Abstract
Background
Oral squamous cell carcinoma (OSCC) accounts over 90% of malignant neoplasms of the oral cavity. This pathological entity is associated to a high mortality rate that has remained unchanged over the past decades. Tumour-associated macrophages (TAMs) are believed to have potential involvement in OSCC progression. However, the molecular networks involved in communication between stroma and cancer cells have not yet been fully elucidated.
Main body
The role of M2 polarized cells in oral carcinogenesis is supported by a correlation between TAMs accumulation into OSCC stroma and poor clinical outcome. Signalling pathways such as the NF-κB and cytokines released in the tumour microenvironment promote a bidirectional cross-talk between M2 and OSCC cells. These interactions consequently result in an increased proliferation of malignant cells and enhances aggressiveness, thus reducing patients’ survival time.
Conclusions
Here, we present a comprehensive review of the role of interleukin (IL)-1, IL-4, IL-6, IL-8, IL-10 and the receptor tyrosine kinase Axl in macrophage polarization to an M2 phenotype and OSCC progression. Understanding the molecular basis of oral carcinogenesis and metastatic spread of OSCC would promote the development of targeted treatment contributing to a more favourable prognosis.
Similar content being viewed by others
Background
Cancer pathogenesis events take place in imbalanced microenvironments, where pathological states do not affect only neoplastic cells [1–3]. Instead, cancerous cells disrupt tissue homeostasis, disturbing different cell types and contributing to disease progression, through interactions with mediators of the immune system [4–6]. Oral squamous cell carcinoma (OSCC) is a solid tumour of epithelial origin that affects more than 400,000 individuals annually worldwide [7]. The mortality rate of this disease has remained largely unchanged for the last decades, with a 5-year survival under 50% [8]. There is compelling evidence that tumour-associated macrophages (TAMs) have potential involvement in the progression and metastatic spread of OSCC. The most important features associated with their presence in the lesion stroma include facilitation of angiogenesis, tumour cell invasion, augmentation of cell motility, persistent growth, and suppression of anti-tumour responses [9–13]. The signals involved in communication between tumour cells and macrophages have not yet been completely elucidated. However, the interaction among tumour and inflammatory cells seems to be bidirectional [14]. Here, we present the strategies by which tumour cells influence macrophage physiology to display a pro-tumour phenotype, and the contribution of TAMs to OSCC progression. Is given an overview of potential markers that could provide support for diagnostic, evaluation of clinical outcome, and be used as valuable antineoplastic targets.
Macrophage differentiation
In adults, inflammatory monocytes (CD64+/CD16- CCR2+ Ly6C+) constitutively originate tissue-resident macrophage populations [15, 16]. The exposure of these cells to microenvironmental stimuli results in complex phenotypic modifications in a time- and location-dependent manner [17–19]. The activation of different regulatory mechanisms and transcription pathways result in a vast spectrum of macrophage subtypes, of which M1 and M2 represent the extreme polarization phenotypes [18, 19]. The M1 polarization state depends on microbial stimulus and a T helper type 1 (TH1) cytokine profile (classical activation pathway). Whereas M2 polarization depends on a T helper type 2 (TH2) cytokine profile (alternative activation pathway) [20]. Interferon-gamma (INF-\( \gamma \)) and interleukin (IL)-4 secretion sustain an M1 and an M2 phenotype commitment, respectively [21]. M1 are innate immune effector cells that fight intracellular microbial challenges by means of reactive oxygen species and nitrogen intermediates. Activation of signal transducer and activator of transcription (STAT)-1 in M1 macrophages is important for optimal TH1 responses [22], such as direct tumour cell death [23, 24]. M2 macrophages block TH1 and differentiate in the tumour stroma from blood monocytes, or resident macrophages in resting state, after making contact with neoplastic cells presenting aberrant production of certain cytokines [18]. Additionally, they promote cancer progression by STAT-3 activation, inducing and maintaining a pro-carcinogenic inflammatory microenvironment [25].
OSCC cells and TAM interactions
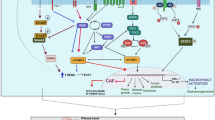
Histopathologically, OSCC presents as fibrous connective tissue with unusual amounts of extracellular matrix rich in fibroblasts, vascular vessels, and inflammatory cells [26]. Among the local milieu of OSCC stromal spaces, rich in perlecans and inflammatory cells, monocytes or resting macrophages are differentiated into LyC16high, CD163+, CD204+, and CD68+ expressing TAMs. These cells are considered of utmost biological importance for disease progression and correlate with increased dedifferentiation in primary tumour sites [27–29]. Moreover, TAMs elicit tumour relapse and/or post-operative cervical lymph node metastasis via angiogenesis and suppression of anti-tumour immunity [9]. An increase in the number of CD163+ macrophages occurs in oral leukoplakia. However, they co-express phosphorylated STAT-1, suggesting that in premalignant lesions TAMs possess an M1 phenotype in a dominant TH1 microenvironment [30]. Polarization to an M2 TAM phenotype probably occurs gradually and early during the onset of cancer. It is suggested that several interleukins (IL-1, IL-4, IL-6, IL-8, and IL-10), and other factors, such as the receptor tyrosine kinase Axl, participate in promoting this phenomenon. In the next sections we propose a topic structured discussion of relevant findings that corroborate this theoretical assumption. Figure 1 briefly reviews the effect of interleukins on TAMs present in OSCC stroma.
Macrophages in resting state suffer microenvironmental effects coordinated by OSCC cells. Interleukin (IL)-1, IL-4, IL-6, IL-8 and IL-10 (not shown), and Gas-6 are produced by OSCC cells and promote macrophage phenotype switching to an M2 polarization state. In turn, TAMs augment the recruitment of chemotactic receptors to tumour sites, induce tumour proliferation, and favour angiogenesis and invasiveness [31, 32, 36, 40–43, 47, 48, 65, 66]
IL-1
Tumour released IL-1 cross-talks to TAMs and induces M2 polarization to an immunosuppressive phenotype via IL-1 receptor (IL-1R) and myeloid differentiation primary response gene 88 (MyD88), which requires I-kappaB kinase beta (IKKβ)-mediated nuclear factor kappa B (NF-κB) [31, 32]. Interleukin-1 beta (IL-1β) is a critical mediator of chronic inflammation and is implicated in OSCC during early and late stages of carcinogenesis. Pro-IL-1β is upregulated in tobacco and betel quid related oral cancer, and is secreted in an inflammasome-dependent manner [33], although it is absent in homeostatic conditions. In the presence of IL-1β TAMs suffer an upregulation of C-X-C motif chemokine receptors (CXCR), especially CXCR4, induced by the activation of extracellular signal regulated MAP kinase (ERK). Macrophages then become attracted by CXCR4 ligands, like stromal cell derived factor-1 alpha (SDF-1α) [34]. SDF-1α is highly inducible in hypoxic and proangiogenic niches, where it reinforces the autocrine/paracrine loop that contributes to an M2 phenotype [35]. Nevertheless, OSCC cells and TAMs together through IL-1β/IL-1R and CXCR4/ SDF-1α and via activation of the ERK signalling pathway produce tumour cell migration and invasion by inducing expression of matrix metalloproteinase (MMP) enzymes MMP-9 and MMP-13 [36]. These important angiogenic modulating enzymes promote the acquisition of vasculature for oxygenation, nutrition, and waste disposal, which are of fundamental importance for tumour growth [2]. Contrarily, the blockade of CXCR4 by the antagonist 1,1′-[1,4-phenylenebis(methylene)]bis-1,4,8,11-tetraazacyclotetradecane octahydrochloride (AMD3100) inhibited SDF-1 mediated lymph node metastasis [37]. Furthermore, a rich IL-1β microenvironment promotes CXCL1 production, and through CXCR2 this induces tyrosine phosphorylation of the endothelial growth factor receptor (EGFR) [33]. As a result, EGFR activates pathways leading to cell growth, DNA synthesis, and the expression of oncogenes like fos and jun [38]. IL-1α is found in tumour cell membranes and in intracellular locations, and is produced in larger amounts than IL-1β in highly metastatic tumours. Despite the lack of evidence that demonstrates its direct participation in OSCC progression through macrophage activation, IL-1α interacts with fibroblasts in the stroma. Therefore, IL-1α acts promoting cell proliferation and upregulating the secretion of IL-8, CXCL-1, and chemokine C-C motif ligand (CCL)-7 [39]. Coincidently, these cytokines are also commonly produced by TAMs, rising the hypothesis of a plausible interaction between OSCC and M2 cells by means of IL-1α.
IL-4
IL-4 is an anti-inflammatory and immunomodulatory cytokine that has been identified as a relevant factor for the activation of TAMs, as well as IL-1. Furthermore, an increased expression of IL-4 receptor alpha (IL-4Rα) correlates with increased OSCC recurrence [40]. Regarding this tumour entity, the interaction between malignant cells and TAMs occurs through the plasminogen activator urokinase (uPA) and its specific receptor uPAR, mainly through the activation of ERK1/2 and increase in the production of IL-4. In OSCC cells this receptor modifies several transduction pathways, affecting neoplastic cell behaviours and acts as a promoter of survival, proliferation, and metastasis [41–43]. The high levels of IL-4 produced modifies the tumour microenvironment and facilitates an increase in arginase-1 levels, considered a biomarker of TAMs [43]. Similarly, this citokine induces cathepsin protease activity in TAMs, where they activate proteins including growth factors, transcription factors, and other proteases, such as MMPs [44]. Cathepsin B is considered a reliable marker for OSCC poor prognosis, correlating to higher tumour grade and lymph node metastasis [45].
IL-6
IL-6 expression in OSCC has been related to high lymph node metastatic rates and poor tumour differentiation, especially in male patients [46]. SDF-1alpha increases secretion of IL-6 in cultured human OSCC cells via CXCR4, ERK, and NF-κB pathways [47], in a similar manner to that seen for IL-1β/IL-1R. Moreover, the aberrant synthesis of IL-6 by neoplastic cells may be controlled by the CXCR4-specific inhibitor AMD3100 [47]. The calcium binding protein S100A9, associated with loss of differentiation and recurrence, tends to be deregulated in both tumour and stromal cells. The expression of S100A9 in monocytes exerts a tumour-promoting effect upon co-culture with oral cancer cells, in particular by releasing IL-6 and the activation of NF-κB or STAT-3 that is not achieved in tumour cell monoculture [48]. In response to apoptotic tumour cell supernatants, signalling patterns were identified that contributed to the TAMs phenotype. Two targets, IL-4Rα and cannabinoid receptor 2 (CB2), were validated and confirmed to regulate both IL-6 and IL-10 production in TAMs, contributing to autocrine/paracrine activation of STAT-3 in macrophages and tumour cells [49]. These findings emphasise the relevance of tumour cells and TAMs interactions for disease progression.
IL-8
IL-8 is a pro-angiogenic, pro-inflammatory mediator important for OSCC angiogenesis progression [50]. The mitogen activated protein kinases (MAPK) pathway is used by OSCC IL-8 to activate angiogenic activity in TAMs [51] augmenting, for example, vascular endothelial growth factor (VEGF) production. The receptors CXCR1 and CXCR2 have been detected in both oral normal keratinocytes and OSCC cells, where they exhibit higher expression. The presence of IL-8 CXC receptors in tumour cells increases ERK phosphorylation and MMP-7 and MMP-9 release, representing a tendency to proliferation, migration, and invasion [52]. Is important to consider that matrix metalloproteinase enzymes are essential for the achievement of a complete angiogenic potential of TAMs. At the same time, the progressive development of the tumour requires vast vasculature. Chronic periodontitis and tobacco consumption have both historically been associated to oral cancer. Then, recent published works propose the following interesting associations that also support the important role of IL-8 in OSCC progression. It is probable that Porphyromonas gingivalis contributes to OSCC progression, increasing IL-8 levels in the microenvironment and upregulating MMPs [53]. Nicotine also increases IL-8 release in OSCC, binding to the nicotine acetylcholine receptor (nAChR) and inducing calcium influx, that phosphorylates Ca(2+)/calmodulin-dependent kinase II (CaMK II) and NF-κB [54].
IL-10
In more dedifferentiated tumour niches the microenvironment progressively acquires an immunosuppressive profile [1–4]. IL-10 is a cytokine that modulates immune responses, causing suppressive regulatory T cell differentiation that contributes to tumour cell proliferation [55]. Since persistent viral infection promotes IL-10 upregulation and impaired T-cell responses [56], it is believed that this cytokine plays a critical role in human papilloma virus (HPV)- and Epstein-Barr virus (EBV)-related OSCC progression [57, 58]. Moreover, IL-10 indicates poor outcomes in HPV-unrelated OSCC, especially when INF-γ secretion [59] and transforming growth factor beta 1 (TGF-β1) levels [60] are low. Receptors for IL-22, a member of the IL-10 family, are highly expressed in OSCC cells, including in metastatic sites, compared to healthy regions. It was observed that in the OSCC MISK81-5 cell line, IL-22 induced the translocation of phosphorylated STAT-3 and upregulated the expression of Bcl-xL, survivin, and c-Myc, all known anti-apoptotic genes, as well as suppressor of cytokine signalling 3 (SOCS3) [61]. In this context, diverse pathways for IL-10 production by TAMs have been described, highlighting their contribution to an immunosuppressive state in the tumour stroma. TAMs present a defective TLR response caused by tumour-selective disruption of the MyD88 signalling cascade, and affect the TIR-domain-containing adapter-inducing interferon-β (TRIF)/TNF receptor associated factors (TRAF3)-dependent pathway in their own favour, leading to favourable transcription at the IL-10 promoter region [62]. In the presence of apoptotic tumour cell-factors like sphingosine-1-phosphate (S1P), TAMs use tyrosine kinase receptor A (TRKA), phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt), and MAPK signalling to induce IL-10 [63].
Gas6/Axl
TAMs acquire, possibly by cancer-derived factors like IL-10, the capacity to produce high levels of Gas-6 that promotes tumour development [64]. At the same time, in a bidirectional interaction, OSCC cells, that also produce Gas-6, polarize TAM toward a tumour-promoter phenotype. In OSCC, Gas-6 cooperates with Axl and achieves biological and clinical relevance by triggering the signalling pathways of PI3/Akt and NF-κB [65]. TAMs and OSCC interact in Gas-6/Axl axis-modulated epithelial-mesenchymal transition by upregulating cadherin, n-cadherin, and vimentin expression, and promoting cell invasion and migration. It was found that Axl expression correlates with clinical stage and lymph node status in OSCC patients. Moreover, TAMs count was associated with phosphorylated Axl immunoactivity in OSCC tissues [13, 65, 66]. Gas-6/Axl and NF-κB may be interesting targets for therapeutic intervention, since NF-κB promotes cancer resistance to apoptosis and production of growth factors in the stroma, which stimulates tumour progression [31].
Role of TAMs in OSCC histopathological diagnosis
Although further clinicopathological studies are needed before interactions between stromal cells and malignant cells can be defined as a key process for OSCC progression, evidence suggests that TAMs play several tumour-promoter roles during carcinogenesis [10, 28]. The presence of these polarized cells should be used as a potential marker to distinguish incipient OSCC from invasive lesions, avoiding underdiagnoses. As indicated by Matwaly et al. [67], the oral mucosa lacks an objective, standard-like structure that is found in other anatomical regions like the oesophagus, which makes the detection of invasiveness in oral cancer demanding. For a better understanding of TAMs in OSCC, more studies are necessary to define, by means of gene profiling, macrophage subpopulations with different tumour promoting abilities. A better indicator of the dynamic regulation of macrophage phenotype may be cellular cytokines, evaluated by means of tests conducted over multiple time points [67]. However, this methodology is time and cost demanding and probably unfeasible in clinical situations, especially in less developed countries where the prevalence of OSCC is higher. However, from the available evidence, it is possible to suggest that screening for TAM markers in oral biopsies certainly may contribute to accurate assessment of OSCC behaviour, being a valuable tool for the estimation of prognosis in cases related and unrelated to viral infection [57–60, 68].
New diagnostic alternatives
Weber et al. [27] propose that even trauma from incisional biopsies might influence tumour biology leading to a worse prognosis and increased risk of developing lymph node metastases in OSCC patients. A wound-healing reaction consecutive to tissue trauma probably provides a microenvironmental stimulus that affects macrophage polarisation [69]. Until the present, diagnostic procedures and therapeutic planning for OSCC have been supported mainly by histopathological findings. Despite being inviable at present, mostly due to the lack of standardized techniques, interpretation, and validation of parameters, the development of new minimal invasive diagnostic strategies should consider the screening of salivary and serum markers that reflect tumour behaviour, associated or not with the improvement of classical techniques like exfoliative cytology. Several studies have demonstrated valuable associations among OSCC clinical stages and prognosis, and salivary or serum markers associated with TAM’s dynamic participation in the tumour stroma [58, 68, 70–73]. Although salivary markers associated to TAM polarization are not yet used as parameters for definitive diagnoses, they should be taken into consideration to evaluate patients with potent malignant disorders, like proliferative verrucous leukoplakia [74], as well as for recurrence in OSCC treated patients.
Targeting TAMs in OSCC therapeutics
TAMs are potential targets for combination therapy in cancer treatment [75]. As we move forward, comprehension of the role of stromal cells in OSCC progression, suggest that therapies that only target TAMs may be possible, leading to an imbalance in tumour growth and invasiveness [75, 76]. However, despite its conceivable relevance, essentially mostly from positive clinical implications, the research in this field is incipient among cancer researchers. Recently a few studies have proposed targeting TAMs pathways to block cancer development [77, 78]. Signalling pathways such as the NF-κB and cytokines released in the tumour microenvironment through OSCC cells and TAMs interactions are attractive targets [79]. Inhibitors of cytokines involved in tumour signalling present potential for use to combat cancer, specially those implicated in promoting a malignancy cycle between OSCC cells and TAMs. Considering that chirurgical approaches are gold standard procedures for OSCC treatment, chemical interventions would be considered of lesser importance. However, it is relevant to underscore that during the last 30 years the disease-free survival and overall survival rates of OSCC patients have remained unchanged, perhaps due to limited care access or professional failures in performing early diagnoses, which is of the utmost relevance for prognosis. For these cases in particular, new therapeutic options are urgently needed.
Conclusions
Impaired tumour-preventive responses in OSCC are promoted by malignant cells and by soluble factors of the microenvironment that attract and polarize macrophages to a tumour-promoting state. Besides, macrophages reinforce the loop that promotes cancer growth and metastasis. This link between inflammation and cancer regulate OSCC progression and signalling pathways that provide a cross-talk between cancer cells and TAMs should be taken into consideration as valuable antineoplastic targets.
Abbreviations
- Akt:
-
Protein kinase B
- AMD3100:
-
1,1′-[1,4-phenylenebis(methylene)]bis-1,4,8,11-tetraazacyclotetradecane octahydrochloride
- Axl:
-
Axl receptor tyrosine kinase
- CaMK II:
-
Ca(2+)/calmodulin-dependent kinase II
- CB2:
-
Cannabinoid receptor 2
- CCL:
-
Chemokine C-C motif ligand
- CXCR:
-
C-X-C motif chemokine receptors
- EBV:
-
Epstein-Barr virus
- EGFR:
-
Endothelial growth factor receptor
- EMT:
-
Epithelial-mesenchymal transition
- ERK:
-
Extracellular signal regulated MAP kinase
- Gas-6:
-
Growth arrest specific gene-6
- GM-CSF:
-
Granulocyte macrophage colony stimulating factor
- HPV:
-
Human papilloma virus
- IKKβ:
-
I-kappaB kinase beta
- IL:
-
Interleukin
- IL-1R:
-
Interleukin-1 receptor
- INF-\( \gamma \) :
-
Interferon-gamma
- MAPK:
-
Mitogen activated protein kinases
- MMP:
-
Matrix metalloproteinase
- MyD88:
-
Myeloid differentiation primary response gene 88
- nAChR:
-
Nicotine acetylcholine receptor
- NF-κB:
-
Nuclear factor kappa B
- OSCC:
-
Oral squamous cell carcinoma
- PI3K:
-
Phosphatidylinositol 3-kinase
- S1P:
-
Sphingosine-1-phosphate
- SDF-1α:
-
Stromal cell derived factor-1 alpha
- SOCS3:
-
Suppressor of cytokine signalling 3
- STAT:
-
Signal transducer and activator of transcription
- TAMs:
-
Tumour-associated macrophages
- TGF-β1:
-
Transforming growth factor beta 1
- TH1:
-
T helper 1
- TH2:
-
T helper 2
- TLR:
-
Toll-like receptors
- TNF:
-
Tumour necrosis factor
- TRAF3:
-
TNF receptor associated factors
- TRIF:
-
TIR-domain-containing adapter-inducing interferon-β
- TRKA:
-
Tyrosine kinase receptor A
- uPA:
-
Plasminogen activator urokinase
- uPAR:
-
Plasminogen activator urokinase receptor
- VEGF:
-
Vascular endothelial growth factor
References
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2010;144(5):646–74.
Sonnenschein C, Soto AM. The aging of the 2000 and 2011 hallmarks of cancer reviews: a critique. J Biosci. 2013;38(3):651–63.
Landskron G, de la Fuente M, Thuwajit P, et al. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185.
Christopher AF, Gupta M, Bansal P.Micronome revealed miR-19a/b as key regulator of SOCS3 during cancer related inflammation of oral squamous cell carcinoma. Gene. 2016;594(1):30-40. doi:10.1016/j.gene.2016.08.044. Epub 2016 Aug 28.
Singh PK, Chandra G, Bogra J, et al. Association of interleukin-6 genetic polymorphisms with risk of OSCC in Indian population. Meta Gene. 2015;4:142–51.
Petersen PE. Oral cancer prevention and control – the approach of the World Health Organization. Oral Oncol. 2008. doi:10.1016/j.oraloncology.2008.05.023.
Surveillance, Epidemiology, and End Results Program of the National Cancer Institute. https://seer.cancer.gov. Accessed 20 Sept 2016.
Wehrhan F, Büttner-Herold M, Hyckel P, et al. Increased malignancy of oral squamous cell carcinomas (OSCC) is associated with macrophage polarization in regional lymph nodes – an immunohistochemical study. BMC Cancer. 2014;14:522.
Chiu KC, Lee CH, Liu SY, et al. Polarization of tumor-associated macrophages and Gas6/Axl signaling in oral squamous cell carcinoma. Oral Oncol. 2015;51(7):683–9.
He KF, Zhang L, Huang CF, et al. CD163+ tumor-associated macrophages correlated with poor prognosis and cancer stem cells in oral squamous cell carcinoma. Biomed Res Int. 2014;2014:838632.
Ni YH, Ding L, Huang XF, et al. Microlocalization of CD68+ tumor-associated macrophages in tumor stroma correlated with poor clinical outcomes in oral squamous cell carcinoma patients. Tumour Biol. 2015;36(7):5291–8.
Lee CH, Liu SY, Chou KC, Yeh CT, et al. Tumor-associated macrophages promote oral cancer progression through activation of the Axl signaling pathway. Ann Surg Oncol. 2014;21(3):1031–7.
Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nature Rev Cancer. 2009;9:239–52.
Grage-Griebenow E, Flad HD, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol. 2001;69(1):11–20.
Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–64.
McWhorter FY, Davis CT, Liu WF. Physical and mechanical regulation of macrophage phenotype and function. Cell Mol Life Sci. 2015;72(7):1303–16.
Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–69.
Melton DW, McManus LM, Gelfond JAL, et al. Temporal phenotypic features distinguish polarized macrophages in vitro. Autoimmunity. 2015;48(3):161–76.
Barros MHM, Hauck F, Dreyer JH, et al. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One. 2013;8(11):e80908.
Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage M1–M2 polarization balance. Front Immunol. 2014;5:614.
Kim HS, Kim DC, Kim H-M, et al. STAT1 deficiency redirects IFN signalling toward suppression of TLR response through a feedback activation of STAT3. Scientific Reports. 2015;5:13414.
Ma J, Liu L, Che G, et al. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer. 2010;10:112.
Mori K, Haraguchi S, Hiori M, et al. Tumor-associated macrophages in oral premalignant lesions coexpress CD163 and STAT1 in a Th1-dominated microenvironment. BMC Cancer. 2015;15:573.
Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9(11):798–809.
Metwaly H, Maruyama S, Yamazaki M, et al. Parenchymal-stromal switching for extracellular matrix production on invasion of oral squamous cell carcinoma. Hum Pathol. 2012;43(11):1973–81.
Weber M, Moebius P, Büttner-Herold M, et al. Macrophage polarisation changes within the time between diagnostic biopsy and tumour resection in oral squamous cell carcinomas—an immunohistochemical study. Br J Cancer. 2015;113(3):510–9.
Weber M, Iliopoulos C, Moebius P, et al. Prognostic significance of macrophage polarization in early stage oral squamous cell carcinomas. Oral Oncol. 2016;52:75–84.
Zhang Q, Liu L, Gong C, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the Literature. PLoS One. 2012;7(12):e50946. doi:10.1371/journal.pone.0050946.
Bôas DS, Takiya CM, Gurgel CA, Cabral MG, Santos JN. Tumor-infiltrating macrophage and microvessel density in oral squamous cell carcinoma. Braz Dent J. 2013;24(3):194-9. doi:10.1590/0103-6440201302049. PMID:23969905.
Hagemann T, Lawrence T, McNeish I, et al. “Re-educating” tumor-associated macrophages by targeting NF-κB. J Exp Med. 2008;205(6):1261–8.
Watari K, Shibata T, Kawahara A, et al. Tumor-derived interleukin-1 promotes lymphangiogenesis and lymph node metastasis through M2-type macrophages. PLoS One. 2014;9(6):e99568.
Lee CH, Chang JS, Syu SH, et al. IL-1β promotes malignant transformation and tumor aggressiveness in oral cancer. J Cell Physiol. 2015;230(4):875–84.
Sun Y, Zhu D, Wang G, et al. Pro-inflammatory cytokine IL-1β up-regulates CXC chemokine receptor 4 via notch and ERK signaling pathways in tongue squamous cell carcinoma. PLoS One. 2015;10(7):e0132677.
Sánchez-Martín L, Estecha A, Samaniego R, et al. The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood. 2011;117(1):88–97.
Yu T, Wu Y, Helman JI, Wen Y, Wang C, Li L. CXCR4 promotes oral squamous cell carcinoma migration and invasion through inducing expression of MMP-9 and MMP-13 via the ERK signaling pathway. Mol Cancer Res. 2011;9(2):161–72.
Uchida D, Onoue T, Kuribayashi N, et al. Blockade of CXCR4 in oral squamous cell carcinoma inhibits lymph node metastases. Eur J Cancer. 2011;47(3):452–9.
Voldborg BR, Damstrup L, Spang-Thomsen M, Poulsen HS. Epidermal growth factor receptor (EGFR) and EGFR mutations, function and possible role in clinical trials. Ann Oncol. 1997;8(12):1197–206.
Bae JY, Kim EK, Yang DH, et al. Reciprocal Interaction between Carcinoma-Associated Fibroblasts and Squamous Carcinoma Cells through Interleukin-1α Induces Cancer Progression. Neoplasia (New York, NY). 2014;16(11):928–38.
Kwon M, Kim JW, Roh JL, Park Y, Cho KJ, Choi SH, Nam SY, Kim SY, Lee BH. Recurrence and cancer-specific survival according to the expression of IL-4Rα and IL-13Rα1 in patients with oral cavity cancer. Eur J Cancer. 2015;51(2):177–85.
Shi Z, Stack MS. Urinary-type plasminogen activator (uPA) and its receptor (uPAR) in squamous cell carcinoma of the oral cavity. Biochem J. 2007;407(2):153–9.
Yoshizawa K, Nozaki S, Kitahara H, et al. Expression of urokinase-type plasminogen activator/urokinase-type plasminogen activator receptor and maspin in oral squamous cell carcinoma: Association with mode of invasion and clinicopathological factors. Oncol Rep. 2011;26(6):1555–60.
Hu J, Jo M, Eastman BM, et al. uPAR Induces Expression of Transforming Growth Factor β and Interleukin-4 in Cancer Cells to Promote Tumor-Permissive Conditioning of Macrophages. Am J Pathol. 2014;184(12):3384–93.
Gocheva V, Wang H-W, Gadea BB, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24(3):241–55.
Yang W-E, Ho C-C, Yang S-F, et al. Cathepsin B Expression and the correlation with clinical aspects of oral squamous cell carcinoma. PLoS One. 2016;11(3):e0152165.
Chen C-J, Sung W-W, Lin Y-M, et al. Gender Difference in the Prognostic Role of Interleukin 6 in Oral Squamous Cell Carcinoma. PLoS One. 2012;7(11):e50104.
Tang C-H, Chuang J-Y, Fong Y-C, Maa M-C, Way T-D, Hung C-H. Bone-derived SDF-1 stimulates IL-6 release via CXCR4, ERK and NF-κB pathways and promotes osteoclastogenesis in human oral cancer cells. Carcinogenesis. 2008;29(8):1483–92.
Fang W-Y, Chen Y-W, Hsiao J-R, et al. Elevated S100A9 expression in tumor stroma functions as an early recurrence marker for early-stage oral cancer patients through increased tumor cell invasion, angiogenesis, macrophage recruitment and interleukin-6 production. Oncotarget. 2015;6(29):28401–24.
Ley S, Weigert A, Hériché JK, et al. RNAi screen in apoptotic cancer cell-stimulated human macrophages reveals co-regulation of IL-6/IL-10 expression. Immunobiology. 2013;218(1):40–51.
Punyani SR, Sathawane RS. Salivary level of interleukin-8 in oral precancer and oral squamous cell carcinoma. Clin Oral Investig. 2013;17(2):517–24.
Nishio Y, Gojoubori T, Kaneko Y, Shimizu N, Asano M. Cancer cell-derived IL-8 induces monocytic THP1 cells to secrete IL-8 via the mitogen-activated protein kinase pathway. Tumour Biol. 2015;36(12):9171–7.
Khurram SA, Bingle L, McCabe BM, Farthing PM, Whawell SA. The chemokine receptors CXCR1 and CXCR2 regulate oral cancer cell behaviour. J Oral Pathol Med. 2014;43(9):667–74.
Ha NH, Park DG, Woo BH, da Kim J, Choi JI, Park BS, Kim YD, Lee JH, Park HR. Porphyromonas gingivalis increases the invasiveness of oral cancer cells by upregulating IL-8 and MMPs. Cytokine. 2016;86:64–72.
Tsunoda K, Tsujino I, Koshi R, et al. Nicotine-Mediated Ca(2+)-Influx Induces IL-8 Secretion in Oral Squamous Cell Carcinoma Cell. J Cell Biochem. 2016;117(4):1009–15.
Gasparoto TH, de Souza Malaspina TS, Benevides L, et al. Patients with oral squamous cell carcinoma are characterized by increased frequency of suppressive regulatory T cells in the blood and tumor microenvironment. Cancer Immunol Immunother. 2010;59(6):819–28.
Brooks DG, Trifilo MJ, Edelmann KH, et al. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12(11):1301–9.
Chuang C-Y, Sung W-W, Wang L, et al. Differential impact of IL-10 expression on survival and relapse between HPV16-positive and -negative oral squamous cell carcinomas. PLoS One. 2012;7(10):e47541.
Polz-Dacewicz M, Strycharz-Dudziak M, Dworzański J, Stec A, Kocot J. Salivary and serum IL-10, TNF-α, TGF-β, VEGF levels in oropharyngeal squamous cell carcinoma and correlation with HPV and EBV infections. Infect Agents Cancer. 2016;11:45.
Wang S, Sun M, Gu C, et al. Expression of CD163, interleukin-10, and interferon-gamma in oral squamous cell carcinoma: mutual relationships and prognostic implications. Eur J Oral Sci. 2014;122(3):202–9.
Gonçalves AS, Arantes DA, Bernardes VF, et al. Immunosuppressive mediators of oral squamous cell carcinoma in tumour samples and saliva. Hum Immunol. 2015;76(1):52–8.
Naher L, Kiyoshima T, Kobayashi I, et al. STAT3 signal transduction through interleukin-22 in oral squamous cell carcinoma. Int J Oncol. 2012;41(5):1577–86.
Banerjee S, Halder K, Bose A, et al. TLR signaling-mediated differential histone modification at IL-10 and IL-12 promoter region leads to functional impairments in tumor-associated macrophages. Carcinogenesis. 2011;32(12):1789–97.
Ley S, Weigert A, Weichand B, Henke N, Mille-Baker B, Janssen RA, Brüne B. The role of TRKA signaling in IL-10 production by apoptotic tumor cell-activated macrophages. Oncogene. 2013;32(5):631–40.
Sica A. Macrophages give Gas(6) to cancer. Blood. 2010;115(11):2122–3. doi:10.1182/blood-2009-12-255869.
Wu G, Ma Z, Hu W, Wang D, Gong B, Fan C, Jiang S, Li T, Gao J, Yang Y. Molecular insights of Gas6/TAM in cancer development and therapy. Cell Death Dis. 2017;8(3):e2700. doi: 10.1038/cddis.2017.113. Review. PMID: 28333143.
Lee CH, Yen CY, Liu SY, Chen CK, Chiang CF, Shiah SG, Chen PH, Shieh YS. Axl is a prognostic marker in oral squamous cell carcinoma. Ann Surg Oncol. 2012;19 Suppl 3:S500–8.
Patankar SR, Wankhedkar DP, Tripathi NS, Bhatia SN, Sridharan G. Extracellular matrix in oral squamous cell carcinoma: Friend or foe? Indian J Dent Res. 2016;27(2):184-9. doi:10.4103/0970-9290.183125.
Jiang T, Liu G, Wang L, Liu H. Elevated Serum Gas6 Is a Novel Prognostic Biomarker in Patients with Oral Squamous Cell Carcinoma. PLoS One. 2015;10(7):e0133940.
Kumar V, Gabrilovich DI. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology. 2014;143(4):512–9.
Cheng Y-SL, Rees T, Wright J. A review of research on salivary biomarkers for oral cancer detection. Clin Trans Med. 2014;3:3.
Rajkumar K, Nandhini G, Ramya R, Rajashree P, Kumar AR, Anandan SN. Validation of the diagnostic utility of salivary interleukin 8 in the differentiation of potentially malignant oral lesions and oral squamous cell carcinoma in a region with high endemicity. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118(3):309–19.
Aziz S, Ahmed SS, Ali A, et al. Salivary Immunosuppressive Cytokines IL-10 and IL-13 Are Significantly Elevated in Oral Squamous Cell Carcinoma Patients. Cancer Invest. 2015;33(7):318–28.
Panneer Selvam N, Sadaksharam J. Salivary interleukin-6 in the detection of oral cancer and precancer. Asia Pac J Clin Oncol. 2015;11(3):236–41.
Bagan L, Sáez GT, Tormos MC, et al. Salivary and serum interleukin-6 levels in proliferative verrucous leukoplakia. Clin Oral Investig. 2016;20(4):737–43.
Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61.
Modi BG, Neustadter J, Binda E, et al. Langerhans Cells Facilitate Epithelial DNA Damage and Squamous Cell Carcinoma. Science (New York, NY). 2012;335(6064):104–8.
Hayashi N, Kataoka H, Yano S, Tanaka M, Moriwaki K, Akashi H, Suzuki S, Mori Y, Kubota E, Tanida S, Takahashi S, Joh T. A novel photodynamic therapy targeting cancer cells and tumor-associated macrophages. Mol Cancer Ther. 2015;14(2):452–60.
Junankar S, Shay G, Jurczyluk J, et al. Real-time intravital imaging establishes tumour-associated macrophages as the extraskeletal target of bisphosphonate action in cancer. Cancer Discov. 2015;5(1):35–42.
Mancino A, Lawrence T. Nuclear factor-kappaB and tumor-associated macrophages. Clin Cancer Res. 2010;16(3):784–9.
Acknowledgement
Not applicable.
Funding
Not applicable.
Availability of data and material
Not applicable.
Authors’ contributions
MNP, KC, FS and MAF were major contributors in writing the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Petruzzi, M.N.M.R., Cherubini, K., Salum, F.G. et al. Role of tumour-associated macrophages in oral squamous cells carcinoma progression: an update on current knowledge. Diagn Pathol 12, 32 (2017). https://doi.org/10.1186/s13000-017-0623-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13000-017-0623-6