Rep. 2016;6:20773.
Zhang B. Transgenic cotton: from biotransformation methods to agricultural application. Methods Mol Biol. 2013;958:3–15.
Cattaneo MG, Yafuso C, Schmidt C, Huang CY, Rahman M, Olson C, et al. Farm-scale evaluation of the impacts of transgenic cotton on biodiversity, pesticide use, and yield. Proc Natl Acad Sci USA. 2006;103:7571–6.
Firoozabady E, Deboer DL, Merlo DJ, Halk EL, Amerson LN, Rashka KE, et al. Transformation of cotton (Gossypium hirsutum L.) by Agrobacterium tumefaciens and regeneration of transgenic plants. Plant Mol Biol. 1987;10:105–16.
Umbeck P, Johnson G, Barton K, Swain W. Genetically transformed cotton (Gossypium hirsutum L.) plants. Bio-Technol. 1987;5:263–6.
Bajwa KS, Shahid AA, Rao AQ, Bashir A, Aftab A, Husnain T. Stable transformation and expression of GhEXPA8 fiber expansin gene to improve fiber length and micronaire value in cotton. Front Plant Sci. 2015;6:838.
Rao AQ, Bakhsh A, Kiani S, Shahzad K, Shahid AA, Husnain T, et al. The myth of plant transformation. Biotechnol Adv. 2009;27:753–63.
Ziemienowicz A. Agrobacterium-mediated plant transformation: factors, applications and recent advances. Biocatal Agric Biotechnol. 2014;3:95–104.
Chen X, Lu X, Shu N, Wang S, Wang J, Wang D, et al. Targeted mutagenesis in cotton (Gossypium hirsutum L.) using the CRISPR/Cas9 system. Sci Rep. 2017;7:44304.
Li C, Unver T, Zhang B. A high-efficiency CRISPR/Cas9 system for targeted mutagenesis in cotton (Gossypium hirsutum L.). Sci Rep. 2017;7:43902.
Wang P, Zhang J, Sun L, Ma Y, Xu J, Liang S, et al. High efficient multisites genome editing in allotetraploid cotton (Gossypium hirsutum) using CRISPR/Cas9 system. Plant Biotechnol J. 2017; 1–14.
Janga MR, Campbell LM, Rathore KS. CRISPR/Cas9-mediated targeted mutagenesis in upland cotton (Gossypium hirsutum L.). Plant Mol Biol. 2017;94:349–60.
Gao W, Long L, Tian XQ, Xu FC, Liu J, Singh PK, et al. Genome editing in cotton with the CRISPR/Cas9 system. Front Plant Sci. 2017;8:1364.
Zhang T, Hu Y, Jiang W, Fang L, Guan X, Chen J, et al. Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nat Biotechnol. 2015;33:531–7.
Li F, Fan G, Lu C, Xiao G, Zou C, Kohel RJ, et al. Genome sequence of cultivated upland cotton (Gossypium hirsutum TM-1) provides insights into genome evolution. Nat Biotechnol. 2015;33:524–30.
Gao X, Britt RC Jr, Shan L, He P. Agrobacterium-mediated virus-induced gene silencing assay in cotton. J Vis Exp. 2011;54:e2938.
Gao X, Shan L. Functional genomic analysis of cotton genes with Agrobacterium-mediated virus-induced gene silencing. Methods Mol Biol. 2013;975:157–65.
Pang J, Zhu Y, Li Q, Liu J, Tian Y, Liu Y, Wu J. Development of Agrobacterium-mediated virus-induced gene silencing and performance evaluation of four marker genes in Gossypium barbadense. PLoS ONE. 2013;8:e73211.
Mustafa R, Shafiq M, Mansoor S, Briddon RW, Scheffler BE, Scheffler J, et al. Virus-induced gene silencing in cultivated cotton (Gossypium spp.) using Tobacco rattle virus. Mol Biotechnol. 2016;58:65–72.
Chen M, Li K, Li H, Song C-P, Miao Y. The glutathione peroxidase gene family in Gossypium hirsutum: genome-wide identification, classification, gene expression and functional analysis. Sci Rep. 2017;7:44743.
Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Calif Agric Exp Stn Circ. 1950;347:1–39.
Li K, Yang F, Zhang G, Song S, Li Y, Ren D, et al. AIK1, a mitogen-activated protein kinase, modulates abscisic acid responses through the MKK5-MPK6 kinase cascade. Plant Physiol. 2017;173:1391–408.
Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–97.
Song CP, Agarwal M, Ohta M, Guo Y, Halfter U, Wang P, et al. Role of an Arabidopsis AP2/EREBP-type transcriptional repressor in abscisic acid and drought stress responses. Plant Cell. 2005;17:2384–96.
Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–7.
Wu HY, Liu KH, Wang YC, Wu JF, Chiu WL, Chen CY, et al. AGROBEST: an efficient Agrobacterium-mediated transient expression method for versatile gene function analyses in Arabidopsis seedlings. Plant Methods. 2014;10:19.
Stachel SE, Messens E, Montagu MV, Zambryski PC. Identification of the signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium tumefaciens. Nature. 1985;318:624–9.
Wang X, Yan Y, Li Y, Chu X, Wu C, Guo X. GhWRKY40, a multiple stress-responsive cotton WRKY gene, plays an important role in the wounding response and enhances susceptibility to ralstonia solanacearum infection in transgenic Nicotiana benthamiana. PLoS ONE. 2014;9:e93577.
Zhang B. Transgenic cotton methods and protocols. New York: Humana Press; 2013. p. 31–79.
Mohammad AM, Bagherieh-Najjar M. Agrobacterium-mediated transformation of plants: basic principles and influencing factor. Afric J Biotechnol. 2009;8:5142–8.
Kavitah G, Taghipour F, Huyop F. Investigation of factors in optimizing Agrobacterium mediated gene transfer in Citrullus lanatus cv round dragon. J Biol Sci. 2010;10:209–16.
Sood P, Bhattacharya A, Sood A. Problems and possibilities of monocot transformation. Biol Plant. 2011;55:1–15.
Janssen BJ, Gardner RC. Localized transient expression of GUS in leaf discs following cocultivation with Agrobacterium. Plant Mol Biol. 1990;14:61–72.
Lacroix B, Citovsky V. The roles of bacterial and host plant factors in Agrobacterium-mediated genetic transformation. Int J Dev Biol. 2013;57:467–81.
Krenek P, Samajova O, Luptovciak I, Doskocilova A, Komis G, Samaj J. Transient plant transformation mediated by Agrobacterium tumefaciens: principles, methods and applications. Biotechnol Adv. 2015;33:1024–42.
Authors’ contributions
YM and CS conceived and designed the experiments. HL, YG, KM, and KL performed the experiments. YM, HL, JG, JB, and KL analyzed the data. YM, HL, JG, and KL contributed reagents/materials/analytical tools. YM, JB and KL wrote the paper. All authors read and approved the final manuscript.
Acknowledgements
We thank Professor Yan Guo from China Agricultural University, for providing the p35S-GFP plasmid.
Competing interests
The authors declare that they have no competing interests.
Accession numbers
Sequence data from this report have been reported in our previous paper [21], GhUBQ1 (ubiquitin 1, EU304080); GhUBQ7 (ubiquitin 7, DQ116441); GhGPX1 (CotAD_10469); GhGPX8 (CotAD_59095); GhWRKY40 (KC414679).
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This work was supported by the National Key Research and Development Program (2016YFD0101006), the National Natural Science Foundation of China (31770300), the Program for Innovative Research Team (in Science and Technology) in University of Henan Province (18IRTSTHN023), and 111 project of China.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1.
Primers used in this study.
Additional file 2.
Pictorial representation of the Agrobacterium-mediated transient transformation of G. hirsutum.
Additional file 3.
Transient GUS expression in cotton plants by GhGPX1pro-GUS-contained EHA105.
Additional file 4.
Relative GhWRKY40 expression levels in EHA105 Agrobacterium-inoculated G. hirsutum plants under different transformation conditions.
Additional file 5.
Transient transformation of cotton seedlings with GhGPX1pro-GUS under vacuum infiltration with or without Agrobacterium in the transformation solution.
Additional file 6.
GUS staining of transiently transformed cotton seedlings.
Additional file 7.
GUS staining of G. hirsutum cultivars CCRI36, Coker201 and TM-1 after transient transformation with A. tumefaciens EHA105 containing the GhGPX1pro-GUS vector.
Additional file 8.
Transformation efficiency.
Additional file 9.
Quantification of relative expression levels for GhGPX1 and GhWRKY40 in Agrobacterium-inoculated G. hirsutum plants using two different endogenous reference genes.
Additional file 10.
GUS staining of plants transiently expressing GhGPX1 promoter constructs using Agrobacterium EHA105-mediated transient transformation at different times after inoculation.
Additional file 11.
Subcellular localization of GFP-tagged GhGPX1 in G. hirsutum plant leaves using Agrobacterium GV3101-mediated transient transformation.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, H., Li, K., Guo, Y. et al. A transient transformation system for gene characterization in upland cotton (Gossypium hirsutum). Plant Methods 14, 50 (2018). https://doi.org/10.1186/s13007-018-0319-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13007-018-0319-2