Abstract
Background
Prolonged over-consumption of a high-fat diet (HFD) commonly leads to obesity and insulin resistance. However, even 3 days of HFD consumption has been linked to inflammation within the key homeostatic brain region, the hypothalamus.
Methods
Mice were fed either a low-fat diet (LFD) or HFD containing 10% or 60% (Kcal) respectively from fat for 3 days. Mice were weighed, food intake measured and glucose tolerance calculated using intraperitoneal glucose tolerance tests (IPGTT). Proteomic analysis was carried out to determine if hypothalamic proteins were changed by a HFD. The direct effects of dietary fatty acids on mitochondrial morphology and on one of the proteins most changed by a HFD, dihydropyrimidinase-related protein 2 (DRP-2) a microtubule-associated protein which regulates microtubule dynamics, were also tested in mHypoE-N42 (N42) neuronal cells challenged with palmitic acid (PA) and oleic acid (OA).
Results
Mice on the HFD, as expected, showed increased adiposity and glucose intolerance. Hypothalamic proteomic analysis revealed changes in 104 spots after 3 days on HFD, which, when identified by LC/MS/MS, were found to represent 78 proteins mainly associated with cytoskeleton and synaptic plasticity, stress response, glucose metabolism and mitochondrial function. Over half of the changed proteins have also been reported to be changed in neurodegenerative conditions such as Alzheimer’s disease. Also,in N42 neurons mitochondrial morphology and DRP-2 levels were altered by PA but not by OA.
Conclusion
These results demonstrate that within 3 days, there is a relatively large effect of HFD on the hypothalamic proteome indicative of cellular stress, altered synaptic plasticity and mitochondrial function, but not inflammation. Changes in N42 cells show an effect of PA but not OA on DRP-2 and on mitochondrial morphology indicating that long-chain saturated fatty acids damage neuronal function.
Similar content being viewed by others
Background
Obesity is increasing not only in Western societies but also in the developing world [1, 2], with obesity related comorbidities and decreased life expectancy putting enormous pressure on health care systems [1, 3, 4]. The overconsumption of energy dense foods, particularly those high in saturated fat and refined sugar appears to be a primary driving force behind the obesity epidemic [5], making it important to understand the mechanisms linking diet with obesity to enable more effective preventative measures to be put in place. Dietary interactions with the hypothalamus appear to be key in the development of obesity. Thus, the aim of this study is to investigate how short-term exposure to a high-fat diet (HFD) influences the proteome of the hypothalamus to better understand how diet interacts with this brain region.
Energy balance is effectively regulated by a well-defined and complex hypothalamic system with leptin acting together with insulin in the hypothalamus not only to inhibit feeding but also to maintain peripheral glucose homeostasis [6, 7]. Nonetheless, in obesity the hypothalamus becomes insensitive to leptin and insulin signifying dysregulation of hypothalamic energy balance. A number of studies in rodents on a HFD have shown inflammation in the hypothalamus, activating both microglia and astrocytes, via the toll like receptor 4 (TLR4) [8, 9] and IKKB/NFκB inflammatory pathway, resulting in leptin and insulin insensitivity [10, 11]. The importance of this pathway is underlined by the fact that blocking or inhibiting diet-induced hypothalamic inflammation prevents leptin insensitivity, glucose intolerance and obesity [10, 12, 13]. More recently, however, the role of TLR4 in this process has been called into question [14]. HFD-induced mitochondrial dysfunction may be the origin of hypothalamic dysfunction and inflammation with excessive dietary intake leading to mitochondrial overload and oxidative stress activating the NFκB inflammatory pathway [15].
Data has emerged which indicates that microglial proliferation is only seen after 8 weeks on HFD despite increased pro-inflammatory gene expression after just 3 days [16]. This indicates that the primary inflammatory response is independent of hypothalamic immune cells with neurons being implicated in triggering microgliosis via advanced glycation end products (AGEs) [17]. In order to explore the initial mechanisms linking a HFD to hypothalamic dysfunction we have used a proteomics approach to identify key proteins changed after just 3 days of a HFD and extended these findings to N42 cells challenged with PA and OA, representative dietary long-chain saturated and monounsaturated fatty acids respectively, and present in large amounts in the HFD, to look at protein changes together with the shape and area occupied by mitochondria indicative of mitochondrial functionality.
Methods
Animals
Male C57Bl/6 J mice, 10 weeks of age (Harlan, Bicester, UK), were first habituated to single housing on grid floors for 1 week then changed from chow and habituated to a semi-purified low-fat diet (LFD) for a further week (10% of energy from fat and 3.8 kcal/g) to avoid any inappetence that may arise in changing directly from chow to a semi-purified diet. Single housing and grid floors were utilised to enable accurate measurement of food intake by weighing unconsumed food, to prevent coprophagia and stress due to dominance. Animals were then randomised into two groups. One group remained on the LFD while the other group were fed a HFD (60% of energy from fat and 5.2 kcal/g) ad libitum for 3 days (D12492 and D12450B, respectively; Research Diets, NJ, US) (see https://researchdiets.com/opensource-diets/dio-series-diets for complete diet composition details and Additional file 1: Table S1 for overview). The difference in fat content is due to increasing the quantity of lard in the diet. Semi–purified diets in comparison to chows have defined ingredients allowing precise diet composition facilitating the replication of experimental conditions. Food intakes and body weights were measured daily (n = 14 per diet). Water was supplied ad libitum but intake was not measured. Mice were killed by exsanguination under terminal anaesthesia. The brains were removed and snap-frozen over dry ice and stored at − 80 °C until proteomic studies were carried out.
Glucose tolerance
Intraperitoneal glucose tolerance tests (IPGTTs) were carried out (n = 8) as a non-recovery procedure after fasting for 5 h. A blood sample (0 mins) was taken prior to an intraperitoneal (IP) glucose injection (1.5 mg/g body weight). Subsequent blood samples were taken from the tail vein at 15, 30, 60 and 120 mins and measured using an Accu Chek Aviva blood glucose monitor (Roche Diagnostics, Burgess Hill, UK). Area under the curve (AUC) was calculated using the trapezoid rule [18]. Data from this group of mice was also used as in a parallel study [19] in accordance with reducing the number of experimental animals (http://www.understandinganimalresearch.org.uk/animals/three-rs/).
Two-dimensional gel electrophoresis (2-DE)
The hypothalamus was dissected from frozen brains (n = 6) using the start of the optic chiasma and the end of the median eminence as the anterior and posterior markers for the first cuts through the brain. The outer edges of the hypothalamus were then used as markers for the side cuts and the top of the third ventricle as a marker for the top of the hypothalamus for the final cut. Hypothalamic tissue was homogenised at a 1:4 ratio of tissue to buffer in 40 mM Tris pH 7.4, 0.1% v/v Triton X-100 containing Roche complete protease inhibitors (Sigma-Aldrich, UK) at the manufacturers recommended concentration. 2-DE was performed essentially as detailed previously with some modifications [20]. Bio-Rad, 17 cm, immobilized pH gradient (IPG) strips (pH 3–10) were used for the separation of proteins in the first dimension. Strips were rehydrated in rehydration buffer (7 M urea; 2 M thiourea; 4% w/v CHAPS; 2% w/v Biolyte; and 50 mM DTT) containing 200 μg of protein sample in a Bio-Rad IEF cell and then focused.
After the first dimension IPG strips were incubated in fresh equilibration buffer (6 M urea; 2% w/v SDS; 0.375 M Tris-HCl, pH 8.8; 20% v/v glycerol; and 130 mM DTT) for 10–15 min at room temperature before transfer to a second equilibration buffer (6 M urea; 2% w/v SDS; 0.375 M Tris-HCl, pH 8.8; 20% v/v glycerol; and 135 mM iodoacetamide) for 10–15 min at room temperature. The strip was then applied to the top of an 18 × 18 cm gel cassette and 5 μl of All Blue Precision Protein Standards (Bio-Rad) was loaded in the reference well. Gels were run at 200 V for 9.5 h or until the bromophenol blue had reached the bottom of the gel. After the second dimension run, the gels were fixed and stained with Coomassie Blue. Twelve gels were run in total each gel representing an individual animal from each treatment group HFD (n = 6) and LFD (n = 6).
Identification of mouse hypothalamic proteins
2-DE gels were analysed using Progenisis Samespots software (Nonlinear Dynamics Ltd., UK). Spots which showed differences in normalised average volume with P < 0.05 by ANOVA in HFD vs. LFD were cut from SDS-PAGE gels. Gel plugs were trypsinized using the MassPrep Station (Waters, Micromass, UK) protocol. Spot identification was carried out using ‘Ultimate’ nanoLC system (LC Packings, UK) and a Q-Trap (Applied Biosystems/MDS Sciex, UK) triple quadrupole mass spectrometer fitted with a nanospray ion source as described previously [20]. The total ion current (TIC) data were submitted for database searching using the MASCOT search engine (Matrix Science, UK) using the MSDB database.
Cell culture and reagents
The N42 clonal hypothalamic neuronal cell line (mHypoE-N42) (Cellution Biosystems Inc. Burlington, Canada) was cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen Life Technologies, Paisley, UK), supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Invitrogen Life Technologies, Paisley, UK) and maintained at 37o C under a 5% CO2 atmosphere. This cell line is derived from mouse hypothalamic primary cultures by retroviral transfer of SV40 T-Ag and expresses enzymatic markers, receptors and neuropeptides which makes it a valuable tool to study hypothalamic metabolic pathways [21]. Information regarding genes expressed in this cell line can be found at (https://www.cedarlanelabs.com/Products/Detail/CLU122?lob=Cellutions). The fatty acids PA and OA and fatty acid free bovine serum albumin (BSA) were from Sigma Aldrich (St. Louis, MO, USA).
Fatty acid-BSA conjugation
PA and OA were conjugated to fatty acid free BSA as described previously [22, 23] with some modifications detailed below. PA and OA were dissolved in 0.1 M NaOH in a shaking water bath and solubilised at 70o C or 37o C respectively in order to yield a final concentration of 20 mM. A 0.5 mM fatty acid free BSA solution was prepared by dissolving BSA in deionised water at 55o C then mixing with PA and OA in order to obtain a 1:4 BSA to fatty acid molar ratio (0.5 mM BSA, 2 mM fatty acid). The PA- and OA-BSA mixtures were vortexed for 10 s followed by 10 min incubation at 55 o C or 37o C respectively before being cooled to room temperature and sterilised by passing through a 0.22 μm pore size membrane filter and stored at -20o C until use.
Western blotting
Lysates were prepared from N42 neurons after a 6 h incubation with 50 μM BSA, 200 μM OA or 200 μM PA in serum-free medium. This concentration of fatty acid was chosen as it does not cause toxicity up to 24 h of treatment in a neuronal cell line and falls within the range of systemic concentrations of free fatty acids reported [22, 24].
Cells were then scraped into phosphate-buffered saline (PBS) and pelleted by centrifugation, M-PER mammalian protein extraction reagent (Thermo scientific) was added before sonication using a Sanyo Soniprep 1500 to ensure complete cell lysis. Protein concentrations were determined using the Pierce 660 nm protein assay reagent (Thermo Scientific). After addition of 4X Laemmli sample buffer (Bio-Rad, UK) containing 2-mercaptoethanol the protein samples were loaded on 10% mini-PROTEANTGX Precast Gels (Bio-Rad) and separated by electrophoresis and then transferred onto PVDF membranes.
Immuno-detection used anti- DRP-2 primary antibody (Rb mAb to CRMP2 ab129082, abcam UK) and peroxidase linked secondary antibody (Goat pAb to Rb IgG (HRP) ab98467, abcam, UK). The blots were developed using the Opti4CN substrate kit (Bio-Rad) following the manufacturer’s recommended protocol and imaged using a Fujifilm LAS-3000 Imager. After imaging membranes were stripped using Restore™ Western Blot Stripping Buffer (Thermo Scientific) and re-probed using a primary antibody to beta-actin (Rb pAB to beta-actin ab8227, abcam UK). Blot images were analysed using Image-J [25]. For each blot lane DRP-2 bands were normalised to the respective beta-actin loading control band prior to semi-quantitative analysis where lysates from cells treated with BSA alone were considered as equivalent to 1 and those from cells treated with PA and OA scored accordingly (n = 4).
Mitochondrial staining
N42 hypothalamic neurons were cultured on glass coverslips in 24 well plates to 70% confluency. Cells were challenged with either 50 μM BSA, 200 μM OA or 200 μM PA in serum-free medium for 6 h. Cells were then stained with 500 nM MitoTracker® Red CMXRos (Thermo Scientific, UK) for 45 min, washed and fixed using 4% paraformaldehyde for 20 min on ice. Cover slips were then mounted on slides using Vectashield with DAPI (Vector Laboratories, Burlingame, CA, USA). Images were captured using a Leica DMR microscope fitted with a QImaging QICAM FAST 1394 digital camera. Neuronal mitochondrial content was analysed using the ImageJ mitophagy macro [26]. The percentage of the cell area occupied by the mitochondria was used as a measure of cellular mitochondrial content.
Statistical analysis
Body weight, food intake and IPGTT data are represented as mean ± SEM and were analysed using GenStat (GenStat, 10th Edition, VSN International Ltd., Oxford) by Student’s T tests. Mitochondria neuronal content was analysed using a one-way analysis of variance (ANOVA) followed by a Tukey multiple comparison test. P < 0.05 was considered statistically significant.
Results
Body weight and food intake
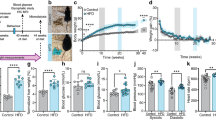
The body weight of mice fed a HFD were significantly increased after 2 days (P < 0.05) and continued to increase up to 3 days on diet (P < 0.01) (Fig. 1a). Food intake (g) dropped significantly in HFD fed mice at day 1 (P < 0.01) but returned to LFD levels by 2 and 3 days of diet (Fig. 1b).
a Body weight of HFD mice was significantly higher than that of LFD mice after 2 and 3 days on diet b The intake of the HFD fed mice decreased after 1 day on diet but returned to LFD levels on days 2 and 3 (n = 6).c IPGTT in LFD and HFD fed mice after 3 days on diet. IPGTT was carried out as a non-recovery procedure as the effect of fasting and glucose administration can alter proteins for some time afterwards. Glucose levels at all time points tested was significantly higher in HFD fed mice (n = 8) (● HFD; ● LFD) as was AUC shown in (d). (* P < 0.05, **P < 0.01, ***P < 0.01)
Glucose tolerance
Basal glucose levels were higher in HFD fed mice after 3 days on diet (P < 0.05) and circulating glucose levels were higher at all time points tested after glucose challenge (IPGTT) (*P < 0.05, **P < 0.01, ***P < 0.001) as was the total AUC in HFD fed mice (P < 0.001) (Fig. 1c & d).
Hypothalamic proteomics
2-DE analysis of mouse hypothalamic tissue revealed a total of 104 protein spots, from a total of 1147, that were significantly changed (P < 0.05) between the LFD and HFD fed mice after 3 days on diet (Additional file 2: Figure S1). These were further analysed by LC-MS/MS and identified as 78 unique proteins (Table 1 and Fig. 2).
Protein analysis according to function
We divided the proteins identified according to function. The changed proteins were found to be mainly associated with the cytoskeleton and synaptic plasticity (37 spots corresponding to 25 proteins), cellular stress responses (32 spots corresponding to 22 proteins), glucose metabolism (14 spots corresponding to 10 proteins). In addition, 28 spots corresponding to 26 proteins did not belong to any of these three functional categories. There are 5 proteins which fall into more than one functional category and these are marked with an asterisk next to the UniProt identifier and the other functional category listed. Mitochondrial proteins and those associated with mitochondrial function (10 proteins) and 3 astrocyte specific proteins are also identified. Additionally, many of the proteins changed in the present study have also been associated with neurodegenerative diseases, particularly Alzheimer’s disease (49 proteins) (Table 1 and Fig. 2).
Western blotting
Immunoblotting of cell lysates from N42 hypothalamic neurons revealed that staining of the bands corresponding to DRP-2 protein was lower by ~ 38% (P < 0.05), in cells challenged with PA whereas those challenged with OA showed no significant changes in DRP-2 compared with control cells (Fig. 3a-b).
a Representative immunoblots showing changes in DRP-2 and beta-actin protein expression in response to fatty acid free BSA, PA and OA challenge in N42 cells b Quantification of DRP-2 bands normalised to beta-actin (n = 4 plates) BSA - bovine serum albumin, DRP-2 - dihydropyrimidinase-related protein 2, PA - palmitic acid, OA - oleic acid (* P < 0.05). c-e Representative fluorescence microscopy images of N42 hypothalamic neuronal mitochondria using MitoTracker® Red CMXRos. The red colour corresponds to mitochondria. Cells were challenged with, c fatty acid free BSA, d 200 μM PA and e 200 μM OA (Bar = 10 μm. Magnification = X100). f The percentage (%) area of the cell occupied by mitochondria after challenge. BSA - bovine serum albumin, PA - palmitic acid, OA - oleic acid (*P < 0.05, **P < 0.01, ***P < 0.001)
Mitochondrial morphology and area occupied
Changes in mitochondrial shape were seen in cells challenged with PA but not after OA challenge. Mitochondria in PA challenged cells appeared rounded and isolated compared to control or OA challenged mitochondria which had an elongated and branched appearance (Fig. 3a-c). The percentage of the cell area occupied by mitochondria was significantly decreased when N42 hypothalamic neurons were challenged with PA (P < 0.05). The area occupied by mitochondria was increased when cells were challenged with OA relative to control cells (P < 0.01) (Fig. 3d).
Discussion
C57Bl/6 J mice fed a semi-purified diet have been widely used in diet-induced obesity studies [20, 27] and in the present study HFD fed mice gained weight and developed glucose intolerance within 3 days as reported previously [20] confirming the reproducibility of the model and implying impaired hypothalamic function. Interestingly, in that study blood triglyceride levels were unchanged after 3 days on a HFD [20]. Evidence exists for the rapid, within 3 days, induction of hypothalamic insulin and leptin insensitivity [11, 28] by a HFD coupled with hypothalamic inflammation [8], endoplasmic reticulum (ER) stress [29, 30] and mitochondrial dysfunction [31].
In the present study, proteomic analysis of the hypothalamus confirmed the rapidity of HFD-induced changes and secondly demonstrated the large number of hypothalamic proteins (104 spots corresponding to 78 proteins) changed in response to a HFD. The validity of using a proteomics approach to interrogate hypothalamic changes is reinforced by the fact that as highly polarised cells, neurons, the major cell type present in the brain, are more likely to demonstrate translational modification of proteins at sites distant from the cell body to rapidly respond to stimuli rather than transcriptional changes and the subsequent transport of proteins from the cell body.
Unsurprisingly proteins involved in energy metabolism were altered in HFD. These include phosphoglucomutase-1 (PGM1), reported to sustain cell growth during nutritional changes by regulating the balance between glucose-1-phosphate and glucose-6-phosphate [32] and is differentially expressed in the brains of patients with Alzheimer’s disease [33]. A reduction of glucose utilisation is one of the earliest signs of Alzheimer’s disease with glucose metabolism adapting to oxidative stress by lowering levels of glycolysis and oxidative phosphorylation and increasing the generation of reducing factors such as nicotinamide adenine dinucleotide phosphate (NADPH) through the pentose phosphate pathway [34]. Two other enzymes altered by HFD are triosephosphate isomerase and phosphoglycerate mutase 1 both involved with the regulation of the glycolytic pathway. Mitochondrial aconitate hydratase, which catalyses the conversion of citrate to isocitrate in the tricarboxylic acid cycle showed the most significant change in HFD fed mice. It is linked to Alzheimer’s disease demonstrating lower activity in response to oxidative stress [34, 35] and loss of function due to oxidative damage in aging rat brain [36]. Isocitrate dehydrogenase which showed changes in two spots in HFD fed mice is also down-regulated in Alzheimer’s disease [37].Changes in these enzymes in response to HFD point to adaptations in metabolic pathways to overcome oxidative stress similar to those observed in the early stages of Alzheimer’s disease.
Glucose metabolism in the hypothalamus is likely impacted by the increase in circulating glucose seen on a HFD after 3 days. The entry of glucose into the brain is mediated by the non-insulin dependent glucose transporter, GLUT1 with brain glucose levels rising in parallel to circulating glucose concentrations. Excess glucose is neurotoxic via the polyol pathway, changing intracellular tonicity and increasing toxic AGEs which in combination with a HFD promote microglial reactivity [17].
Other protein changes are in pathways not previously thought to be part of the hypothalamic response to a HFD. These include 25 proteins associated with neurogenesis, synaptogenesis, neurite outgrowth and axonal and dendritic cytoskeletal proteins, implying that neuronal remodelling and changes in synaptic connectivity are changed and may be compromised. Notable amongst these are the collapsin response mediator family of proteins (CRMPS - also known as dihydropyrimidinase-related proteins - DPYL and DRPs), consisting of five closely sequence related, phosphoproteins. Single spots representing DRP-1 and 5 are changed together with 9 separate spots corresponding to DRP-2 demonstrating a large number of post-translational changes induced by a HFD. DRP-2 regulates microtubule dynamics and promotes the differentiation of axons from neurites by binding to tubulin dimers. This promotes microtubule assembly and stability [38] which in turn promotes axon elongation. Phosphorylation of DRP-2 lowers its binding affinity to tubulin and is key in the regulation of dendritic spine formation [39]. Because of this DRP-2 was selected to further study the effect of fatty acids in N42 hypothalamic cells where PA challenge altered expression of DRP-2 immunoreactive bands while OA had no effect supporting the contention that long-chain saturated fatty acids damage hypothalamic neuronal function [40].
Dendritic spines are small, highly dynamic, protrusions on the surface of dendrites, which form the postsynaptic component of excitatory synapses [41] and their formation in the hypothalamus is necessary for the activation of agouti-related peptide (AgRP) neurons by fasting [42]. Formation is dependent on cytoskeletal remodelling of actin [43] the most prominent cytoskeletal protein at synapses. Indeed a large number of proteins identified as changed by a HFD are associated with actin organisation, including F-actin-capping protein subunits alpha 1 and beta, profilin-1, profilin-2, plastin-3 and tropomyosin alpha-1 chain. Profilin-1 and 2 bind actin at synapses where they act both as stable structural components and as regulators of actin filament branching providing a modulatory component for the efficacy of pre- and post-synaptic terminals with actin being most enriched in dendritic spines [44, 45]. Also changed on a HFD was actin related protein 3 which functions as ATP-binding component of the Arp2/3 complex involved in regulation of actin polymerization important in dendritic spine formation [46]. Fascin appears twice on the list of proteins changed by a HFD and is important in the cross-linking of filamentous actin into ordered bundles present in cytoskeletal processes and in the function and architecture of cell protrusions again indicating changes in neuronal plasticity in response to a HFD.
Ornithine aminotransferase is also changed, and in the brain is involved in the synthesis of glutamate and gamma-aminobutyric acid GABA [47], two important neurotransmitters localised to synaptosomes [48], again indicating that communication between neurons is altered by a HFD.
Thus, a HFD has a rapid and profound effect on the hypothalamus, altering proteins involved in glucose metabolism linked to oxidative stress and other stress-related proteins. Unexpectedly a large number of cytoskeletal proteins involved in neuronal remodeling and synaptic plasticity were also changed indicating that this area of the brain was undergoing rapid structural changes in response to a HFD. Previously, it has been shown that in rodents susceptible to a HFD that synapses were lost from pro-opiomelanocortin (POMC) neurons which became sheathed in glia after 3 months on the diet [49].
Many of the proteins that were changed are also reported as altered in neurodegenerative diseases particularly Alzheimer’s disease (44 proteins). This may be due to the fact that both a HFD and Alzheimer’s disease are associated with neuro-inflammation and these changes are secondary to the pro-inflammatory condition or there may be a link between the neuronal effects of a HFD which leads to an Alzheimer’s type pathology as is borne out by the well documented connection between obesity, type 2 diabetes, cognitive decline and Alzheimer’s disease [50, 51]. Nonetheless, proteins which are associated with inflammation were not detected as changed by HFD in the present study and only 3 astrocyte specific proteins were identified.
The brain utilises high levels of energy compared to other organs and also contains elevated concentrations of lipids which are susceptible to peroxidation by reactive oxygen species (ROS) produced as a by-product of oxidative metabolism [52]. HFD-induced obesity is associated with oxidative stress and mitochondrial dysfunction, which are linked to neurodegeneration [53]. The effect of the long-chain saturated fatty acid, PA but not the monounsaturated fatty acid, OA, on mitochondrial function in neuronal cells is shown by distinct changes in mitochondrial morphology and area which are potentially indicative of fragmentation and loss of functionality.
Conclusions
In conclusion, changes to synaptic plasticity and neuronal function appear to precede HFD-induced inflammation in the hypothalamus. Indeed at 3 days on a HFD no changes in any protein specifically related to inflammation were seen. Nonetheless, many proteins associated with cellular stress (22 proteins) were found to be changed in response to the diet indicating that oxidative stress in neurons may precede, and thus be, causative in hypothalamic inflammation. Further, experiments on N42 cells using the representative long-chain saturated fatty acid, PA, and the monounsaturated fatty acid, OA, confirm that the long-chain saturated fatty acids, rather than lipids per se, are causative in the changes seen with a HFD as shown by changes in mitochondrial morphology and immunoreactive DRP-2 levels.
Abbreviations
- 2-DE:
-
Two-dimensional gel electrophoresis
- AgRP:
-
Agouti-related peptide
- ANOVA:
-
Analysis of variance
- AUC:
-
Area under the curve
- DRP-2:
-
Dihydropyrimidinase-related protein 2
- ER:
-
Endoplasmic reticulum
- GABA:
-
Gamma-aminobutyric acid
- GLUT1:
-
Glucose transporter 1
- HFD:
-
High-fat diet
- IKKβ/NKκB:
-
Inhibitor of nuclear factor kappa-B kinase subunit β/nuclear factor kappa-light-chain-enhancer of activated B cells
- IPG:
-
Immobilized pH gradient
- IPGTT:
-
Intraperitoneal glucose tolerance test
- LC − MS/MS:
-
Liquid chromatography coupled with tandem mass spectrometry
- LFD:
-
Low-fat diet
- N42:
-
mHypoE-N42
- Ob-Rb:
-
Long signalling form of the leptin receptor
- POMC:
-
Pro-opiomelanocortin
- ROS:
-
Reactive oxygen species
- TLR4:
-
Toll like receptor 4
References
Butland B, Jebb S, Kopelman P, McPherson K, Thomas S, Mardell J, et al. Tackling obesities:future choices - foresight project report. 2nd ed; 2007.
World Health Organisation. Obesity and overweight. Fact sheet No311. 2014.
Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–25.
Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of type 1 and type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med. 2012;29:855–62.
Jeffery RW, Harnack LJ. Evidence implicating eating as a primary driver for the obesity epidemic. Diabetes. 2007;56(11):2673–6.
Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. 2002;5:566–72.
Koch C, Augustine RA, Steger J, Ganjam GK, Benzler J, Pracht C, et al. Leptin rapidly improves glucose homeostasis in obese mice by increasing hypothalamic insulin sensitivity. J Neurosci. 2010;30:16180–7.
Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–62.
Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29:359–70.
Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2006;103:10741–6.
Koch CE, Lowe C, Pretz D, Steger J, Williams LM, Tups A. High-fat diet induces leptin resistance in leptin-deficient mice. J Neuroendocrinol. 2014;26:58–67.
Cai D, Liu T. Inflammatory cause of metabolic syndrome via brain stress and NF-kappaB. Aging (Albany NY). 2012;4:98–115.
Benzler J, Ganjam GK, Legler K, Stohr S, Kruger M, Steger J, et al. Acute inhibition of central c-Jun N-terminal kinase restores hypothalamic insulin signalling and alleviates glucose intolerance in diabetic mice. J Neuroendocrinol. 2013;25:446–54.
Dalby MJ, Aviello G, Ross AW, Walker AW, Barrett P, Morgan PJ. Diet induced obesity is independent of metabolic endotoxemia and TLR4 signalling, but markedly increases hypothalamic expression of the acute phase protein, SerpinA3N. Sci Rep. 2018;8:15648.
Cunarro J, Casado S, Lugilde J, Tovar S. Hypothalamic mitochondrial dysfunction as a target in obesity and metabolic disease. Front Endocrinol. 2018;9:283.
Baufeld C, Osterloh A, Prokop S, Miller KR, Heppner FL. High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol. 2016;132:361–75.
Gao Y, Bielohuby M, Fleming T, Grabner GF, Foppen E, Bernhard W, et al. Dietary sugars, not lipids, drive hypothalamic inflammation. Mol Metab. 2017;6:897–908.
Atkinson KE. An introduction to numerical analysis; 1989.
McLean FH, Grant C, Morris AC, Horgan GW, Polanski AJ, Allan K, et al. Rapid and reversible impairment of episodic memory by a high-fat diet in mice. Sci Rep. 2018;8:11976.
Williams LM, Campbell FM, Drew JE, Koch C, Hoggard N, Rees WD, et al. The development of diet-induced obesity and glucose intolerance in C57BL/6 mice on a high-fat diet consists of distinct phases. PLoS One. 2014;9:e106159.
Mayer CM, Belsham DD. Palmitate attenuates insulin signaling and induces endoplasmic reticulum stress and apoptosis in hypothalamic neurons: rescue of resistance and apoptosis through adenosine 5′ monophosphate-activated protein kinase activation. Endocrinol. 2010;151:576–85.
Cousin SP, Hugl SR, Wrede CE, Kajio H, Myers MG. Jr, Rhodes CJ. Free fatty acid-induced inhibition of glucose and insulin-like growth factor I-induced deoxyribonucleic acid synthesis in the pancreatic beta-cell line INS-1. Endocrinology. 2001;142:229–40.
Sergi D, Morris AC, Kahn DE, McLean FH, Hay EA, Kubitz P, et al. Palmitic acid triggers inflammatory responses in N42 cultured hypothalamic cells partially via ceramide synthesis but not via TLR4. Nutr Neurosci. 2018:1–14. https://doi.org/10.1080/1028415X.2018.1501533.
Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60:2441–9.
Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671.
Dagda RK, Zhu J, Kulich SM, Chu CT. Mitochondrially localized ERK2 regulates mitophagy and autophagic cell stress. Autophagy. 2008;4:770–82.
Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53:S215–9.
Clegg DJ, Gotoh K, Kemp C, Wortman MD, Benoit SC, Brown LM, et al. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav. 2011;103:10–6.
Contreras C, Gonzalez-Garcia I, Martinez-Sanchez N, Seoane-Collazo P, Jacas J, Morgan DA, et al. Central ceramide-induced hypothalamic lipotoxicity and ER stress regulate energy balance. Cell Rep. 2014;9:366–77.
Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73.
Schneeberger M, Dietrich MO, Sebastián D, Imbernón M, Castaño C, Garcia A, et al. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell. 2013;155:172–87.
Bae E, Kim HE, Koh E, Kim K. Phosphoglucomutase1 is necessary for sustained cell growth under repetitive glucose depletion. FEBS Lett. 2014;588:3074–80.
Minjarez B, Calderón-González KG, Rustarazo MLV, Herrera-Aguirre ME, Labra-Barrios ML, Rincon-Limas DE, et al. Identification of proteins that are differentially expressed in brains with Alzheimer's disease using iTRAQ labeling and tandem mass spectrometry. J Proteome. 2016;139:103–21.
Mamelak M. Sporadic Alzheimer's disease: the starving brain. J Alzheimers Dis. 2012;31:459–74.
Zahid S, Oellerich M, Asif AR, Ahmed N. Differential expression of proteins in brain regions of Alzheimer’s disease patients. Neurochem Res. 2014;39:208–15.
Perluigi M, Di Domenico F, Giorgi A, Schinina M, Coccia R, Cini C, et al. Redox proteomics in aging rat brain: involvement of mitochondrial reduced glutathione status and mitochondrial protein oxidation in the aging process. J Neurosci Res. 2010;88:3498–507.
Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol. 2005;57:695–703.
Fukata Y, Itoh TJ, Kimura T, Menager C, Nishimura T, Shiromizu T, et al. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol. 2002;4:583–91.
Jin X, Sasamoto K, Nagai J, Yamazaki Y, Saito K, Goshima Y, et al. Phosphorylation of CRMP2 by Cdk5 regulates dendritic spine development of cortical neuron in the mouse hippocampus. Neural Plast. 2016. https://doi.org/10.1155/2016/6790743.
Williams LM. Hypothalamic dysfunction in obesity. Proc Nutr Soc. 2012;71:521–33.
Matus A. Growth of dendritic spines: a continuing story. Curr Opin Neurobiol. 2005;15:67–72.
Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, et al. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73:511–22.
Korobova F, Svitkina T. Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol Biol Cell. 2010;21:165–76.
Conde C, Cáceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci. 2009;10:319–32.
Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–56.
Kim IH, Racz B, Wang H, Burianek L, Weinberg R, Yasuda R, et al. Disruption of Arp2/3 results in asymmetric structural plasticity of dendritic spines and progressive synaptic and behavioral abnormalities. J Neurosci. 2013;33:6081–92.
Ginguay A, Cynober L, Curis E, Nicolis I. Ornithine aminotransferase, an important glutamate-metabolizing enzyme at the crossroads of multiple metabolic pathways. Biology. 2017;6:18.
Wong P, McGeer E, McGeer P. A sensitive radiometric assay for ornithine aminotransferase: regional and subcellular distributions in rat brain. J Neurochem. 1981;36:501–5.
Horvath TL, Sarman B, Garcia-Caceres C, Enriori PJ, Sotonyi P, Shanabrough M, et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci U S A. 2010;107:14875–80.
Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J Clin Exp Neuropsychol. 2004;26:1044–80.
Wong RH, Scholey A, Howe PR. Assessing premorbid cognitive ability in adults with type 2 diabetes mellitus--a review with implications for future intervention studies. Curr Diab Rep. 2014;14:547.
Angelova PR, Abramov AY. Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett. 2018. https://doi.org/10.1002/1873-3468.12964.
Zhang X, Dong F, Ren J, Driscoll MJ, Culver B. High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp Neurol. 2005;191:318–25.
Funding
LMW, FMC, CG, ACM and C-DM were funded by the Scottish Government’s Rural and Environment Science and Analytical Services Division (RESAS). FHM was supported by an EASTBIO DTP BBSRC studentship. DS was supported by a SULSA studentship. CR was supported by the HOTSTART Scholarship Programme from the School of Medicine, Medical Sciences and Nutrition, University of Aberdeen.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its Supplementary information files].
Author information
Authors and Affiliations
Contributions
FHM, LMW, FMC, RFL and DS all contributed to planning the study, data analysis and writing the paper. FHM, CG, ACM, CR and DS carried out the experiment. C-DM provided statistical input for the analysis of large data sets. All authors contributed to the analysis and interpretation of data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All studies involving animals were licensed under the Animal (Scientific Procedures) Act of 1986 and in accordance with the European Directive on the Protection of Animals used for Scientific Purposes 2010/63/E following ARRIVE guidelines and had received prior approval from the Rowett Institute of Nutrition and Health’s Ethical Review Committee.
Consent for publication
N/A
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Composition of the semi-purified diets used in the study (DOCX 15 kb)
Additional file 2:
Figure S1. Representative 2D Coomassie stained gel of mouse hypothalamic proteins after 3 days on the HFD. Precision Blue Protein Standards (Bio-Rad) are shown as indicated. Numbered spots indicate those with significantly different average normalised volumes (P < 0.05) (n = 5) in HFD compared to (P < 0.06) LFD fed mice. Proteins were identified by LC/MS/MS. See Table 1 and Fig. 2 for protein identification (PDF 621 kb)
Additional file 3:
Supplementary References (DOCX 27 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
McLean, F.H., Campbell, F.M., Langston, R.F. et al. A high-fat diet induces rapid changes in the mouse hypothalamic proteome. Nutr Metab (Lond) 16, 26 (2019). https://doi.org/10.1186/s12986-019-0352-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12986-019-0352-9