Abstract
Background
Parkinson’s disease (PD) is a neurogenerative disorder implicated in dysfunctions of motor functions, particularly gait and balance. Transcranial direct current stimulation (tDCS) is a noninvasive brain stimulation offered as a potential adjuvant therapy for PD. This systematic review and meta-analysis were conducted to identify whether tDCS alone and combined with additional rehabilitation therapies improve gait and balance among individuals with PD.
Methods
We searched PubMed, Embase, Web of Science, and relevant databases for eligible studies from inception to December 2022. Studies with a comparative design investigating the effects of tDCS on motor functions, including gait and balance among individuals with PD, were included. A meta-analysis was performed for each outcome using a random effects model for subgroup analysis and pooling of overall effect sizes.
Results
A total of 23 studies were included in the meta-analysis. The pooled results revealed that tDCS has moderate overall effects on gait, measured by gait speed (standardized mean deviation [SMD] = 0.238; 95% confidence interval [CI] − 0.026 to 0.502); stride length (SMD = 0.318; 95% CI − 0.015 to 0.652); cadence (SMD = − 0.632; 95% CI − 0.932 to − 0.333); freezing of gait questionnaire scores (SMD = − 0.360; 95% CI − 0.692 to − 0.027); step length (SMD = 0.459; 95% CI − 0.031 to 0.949); walking time (SMD = − 0.253; 95% CI − 0.758 to 0.252); stride time (SMD = − 0.785; 95% CI: − 1.680 to 0.111); double support time (SMD = 1.139; 95% CI − 0.244 to 0.523); and balance, measured by timed up and go (TUG) test (SMD = − 0.294; 95% CI − 0.516 to − 0.073), Berg balance scale (BBS) scores (SMD = 0.406; 95% CI − 0.059 to 0.87), and dynamic gait index (SMD = 0.275; 95% CI − 0.349 to 0.898). For the subgroup analysis, gait and balance demonstrated moderate effect sizes. However, only cadence, stride time, and TUG indicated a significant difference between real and sham tDCS (P = 0.027, P = 0.002, and P = 0.023, respectively), whereas cadence and BBS (P < 0.01 and P = 0.045, respectively) significantly differed after real tDCS plus other therapies rather than after sham tDCS plus other therapies.
Conclusions
Our results indicated that tDCS is significantly associated with gait and balance improvements among individuals with PD. The findings of this study provide more proof supporting the effectiveness of tDCS, encouraging tDCS to be utilized alone or in combination with other therapies in clinical practice for PD rehabilitation.
Similar content being viewed by others
Background
Parkinson’s disease (PD) is the second most common neurodegenerative disorder and the fastest growing in terms of prevalence, disability, and death among neurological diseases, according to the Global Burden of Disease Study reported in 2019 [1,2,3]. The prevalence of PD increases with age and accounts for up to 4% of individuals in the oldest age groups [4]. PD affects nearly 1% of the population above 60 years old [5] and is expected to increase as the older adult population grows. Consequently, healthcare systems and society are heavily burdened by lost productivity and medical costs [6]. PD is primarily caused by the loss of dopaminergic cells in the substantia nigra pars compacta, which results in reduced dopamine input to the striatum and contributes to excess activation of the inhibitory output of the basal ganglia (BG) [7, 8]. Because the BG is connected with the cortex and cerebellum to form a fundamental circuit, the abnormal inhibition from BG might influence the cortex and cerebellum through the anatomically segregated BG pathway [9,10,11]. Hence, dysfunction between BG, cortex, and cerebellum (BG–Ctx–Cer) is related to the induction of key PD symptoms, including muscular rigidity, tremor, bradykinesia and postural instability. These motor symptoms can lead to gait and balance deficits, which subsequently can increase fall risk, reduce the quality of life, and increase the mortality rate of patients with PD [11, 12].
Although pharmacology is the gold standard in PD treatment, medications based on dopamine replacement can only control PD and have enormous effects on motor symptoms during the early stages. However, gait and balance are significantly impaired during the late stages and do not respond well to medications such as levodopa [13]. Growing evidence highlights that the potential invasive and noninvasive neuromodulation approaches target various areas in the brain, typically the BG–Ctx–Cer system in patients with PD [11, 14,15,16].
Transcranial direct current stimulation (tDCS) is a noninvasive brain stimulation technique that applies an anodal or cathodal charge of a weak electrical current over the targeted cortex through two or more electrodes. tDCS can excite or inhibit widespread neuronal activity and trigger dopamine releases through motor networks in the BG–Ctx–Cer system and through other motor cortical areas [14, 17, 18].
Numerous studies have shown that tDCS benefits motor functions, including walking, upper limb functions, and functional locomotion in PD [19,20,21,22,23,24,25]. Furthermore, tDCS can be utilized as an adjuvant therapy for PD, often being applied either alone or in combination with with other rehabilitation therapies. However, no systematic review or meta-analysis has specifically explored the effects of tDCS on gait and balance, particularly when tDCS is used as a standalone treatment or in combination with other rehabilitative therapies. In the present systematic review and meta-analysis, we elucidated whether tDCS alone and in combination with other rehabilitation therapies improves gait and balance among individuals with PD. Additionally, we addressed whether the effect of tDCS combined with rehabilitation therapies is superior to rehabilitation therapies. Our findings could provide comprehensive evidence of the effects of tDCS on motor functions and could be valuable for guiding future treatments and research in tDCS.
Methods
The current systematic review and meta-analysis were performed in accordance with the guidelines of The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Additional file 1: Table S1. PRISMA Checklist 2020) [26]. The study protocol was registered with the International Prospective Register of Systematic Reviews under the registration number CRD42022329764 on May 7, 2022.
Search strategy
Two authors (TXDN and PTM) independently searched three different electronic databases, including PubMed, Embase, and Web of Science, for eligible articles from inception until December 2022. The following terms were used for electronic searching: ((“transcranial direct current stimulation” OR “tDCS” OR “transcranial electrical stimulation” OR “tES”)) AND ((“gait” OR “walking” OR "walk” OR “Spatiotemporal” OR “balance” OR “postural control” OR “postural stability” OR “posture”)) AND ((“Parkinson’s disease” OR “Parkinson” OR “PD” OR “Parkinson disease” OR “Parkinsonism” OR “Parkinsonian”)). Moreover, queries for reference lists of relevant systematic reviews were manually conducted to gather additional eligible studies.
Selection criteria
Two authors (TXDN and PTM) independently screened the titles, abstracts, and full texts to identify eligible studies for inclusion in this systematic review and meta-analysis. Studies were considered to include if they met the following criteria: (1) recruited patients diagnosed with PD according to UK PD Society Brain Bank clinical diagnostic criteria [27] and did not have comorbid neurological diseases; (2) investigated the effects of tDCS alone or in combination with rehabilitative therapies such as gait training, physical training, dance, aerobic exercises, and strength exercises; (3) included a comparator group comprising PD patients who received sham tDCS, standard care, placebo, or other rehabilitative therapies excluding tDCS; (4) measured outcomes of gait (spatiotemporal gait parameters, freezing of gait questionnaire [FOG-Q], FOG provoking test, walking time, 10-min walking test [10MWT], and 6-m walking test [6MWT]), static balance (center of pressure [CoP] velocity), and dynamic balance (timed up and go [TUG] test, Berg balance scale [BBS], balance evaluation systems test [BESTest], MiniBESTest, functional reach test [FRT], dynamic gait index [DGI], and functional gait assessment [FGA]); (5) were a clinical randomized control trial (RCT), quasi RCT, crossover RCT study, or comparative study; and (6) were published in English.
Studies were considered excluded if they: (1) were a preclinical study; (2) had no control group; (3) were conference abstracts, communications, a letter with no empirical data, or commentary; or (4) did not include the full text.
Risk of bias and quality assessment
The included studies, which were randomized control trials, were evaluated according to 11 metrics on the Physiotherapy Evidence Database (PEDro) scale [28, 29]. One point was given for each satisfying criterion (except for the first item, which required a YES or NO response). The score ranged from 0 to 10, with a score of 9–10 indicating excellent quality, a score of 6–8 indicating good quality, a score of 4–5 indicating fair quality, and a score of < 4 indicating poor quality. Moreover, the 12-item methodological index for nonrandomized studies [30] was used to evaluate the methodology of nonrandomized studies. The maximum score was 24, and each item was scored from 0 to 2. The higher the score was represented the higher the quality of the study. These scales can be applied to assess the internal and external validity of a clinical trial. Additionally, we identified the evidence level of studies according to the “Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence” [31], which can assist decision-making in clinical scenarios. The score was independently rated by two authors (TXDN and PTM). Any disagreements on the risk of bias or quality assessments were resolved by a third author or the research team.
Data extraction
Two authors (TXDN and PTM) performed data extraction independently using a predefined format. Any discrepancies that arose during this process were resolved through discussion. The following data elements were extracted from the included studies: (1) study source (authors, publication year), (2) methods (study designs), (3) participant information (number of participants in each group, mean age, Hoehn & Yahr scores, Unified Parkinson Disease Rating Scale motor section (UPDRS III) scores, medication during the intervention, disease duration), (4) interventions (type of intervention of experimental and control groups, electrode montage, intensity, duration, number of sessions), and (5) outcome measures.
The means, standard deviations (SD), and sample size for each outcome measure were coded and organized in a spreadsheet for meta-analysis [32, 33]. If mean and standard deviations were not available in the included studies, data presented in the form of standard errors, confidence intervals, or medians with ranges were converted into mean and SD format using established statistical formulas as recommended in the literature [34]. In the event of missing data, authors were contacted; if authors did not respond, data values presented as graphs were extracted using the GRABIT software (MathWorks, Inc., Natick, Massachusetts, USA).
Data synthesis
All statistical data analyses were carried out by Comprehensive Meta-Analysis version 2 software (Biostat, Englewood, NJ, USA). The standardized mean difference (SMD) with 95% confidence intervals (CIs) for each included study was calculated using Cohen’s d method based on the mean and SD. Subsequently, the subgroup analysis for interventions was conducted and the overall effect sizes were pooled for each outcome variable by using a random-effect model. An SMD value of 0.20 or less indicated a small effect size, a value around 0.50 indicated a moderate effect size, and a value of 0.80 or greater indicated a large effect size [35].
The heterogeneity among the results of included studies was determined based on values of Q and I2 statistics [36]. A P value of ≤ 0.05 from Q statistic and an I2 value greater than 50% was considered an indicator of significant heterogeneity [37]. If a significant heterogeneity was observed between the studies, the researchers eliminated outliers or subgroups to reduce inconsistencies. We also assessed publication bias through visual inspection of funnel plots and statistical tests, including both Egger’s and Begg’s tests [38, 39], when at least ten studies were included in the meta-analysis following the Cochrane Collaboration guideline [40]. The statistical significance was set at the level of 0.05 (P ≤ 0.05) for all calculations.
Results
Study identification
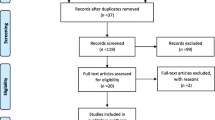
The search yielded a total of 351 records from the PubMed, Embase, and Web of Science databases and the reference lists of relevant systematic reviews (Fig. 1). We then screened titles and abstracts of 196 records after removing 155 duplicates. Altogether, 140 records were excluded. Then, we evaluated the full text of 56 records. After the full-text reading, it is found that 31 texts did not meet the inclusion criteria; 21 records were conference abstracts with no full text available, three were short communications, two were published in Chinese, two produced no relevant outcomes, two were noncontrolled trials, and one was a case study. Overall, 25 studies were eligible and were enlisted in this systematic review. Eleven studies were RCTs, and 14 studies were crossover RCTs. Since two studies were not able to extract appropriate data, a meta-analysis was performed from the data of 23 studies.
Study characteristics
The demographic characteristics, intervention and comparator descriptions, and outcome measures are illustrated in Table 1.
Participants
In total, 569 individuals with PD across the included studies were included, with an average age of 50 and 79 years. Of the total number of participants included, the mean Hoehn & Yahr (H&Y) scores were from 1 to 4, the mean PD duration extended from 1.2 to 17.7 years, and the UPDRS III scores ranged from 7.2 to 55.2. All participants were in an ON-medication state for the entire study.
Interventions
Among 25 studies included in the systematic review, four of which [41,42,43,44] included more than one comparison. Seventeen trials used real tDCS compared with sham tDCS [41,42,43,44,45,46,47,48,49,50,51,52,53,54], and another fourteen trials compared real tDCS plus other rehabilitative therapies with sham tDCS plus other rehabilitation therapies, such as gait training [41, 55,56,57], physical therapy [58,59,60], repetitive transcranial magnetic stimulation (rTMS) [61, 62], aerobic exercise [63], dual-task [51, 64], visual cueing [65], and biking and Wii games [42]. In the studies that combined two interventions, the participants received tDCS protocols either simultaneously with or before with other therapies. Anodal tDCS electrodes were mainly placed over different target areas of the motor cortex (the primary motor cortex, dorsolateral prefrontal cortex, or supplementary motor cortex) according to the 10–20 international electroencephalography system. Most studies offered single-session interventions, and the frequency of intervention in other nine studies extended from 5 to 20 sessions [45, 50, 55, 56, 58,59,60,61, 65]. The total intervention duration per session ranged between 7 and 60 min, in which the most minor and most prolonged periods of tDCS were 7 [49], and 30 min [51, 60], respectively.
Outcomes
Among the gait spatiotemporal parameters, gait speed was included the most (13 studies), followed by cadence (10 studies), stride length (10 studies), and other parameters (step length, walking time, step time, and double support time). Additionally, the FOG-Q was used in four studies, and the FOG provoking test was used in two studies to measure the FOG severity score. Test duration was also used to assess FOG status during walking. However, only one study evaluated static balance by using peak CoP velocity. TUG tests were conducted in 14 studies, and BBSs were used in three studies to measure dynamic balance. Finally, two studies used the DGI to measure balance.
Risk of bias and quality of included studies
Since all included studies were RCTs, PEDro scale was used to evaluate the risk of bias in each included study. The average score was 7.08 ± 1.11, indicating good quality. In total, three studies scored a 9, indicating excellent quality; 20 studies scored 6–8, indicating that 80% of studies demonstrated good quality; and two studies demonstrated fair quality (Table 2). Only five studies reported allocation concealment [47, 50, 55, 59, 61], and two studies (8%) [50, 59] used an intention-to-treat analysis. Assessors and participants could not be blinded in 10 and 3 studies, respectively. Although blinded therapists often face challenges during the intervention, eight studies (32%) reported success in including blinded therapists. All 25 studies were determined to be level 2 on the "Oxford Centre for Evidence-Based Medicine Levels of Evidence".
Effects of tDCS alone and in combination with rehabilitation therapies
Results of subgroup analysis
The effects of tDCS for each outcome are summarized in Table 3.
Real tDCS versus sham tDCS
The effects of tDCS alone on gait were assessed by measuring gait speed (seven studies), stride length (five studies), cadence (five studies), FOG-Q (two studies), walking time (three studies), and stride time (three studies). Compared with a control group receiving sham tDCS, PD patients in the real tDCS group exhibited greater gait speed and stride length and lower cadence, FOG-Q, walking time, and stride time with moderate effect sizes. Real tDCS significantly affected the decrease in cadence and stride time (P = 0.027 and P = 0.002, respectively). To evaluate the effect of tDCS alone on balance, 12 studies used TUG tests, one used the BBS, and one used the DGI. The results indicated that real tDCS is associated with greater balance. However, a statistically significant difference was found only in the TUG tests (P = 0.023).
Real tDCS plus other therapies versus sham tDCS with other therapies
The effects of tDCS with other therapies on gait were assessed by measuring gait speed (six studies), stride length (five studies), cadence (five studies), FOG-Q (two studies), step length (three studies), stride time (one studies), and double support time (three studies). The effects on balance were assessed using TUG tests (eight studies), BBS scores (two studies), and the DGI (one study). The pooled results indicated that the participants in the tDCS plus other therapies group exhibited greater improvements in gait (cadence, P < 0.01) and balance (BBS, P = 0.045) than those in the sham tDCS with other therapies group, indicating that tDCS can induce additional effects and promote other therapies in PD rehabilitation.
Overall effects of tDCS
Gait
The results of the pooled analysis revealed the moderate effects of the tDCS group on the changes in gait speed (SMD = 0.238; 95% CI − 0.026 to 0.502), stride length (SMD = 0.318; 95% CI − 0.015 to 0.652), cadence (SMD = − 0.632; 95% CI − 0.932 to − 0.333), FOG-Q (SMD = − 0.360; 95% CI − 0.692 to − 0.027), step length (SMD = 0.459; 95% CI − 0.031 to 0.949), walking time (SMD = − 0.253; 95% CI − 0.758 to 0.252), stride time (SMD = − 0.785; 95% CI − 1.680 to 0.111), and double support time (SMD = 1.139; 95% CI − 0.244 to 0.523). However, only cadence and FOG-Q significantly improved after tDCS compared with the control group (P < 0.001, P = 0.034, respectively) (Figs. 2, 3, 4, 5, Additional file 2: Figs. S1–4). No heterogeneity was present among studies for all outcome measures of gait (I2 = 0%, P > 0.05). Publication bias was assessed through funnel plot, Egger’s, and Begg’s tests. The analyses revealed that Egger’s test (P = 0.018) and Begg’s test (P < 0.001) indicated a significant publication bias for gait speed, with one study falling outside the funnel plot. This outlier study included a lengthier intervention protocol than the other studies, which involved three weeks of tDCS combined with dual-task gait training. In addition, no publication bias was observed for cadence and stride length (Additional file 3: Figs. S5–7).
Forest plot of standardized mean difference (SMD) and their 95% CI for gait speed. Black squares represent the SMD in individual trials. Horizontal lines represent 95% confidence interval (CI). The blue rhombus at the bottom indicates an overall pooled effect. tDCS: Transcranial direct current stimulation. The subjects received real tDCS showing an improvement in gait speed. However, this improvement did not reveal statistical significance compared to sham treatment patients (P = 0.077)
Forest plot of standardized mean difference (SMD) and their 95% CI for stride length. Black squares represent the SMD in individual trials. Horizontal lines represent 95% confidence interval (CI). The blue rhombus at the bottom indicates an overall pooled effect. tDCS: Transcranial direct current stimulation. Similarly, the subjects in real tDCS showed an improvement in stride length. However, this improvement did not reveal statistical significance compared to patients in the sham treatment group (P = 0.062)
Forest plot of standardized mean difference (SMD) and their 95% CI for cadence. Black squares represent the SMD in individual trials. Horizontal lines represent 95% confidence interval (CI). The blue rhombus at the bottom indicates an overall pooled effect. tDCS: Transcranial direct current stimulation. Subjects who received either real tDCS alone or combined with additional therapies had distinctly reduced cadence during walking. This shows strong evidence that tDCS has a substantial beneficial effect on cadence parameters (P < 0.001)
Forest plot of standardized mean difference (SMD) and their 95% CI for freezing of gait questionnaire. Black squares represent the SMD in individual trials. Horizontal lines represent 95% confidence interval (CI). The blue rhombus at the bottom indicates an overall pooled effect. tDCS: Transcranial direct current stimulation. The pooled results showed that tDCS indeed reduces the freezing during gait as measured by the freezing of gait questionnaire with a moderate effect size of 0.360 (P = 0.034)
Balance
tDCS remarkably improved the balance of PD patients compared with the control group, which was indicated by the decrease in time required to complete the TUG test (SMD = − 0.294; 95% CI − 0.516 to − 0.073, P = 0.009, Fig. 6). Additionally, the meta-analysis results revealed a nonsignificant difference in BBS scores (SMD = 0.406; 95% CI − 0.059 to 0.87, P = 0.087, Fig. 7) and the DGI (SMD = 0.275; 95% CI − 0.349 to 0.898, P = 0.388, Additional file 4: Fig. S8) between the tDCS group and control group. No publication bias (P > 0.05 in Egger’s and Begg’s tests) for the timed up and go test (Additional file 5: Fig. S9) or no heterogeneity (I2 = 0%, P > 0.05) was present among the included studies for all outcome measures.
Forest plot of standardized mean difference (SMD) and their 95% CI for timed up and go test. Black squares represent the SMD in individual trials. Horizontal lines represent 95% confidence interval (CI). The blue rhombus at the bottom indicates an overall pooled effect. tDCS: Transcranial direct current stimulation. The results of this meta-analysis show robust evidence that tDCS significantly improved the balance of PD patients compared with controls, as indicated by a reduction in the time required to complete the TUG test (P = 0.009)
Forest plot of standardized mean difference (SMD) and their 95% CI for Berg balance scale. Black squares represent the SMD in individual trials. Horizontal lines represent 95% confidence interval (CI). The blue rhombus at the bottom indicates an overall pooled effect. tDCS: Transcranial direct current stimulation. The overall meta-analysis result from studies which compared with patients in the sham group with patients who received either tDCS alone or tDCS combined with additional rehabilitation therapies did not show a significant improvement in balance measured by Berg balance scale (P = 0.087)
Discussion
The current systematic review and meta-analysis summarized the available data on the effectiveness of tDCS alone and in combination with other therapies for patients with PD. Although two studies provided figures with data, we were unable to extract data by using GRABIT; consequently, the data were not included in the meta-analysis [46, 64]. Therefore, we conducted a meta-analysis on 11 outcome measures, including 75 comparisons from 23 studies. Studies were scored from fair quality to excellent quality. Evidence supported that tDCS-induced therapeutic effects play a critical role in managing the motor symptoms of patients with PD. Altogether, the key findings of this review indicated that tDCS protocols greatly affect the gait and balance of patients with PD who are over 50 years old and with mild to severe disease for less than 17 years.
To our knowledge, six meta-analyses [19, 20, 24, 66,67,68] have been conducted on the effects of tDCS on motor function among patients with PD. These meta-analyses focused on specific aspects of tDCS, such as single versus multitarget regions [24] and real versus sham tDCS combined with gait training [19, 68]. In a meta-analysis of 21 studies that enrolled 736 patients with PD, the authors reported insufficient evidence to conclude that tDCS could improve motor functions [67]. The authors proposed that several factors contributed to the tDCS-induced effects on motor functions, including tDCS parameters, stimulation areas, and patient features. Nevertheless, our findings are in agreement with those of other studies [20, 66] that revealed the therapeutic effects of tDCS on gait, balance, and functional mobility but did not reveal any significant difference compared with the control group. However, our meta-analysis was more rigorous than other meta-analyses. We included the broadest range of studies and outcome measures to provide comprehensive evidence that can support decision-making in clinical practices. Additionally, the subgroup analyses were performed to examine the effects of tDCS with and without other therapies, which can benefit future research on tDCS.
Gait and balance deficits are a hallmark of disease progression [69]. These deficits eventually become refractory motor complications and can lead to disability among patients with PD [45]. In advanced stages of PD, patients typically exhibit abnormal gait patterns such as reduced gait speed and step length, increased cadence, and double-limb support [70, 71]. Posture control when standing up, the narrowing of the support base while walking, and postural instability in the mediolateral plane when turning worsen as PD progresses [72]. Additionally, FOG commonly occurs when patients walk, turn, and traverse narrow hallways, all of which increase fall risk [71, 72]. These gait and balance impairments arise from various pathological mechanisms involving the BG network [73]. As a clinically noninvasive brain stimulation procedure, tDCS effectively rehabilitates gait and balance and produces noticeable results by applying an anodal charge over the targeted cortex. The beneficial effects of tDCS on gait and balance can be explained by two mechanisms. Applied anodal tDCS on motor cortices could induce dopamine releases in the BG by activating glutamatergic corticostriatal projections and could modulate the functional connectivity in corticostriatal and thalamocortical circuits. Most studies took advantage of the immediate mechanisms of tDCS and supplied a single session of tDCS to examine short-term improvements. However, it should be noted that the positive changes in gait and balance after tDCS were inconsistent with the stimulation area and intensity. In one study by Wong et al. [44], tDCS was applied separately over the primary motor cortex (M1), dorsolateral prefrontal cortex (DLPFC), and the cerebellum within 20 min. Despite the differences between pre- and post-intervention found in the majority of gait parameters (gait speed, cadence, and step length), none of the groups exhibited significant differences, including the sham group. Their results also supported that tDCS targeting M1 or DLPFC can improve gait in a single walking task. Another study compared single-target (M1) and multitarget (M1 and DLPFC) tDCS protocols. This study indicated that simultaneously stimulating M1 and DLPFC at an intensity of 1.5 mA for 20 min, rather than only M1, was more effective in alleviating FOG severity and balance, which was reflected by gait speed and TUG test results [43]. Another study performed anodal tDCS over M1 with 1 mA, 2 mA, and sham tDCS during separate 20-min sessions [46]. A better postural response to external perturbations among patients with PD was observed for 2 mA but not for 1 mA or sham. These observations demonstrated the substantial heterogeneity in tDCS protocols employed across the included study. Accordingly, it is critically important to establish investigations that focus on optimizing tDCS treatment protocols and investigating whether these various parameters have a notable influence on the effects of tDCS.
Regarding the combination of tDCS and other therapies, the action mechanism of tDCS could promote the inherent positive effects of rehabilitation therapies on motor performances in patients with PD. Kaski et al. [41] revealed that applying both tDCS and physical training was more effective in improving gait functions than training or tDCS alone. Furthermore, Conceicao et al. determined that the gait variability, executive control of walking and processing speed were enhanced by applying one session of anodal tDCS during aerobic exercise [63]. This study also highlighted that the addition of tDCS to aerobic exercise could modulate cholinergic activity, which affects gait disturbances in patients with PD. Additionally, numerous studies in our meta-analysis have confirmed that combined gait and balance training with tDCS improved gait speed [41, 59], stride length [41], double support time [59], cadence and step length [55, 60], TUG test results [56, 59], and BBS scores [55]. These findings support the benificial effects of tDCS with other therapies on gait and balance among patients with PD.
There are a number of limitations listed in the current study. First, half of the included studies were crossover designs with a 1-week washout that may have resulted in a carry-over effect. Nevertheless, the effect of tDCS would not be prolonged for a substantial period. Second, the validity of our results may be influenced by the fact that most of the included studies had a small number of participants. Third, many studies did not report using an intention-to-treat analysis or having allocation concealment or blinding (including participant, therapist, and assessor), which could have produced biases in the original studies and influenced the results of this meta-analysis. Fourth, the variety of tDCS protocols, such as intervention length, electrode montages, and additional therapies, may have affected the consistency among studies. Fortunately, no significant heterogeneity was observed in any analysis. Fifth, we were unable to investigate the effects of tDCS on each stage of the disease due to substantial variation in disease severity and the insufficient data reported in the included studies. Finally, the effects of tDCS on gait and balance were moderate, but effect sizes were almost entirely smaller than 0.5 and, in some cases, did not significantly differ from the control group. Therefore, future studies could further investigate under a larger sample size and be more methodologically rigorous when studying the effects tDCS in individuals with PD.
Conclusions
Gait and balance impairments are incredibly challenging to address in PD rehabilitation. tDCS is an adjuvant treatment that has demonstrated benefits for improving motor and non-motor functions in PD patients. The results of our systematic review and meta-analysis offer substantial evidence that tDCS, whether used alone or in combination with other therapies, significantly enhances gait and balance in individuals with PD compared to sham tDCS or sham tDCS combined with other therapies. Nevertheless, it is crucial to recognize that the optimal protocol for tDCS in the treatment of PD has not yet been established. Consequently, further research is essential to identify the therapeutic protocols that are critical for maximizing the efficacy of tDCS.
Clinical implication
To date, growing evidence uncovers the potential benefits of tDCS in various neurological conditions, including PD. It is thus becoming more critical to incorporate its significance into therapeutic practice. Our systematic review and meta-analysis of tDCS effects are practically meaningful to research and clinical applications. Our study conclusively demonstrates that tDCS, whether used alone or in combination with other therapies, is efficacious in improving certain aspects of gait and balance in individuals with PD. These findings hold significant clinical relevance as they inform healthcare decision-making for clinicians and patients, shedding light on the advantages and therapeutic benefits of tDCS among a variety of existing non-invasive brain stimulation techniques. In particular, these findings facilitate the integration of tDCS as a valuable component within a comprehensive PD rehabilitation program. However, it is essential to note that the optimal protocol of tDCS is not yet established for treating PD. Therefore, further research is necessary to elucidate the specific protocol, including targeted area, intensity, duration, and targeted stage of the disease, to maximize the benefit impacts of tDCS.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Additional information files.
Abbreviations
- PD:
-
Parkinson’s disease
- BG:
-
Basal ganglia
- BG–Ctx–Cer:
-
Basal, cortex, and cerebellum
- M1:
-
Primary motor cortex
- DLPFC:
-
Dorsolateral prefrontal cortex
- tDCS:
-
Transcranial direct current stimulation
- tES:
-
Transcranial electrical stimulation
- rTMS:
-
Repetitive transcranial magnetic stimulation
- atDCS:
-
Anodal tDCS
- FOG-Q:
-
Freezing of gait questionnaire
- 10MWT:
-
Ten-meter walking test
- 6MWT:
-
Six minutes waking test
- CoP:
-
Center of pressure
- TUG:
-
Timed up and go test
- BBS:
-
Berg balance scale
- BESTest:
-
Balance evaluation systems test
- FRT:
-
Functional reach test
- DGI:
-
Dynamic gait index
- FGA:
-
Functional gait assessment
- UPDRS III:
-
Unified Parkinson Disease Rating Scale Motor section
- H&Y score:
-
Hoehn & Yahr score
- RCT:
-
Randomized control trial
- SMD:
-
Standardized mean difference
- CI:
-
Confidence interval
- SD:
-
Standard deviation
- IG:
-
Intervention group
- CG:
-
Control group
References
Dorsey ER, Elbaz A, Nichols E, Abbasi N, Abd-Allah F, Abdelalim A, Adsuar JC, Ansha MG, Brayne C, Choi JY, Collado-Mateo D. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939–53. https://doi.org/10.1016/s1474-4422(18)30295-3.
Kalia LV, Lang AE. Parkinson’s disease. Lancet (London, England). 2015;386:896–912. https://doi.org/10.1016/s0140-6736(14)61393-3.
Zhong QQ, Zhu F. Trends in prevalence cases and disability-adjusted life-years of Parkinson’s disease: findings from the global burden of disease study 2019. Neuroepidemiology. 2022. https://doi.org/10.1159/000524208.
de Lau LML, Breteler MMB. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–35. https://doi.org/10.1016/S1474-4422(06)70471-9.
Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm (Vienna, Austria : 1996). 2017;124:901–5. https://doi.org/10.1007/s00702-017-1686-y.
Findley LJ. The economic impact of Parkinson’s disease. Parkinsonism Relat Disord. 2007;13:S8-s12. https://doi.org/10.1016/j.parkreldis.2007.06.003.
Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, Agid Y, DeLong MR, Obeso JA. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci. 2010;11:760–72. https://doi.org/10.1038/nrn2915.
Magrinelli F, Picelli A, Tocco P, Federico A, Roncari L, Smania N, Zanette G, Tamburin S. Pathophysiology of motor dysfunction in Parkinson’s disease as the rationale for drug treatment and rehabilitation. Parkinsons Dis. 2016;2016:9832839. https://doi.org/10.1155/2016/9832839.
Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. https://doi.org/10.1146/annurev.ne.09.030186.002041.
Obeso JA, Rodríguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C, Rodriguez M. Functional organization of the basal ganglia: therapeutic implications for Parkinson’s disease. Mov Dis. 2008;23(Suppl 3):S548-559. https://doi.org/10.1002/mds.22062.
Caligiore D, Helmich RC, Hallett M, Moustafa AA, Timmermann L, Toni I, Baldassarre G. Parkinson’s disease as a system-level disorder. NPJ Parkinsons Dis. 2016;2:16025. https://doi.org/10.1038/npjparkd.2016.25.
McGregor MM, Nelson AB. Circuit mechanisms of Parkinson’s disease. Neuron. 2019;101:1042–56. https://doi.org/10.1016/j.neuron.2019.03.004.
Keus S, Munneke M, Graziano M. European physiotherapy guideline for Parkinsons disease. Amsterdam: KNGF/ParkinsonNet; 2014.
Madrid J, Benninger DH. Non-invasive brain stimulation for Parkinson’s disease: clinical evidence, latest concepts and future goals—a systematic review. J Neurosci Methods. 2021;347: 108957. https://doi.org/10.1016/j.jneumeth.2020.108957.
Benninger DH, Hallett M. Non-invasive brain stimulation for Parkinson’s disease: current concepts and outlook 2015. NeuroRehabilitation. 2015;37:11–24. https://doi.org/10.3233/nre-151237.
da Silva Machado CB, da Silva LM, Gonçalves AF, Andrade PR, Mendes C, de Assis T, Godeiro Júnior CO, Andrade SM. Multisite non-invasive brain stimulation in Parkinson’s disease: a scoping review. NeuroRehabilitation. 2021;49:515–31. https://doi.org/10.3233/nre-210190.
Fregni F, Boggio PS, Santos MC, Lima M, Vieira AL, Rigonatti SP, Silva MTA, Barbosa ER, Nitsche MA, Pascual-Leone A. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Mov Disord. 2006;21:1693–702. https://doi.org/10.1002/mds.21012.
Santos Ferreira I, Teixeira Costa B, Lima Ramos C, Lucena P, Thibaut A, Fregni F. Searching for the optimal tDCS target for motor rehabilitation. J Neuroeng Rehabil. 2019;16:90. https://doi.org/10.1186/s12984-019-0561-5.
Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for idiopathic Parkinson’s disease. Cochrane Database Syst Rev. 2016;7:10916. https://doi.org/10.1002/14651858.CD010916.pub2.
Lee HK, Ahn SJ, Shin YM, Kang N, Cauraugh JH. Does transcranial direct current stimulation improve functional locomotion in people with Parkinson’s disease? A systematic review and meta-analysis. J Neuroeng Rehabil. 2019;16:84. https://doi.org/10.1186/s12984-019-0562-4.
Orrù G, Baroni M, Cesari V, Conversano C, Hitchcott PK, Gemignani A. The effect of single and repeated tDCS sessions on motor symptoms in Parkinson’s disease: a systematic review. Arch Ital Biol. 2019;157:89–101. https://doi.org/10.12871/00039829201925.
Simpson MW, Mak M. The effect of transcranial direct current stimulation on upper limb motor performance in Parkinson’s disease: a systematic review. J Neurol. 2020;267:3479–88. https://doi.org/10.1007/s00415-019-09385-y.
Cammisuli DM, Cignoni F, Ceravolo R, Bonuccelli U, Castelnuovo G. Transcranial direct current stimulation (tDCS) as a useful rehabilitation strategy to improve cognition in patients with Alzheimer’s disease and Parkinson’s disease: an updated systematic review of randomized controlled trials. Front Neurol. 2021;12: 798191. https://doi.org/10.3389/fneur.2021.798191.
de Oliveira PCA, de Araújo TAB, Machado D, Rodrigues AC, Bikson M, Andrade SM, Okano AH, Simplicio H, Pegado R, Morya E. Transcranial direct current stimulation on Parkinson’s disease: systematic review and meta-analysis. Front Neurol. 2021;12: 794784. https://doi.org/10.3389/fneur.2021.794784.
Pol F, Salehinejad MA, Baharlouei H, Nitsche MA. The effects of transcranial direct current stimulation on gait in patients with Parkinson’s disease: a systematic review. Transl Neurodegener. 2021;10:22. https://doi.org/10.1186/s40035-021-00245-2.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. https://doi.org/10.1136/bmj.n71.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. https://doi.org/10.1136/jnnp.55.3.181.
https://pedro.org.au/wp-content/uploads/PEDro_scale.pdf. Accessed 5 May 2022.
de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55:129–33. https://doi.org/10.1016/s0004-9514(09)70043-1.
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–6. https://doi.org/10.1046/j.1445-2197.2003.02748.x.
Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, Moschetti I, Phillips B, Thornton H. Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence (Background Document)”. Oxford Centre for Evidence-Based Medicine. 2011. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/explanation-of-the-2011-ocebm-levels-of-evidence/. Accessed 5 May 2022.
Higgins J, Li T, Deeks J, Higgins JPT. Chapter 6. Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). Hoboken: Wiley; 2019.
Higgins JP, Eldridge S, Li T. Including variants on randomized trials. In: Julian PT, editor. Cochrane handbook for systematic reviews of interventions. Hobeken: Wiley; 2019. p. 569–93.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. https://doi.org/10.1186/1471-2288-5-13.
Cohen J. Statistical power analysis. Curr Dir Psychol Sci. 1992;1:98–101. https://doi.org/10.1111/1467-8721.ep10768783.
Rücker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med Res Methodol. 2008;8:79. https://doi.org/10.1186/1471-2288-8-79.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. https://doi.org/10.1002/sim.1186.
Page MJ, Sterne JAC, Higgins JPT, Egger M. Investigating and dealing with publication bias and other reporting biases in meta-analyses of health research: a review. Res Synth Methods. 2021;12:248–59. https://doi.org/10.1002/jrsm.1468.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Sterne J, Egger M, Moher D. Addressing reporting biases. Hoboken: Wiley; 2008. p. 297–333.
Kaski D, Dominguez RO, Allum JH, Islam AF, Bronstein AM. Combining physical training with transcranial direct current stimulation to improve gait in Parkinson’s disease: a pilot randomized controlled study. Clin Rehabil. 2014;28:1115–24. https://doi.org/10.1177/0269215514534277.
Criminger C, Swank C, Mehta J. Does transcranial direct current stimulation plus concurrent activity lessen dual task cost in people with Parkinson’s disease? Brain Stimul. 2017;10:405–6. https://doi.org/10.1016/j.brs.2017.01.202.
Dagan M, Herman T, Harrison R, Zhou J, Giladi N, Ruffini G, Manor B, Hausdorff JM. Multitarget transcranial direct current stimulation for freezing of gait in Parkinson’s disease. Mov Disord. 2018;33:642–6. https://doi.org/10.1002/mds.27300.
Wong PL, Yang YR, Huang SF, Fuh JL, Chiang HL, Wang RY. Transcranial direct current stimulation on different targets to modulate cortical activity and dual-task walking in individuals With Parkinson’s disease: a double blinded randomized controlled trial. Front Aging Neurosci. 2022;14: 807151. https://doi.org/10.3389/fnagi.2022.807151.
Benninger DH, Lomarev M, Lopez G, Wassermann EM, Li X, Considine E, Hallett M. Transcranial direct current stimulation for the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2010;81:1105–11. https://doi.org/10.1136/jnnp.2009.202556.
Beretta VS, Vitório R, Nóbrega-Sousa P, Conceição NR, Orcioli-Silva D, Pereira MP, Gobbi LTB. Effect of different intensities of transcranial direct current stimulation on postural response to external perturbation in patients with Parkinson’s disease. Neurorehabil Neural Repair. 2020;34:1009–19. https://doi.org/10.1177/1545968320962513.
Bueno ME, do Nascimento Neto LI, Terra MB, Barboza NM, Okano AH, Smaili SM. Effectiveness of acute transcranial direct current stimulation on non-motor and motor symptoms in Parkinson’s disease. Neurosci Lett. 2019;696:46–51. https://doi.org/10.1016/j.neulet.2018.12.017.
Lattari E, Costa SS, Campos C, de Oliveira AJ, Machado S, Maranhao Neto GA. Can transcranial direct current stimulation on the dorsolateral prefrontal cortex improves balance and functional mobility in Parkinson’s disease? Neurosci Lett. 2017;636:165–9. https://doi.org/10.1016/j.neulet.2016.11.019.
Manenti R, Brambilla M, Rosini S, Orizio I, Ferrari C, Borroni B, Cotelli M. Time up and go task performance improves after transcranial direct current stimulation in patient affected by Parkinson’s disease. Neurosci Lett. 2014;580:74–7. https://doi.org/10.1016/j.neulet.2014.07.052.
Manor B, Dagan M, Herman T, Gouskova NA, Vanderhorst VG, Giladi N, Travison TG, Pascual-Leone A, Lipsitz LA, Hausdorff JM. Multitarget transcranial electrical stimulation for freezing of gait: a randomized controlled trial. Mov Disord. 2021;36:2693–8. https://doi.org/10.1002/mds.28759.
Mishra RK, Thrasher AT. Transcranial direct current stimulation of dorsolateral prefrontal cortex improves dual-task gait performance in patients with Parkinson’s disease: a double blind, sham-controlled study. Gait Posture. 2021;84:11–6. https://doi.org/10.1016/j.gaitpost.2020.11.012.
Da Silva DCL, Lemos T, de Sá Ferreira A, Horsczaruk CHR, Pedron CA, de Carvalho Rodrigues E, de Oliveira LAS. Effects of acute transcranial direct current stimulation on gait kinematics of individuals with parkinson disease. Top Geriatr Rehabil. 2018;34:262.
Swank C, Mehta J, Criminger C. Transcranial direct current stimulation lessens dual task cost in people with Parkinson’s disease. Neurosci Lett. 2016;626:1–5. https://doi.org/10.1016/j.neulet.2016.05.010.
Valentino F, Cosentino G, Brighina F, Pozzi NG, Sandrini G, Fierro B, Savettieri G, D’Amelio M, Pacchetti C. Transcranial direct current stimulation for treatment of freezing of gait: a cross-over study. Mov Disord. 2014;29:1064–9. https://doi.org/10.1002/mds.25897.
Costa-Ribeiro A, Maux A, Bosford T, Aoki Y, Castro R, Baltar A, Shirahige L, Moura Filho A, Nitsche MA, Monte-Silva K. Transcranial direct current stimulation associated with gait training in Parkinson’s disease: a pilot randomized clinical trial. Dev Neurorehabil. 2017;20:121–8. https://doi.org/10.3109/17518423.2015.1131755.
Na Y, Kim J, Lee SH, Kim J, Lee J, Shin SY, Chang WH, Cho JW, Kim YH. Multichannel transcranial direct current stimulation combined with treadmill gait training in patients with Parkinson’s disease: a pilot study. Front Neurol. 2022;13: 804206. https://doi.org/10.3389/fneur.2022.804206.
Fernández-Lago H, Bello O, Mora-Cerdá F, Montero-Cámara J, Fernández-Del-Olmo M. Treadmill walking combined with anodal transcranial direct current stimulation in Parkinson disease: a pilot study of kinematic and neurophysiological effects. Am J Phys Med Rehabil. 2017;96:801–8. https://doi.org/10.1097/phm.0000000000000751.
Manenti R, Brambilla M, Benussi A, Rosini S, Cobelli C, Ferrari C, Petesi M, Orizio I, Padovani A, Borroni B, et al. Mild cognitive impairment in Parkinson’s disease is improved by transcranial direct current stimulation combined with physical therapy. Mov Disord. 2016;31:715–24. https://doi.org/10.1002/mds.26561.
Schabrun SM, Lamont RM, Brauer SG. Transcranial direct current stimulation to enhance dual-task gait training in Parkinson’s disease: a pilot RCT. PLoS ONE. 2016;11: e0158497. https://doi.org/10.1371/journal.pone.0158497.
Yotnuengnit P, Bhidayasiri R, Donkhan R, Chaluaysrimuang J, Piravej K. Effects of transcranial direct current stimulation plus physical therapy on gait in patients with Parkinson disease: a randomized controlled trial. Am J Phys Med Rehabil. 2018;97:7–15. https://doi.org/10.1097/phm.0000000000000783.
Chang WH, Kim MS, Park E, Cho JW, Youn J, Kim YK, Kim YH. Effect of dual-mode and dual-site noninvasive brain stimulation on freezing of gait in patients with Parkinson disease. Arch Phys Med Rehabil. 2017;98:1283–90. https://doi.org/10.1016/j.apmr.2017.01.011.
von Papen M, Fisse M, Sarfeld AS, Fink GR, Nowak DA. The effects of 1 Hz rTMS preconditioned by tDCS on gait kinematics in Parkinson’s disease. J Neural Transm (Vienna, Austria : 1996). 2014;121:743–54. https://doi.org/10.1007/s00702-014-1178-2.
Conceição NR, Gobbi LTB, Nóbrega-Sousa P, Orcioli-Silva D, Beretta VS, Lirani-Silva E, Okano AH, Vitório R. Aerobic exercise combined with transcranial direct current stimulation over the prefrontal cortex in Parkinson Disease: effects on cortical activity, gait, and cognition. Neurorehabil Neural Repair. 2021;35:717–28. https://doi.org/10.1177/15459683211019344.
Mishra RK, Thrasher AT. Effect of concurrent transcranial direct current stimulation on instrumented timed up and go task performance in people with Parkinson’s disease: a double-blind and cross-over study. J Clin Neurosci. 2022;100:184–91. https://doi.org/10.1016/j.jocn.2022.04.029.
Lee SA, Kim MK. The effect of transcranial direct current stimulation combined with visual cueing training on motor function, balance, and gait ability of patients with Parkinson’s disease. Medicina (Kaunas). 2021. https://doi.org/10.3390/medicina57111146.
Goodwill AM, Lum JAG, Hendy AM, Muthalib M, Johnson L, Albein-Urios N, Teo WP. Using non-invasive transcranial stimulation to improve motor and cognitive function in Parkinson’s disease: a systematic review and meta-analysis. Sci Rep. 2017;7:14840. https://doi.org/10.1038/s41598-017-13260-z.
Liu X, Liu H, Liu Z, Rao J, Wang J, Wang P, Gong X, Wen Y. Transcranial direct current stimulation for Parkinson’s disease: a systematic review and meta-analysis. Front Aging Neurosci. 2021;13: 746797. https://doi.org/10.3389/fnagi.2021.746797.
Nascimento LR, do Carmo WA, de Oliveira GP, Arêas F, Dias FM. Transcranial direct current stimulation provides no clinically important benefits over walking training for improving walking in Parkinson’s disease: a systematic review. J Physiother. 2021;67:190–6. https://doi.org/10.1016/j.jphys.2021.06.003.
Boonstra TA, van der Kooij H, Munneke M, Bloem BR. Gait disorders and balance disturbances in Parkinson’s disease: clinical update and pathophysiology. Curr Opin Neurol. 2008;21:461–71. https://doi.org/10.1097/WCO.0b013e328305bdaf.
Raccagni C, Nonnekes J, Bloem BR, Peball M, Boehme C, Seppi K, Wenning GK. Gait and postural disorders in parkinsonism: a clinical approach. J Neurol. 2020;267:3169–76. https://doi.org/10.1007/s00415-019-09382-1.
Mirelman A, Bonato P, Camicioli R, Ellis TD, Giladi N, Hamilton JL, Hass CJ, Hausdorff JM, Pelosin E, Almeida QJ. Gait impairments in Parkinson’s disease. Lancet Neurol. 2019;18:697–708. https://doi.org/10.1016/s1474-4422(19)30044-4.
Son M, Youm C, Cheon S, Kim J, Lee M, Kim Y, Kim J, Sung H. Evaluation of the turning characteristics according to the severity of Parkinson disease during the timed up and go test. Aging Clin Exp Res. 2017;29:1191–9. https://doi.org/10.1007/s40520-016-0719-y.
Takakusaki K. Functional neuroanatomy for posture and gait control. J Mov Disord. 2017;10:1–17. https://doi.org/10.14802/jmd.16062.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Science and Technology Council, Taiwan (Grant no. NSTC 111-2314-B-182-035-MY3, 112-2314-B-182-022, 112-2321-B-002-021, MOST 109-2314-B-182-029-MY3) and Chang Gung Medical Foundation, Taiwan (CMRPD1M0231, CMRPD1M0701, CMRPD1M0252, and CMRPD1N0301, and CORPD1N0061).
Author information
Authors and Affiliations
Contributions
THH and TXDN provided the conception and study design. TXDN and PTM performed the literature search, quality assessment of included studies, data extraction, data, and interpretation. TXD Nguyen wrote the manuscript. THH and YJC contributed to the critical revision of the manuscript. All authors approved the final manuscript to be published.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
No conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
PRISMA Checklist 2020.
Additional file 2: Figure S1.
Forest plot of standardized mean difference (SMD) and their 95% CI for step length. Figure S2. Forest plot of standardized mean difference (SMD) and their 95% CI for walking time. Figure S3. Forest plot of standardized mean difference (SMD) and their 95% CI for stride time. Figure S4. Forest plot of standardized mean difference (SMD) and their 95% CI for double support time.
Additional file 3: Figure S5.
Funnel plot of gait speed. Figure S6. Funnel plot of stride length. Figure S7. Funnel plot of cadence.
Additional file 4: Figure S8.
Forest plot of standardized mean difference (SMD) and their 95% CI for dynamic gait index.
Additional file 5: Figure S9.
Funnel plot of timed up and go test.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nguyen, T.X.D., Mai, P.T., Chang, YJ. et al. Effects of transcranial direct current stimulation alone and in combination with rehabilitation therapies on gait and balance among individuals with Parkinson’s disease: a systematic review and meta-analysis. J NeuroEngineering Rehabil 21, 27 (2024). https://doi.org/10.1186/s12984-024-01311-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12984-024-01311-2