Abstract
Background
Many diagnostic robotic devices have been developed to quantify viscoelastic properties and spasticity of patients with upper motor neuron lesions. However, in clinical practice, subjective and nonvalid clinical scales are still commonly used. To understand the limited use of diagnostic robotic devices assessing viscoelastic joint properties and spasticity in clinical practice, we evaluate the diagnostic level of evidence of studies on these devices.
Method
A systematic literature review was performed using multiple databases. Two of the authors independently screened all articles. Studies investigating human subjects diagnosed with stroke or cerebral palsy, measured with a mechanical device to assess viscoelastic joint properties and/or spasticity of an extremity. All articles were assigned a diagnostic level of evidence, which was established with a classification strategy based on the number of participants and the design of the study, from a Level 0 (less than 10 subjects) to a Level IV, reporting the long-term clinical consequences in daily care.
Results
Fifty-nine articles were included. Most studies measured the upper limb (64%) in stroke patients (81%). The highest level of evidence found was Level IIa (53%); these studies correlated the test values of the robotic device with a clinical test or within subgroups. Level 0 (30%) and Level I (17%; determining the range of values of the robotic test) were also common. None of the studies tested their device for diagnostic accuracy (Level III), clinical added value (Level IV).
Conclusion
The diagnostic evidence needed for implementing robotic devices in clinical practice is lacking. Our findings indicate that more effort should be invested in studying diagnostic accuracy (Level III) or added value for clinical care (Level IV); only these studies can provide clinicians with evidence that robotic devices have added value above the currently-used clinical scales.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
In patients with upper motor neuron disease, non-neural (i.e. altered tissue viscoelastic joint properties) and neural (i.e. improper muscle activation, caused by e.g. spasticity) properties may contribute to joint stiffness [1]. These impairments are common as over eighty-five percent of all patients with upper motor neuron disease suffer from a paretic upper or lower extremity resulting in pain, difficulty moving, or poor hygiene [2, 3]. However, since impairments in non-neural and neural joint properties require different treatments, reliable, valid, and user-independent assessment is needed to support clinicians in selecting treatment plans.
Frequently used clinical tools to measure viscoelastic joint properties (e.g. Modified Tardieu Scale (MTS) and goniometer) and spasticity (e.g. Modified Ashworth Scale (MAS) and Modified Tardieu Scale (MTS)) of an extremity are subjected to the biases inherent to human perception. For measuring spasticity with a clinical measurement tool such as the MAS, studies show only fair to good intra-rater agreement scores [4, 5]. Fleuren et al. [6], due to its poor reliability and validity, stated that the MAS should not be used to evaluate spasticity anymore. Further, viscoelastic joint properties and spasticity cannot be differentiated with clinical tools [5, 7], which hampers individually tailored treatment plans.
Robotic measurement tools have the potential of more quantitative, objective, operator-independent assessment of viscoelastic joint properties and spasticity by imposing controlled positions or forces and measuring a person's individual responses with sensors. For example, during a velocity-controlled passive stretch, force sensors can measure the joint-muscle resistance during the whole movement trajectory [8,9,10]; changes in resistance that result from involuntary muscle activation can be a measure of spasticity [11].
Robotic measurement tools are not commonly used in clinical practice [12]. This may be due to multiple reasons, such as high purchase costs, relatively complex ease of use, and the relatively long measurement time. Another reason may be that no evidence is available of studies investigating the clinical added value of these robotic measurement tools.
Different ways of studying a diagnostic instrument's potential or diagnostic value are possible, depending on the kind of research question. To convert the diagnostic question into the appropriate research design, Sackett and Haynes [13] suggested four relevant questions that need to be answered in four different phases. Phase 1 Question: Do test results in affected patients differ from those in normal individuals? Phase 2 Question: Are patients with certain test results more likely to have the target disorder? Phase 3 Question: Do test results distinguish patients with and without the target disorder among those in whom it is clinically sensible to suspect the disorder? Phase 4 Question: Do patients undergoing the diagnostic test fare better than similar untested patients? The type of questions provide an increasing detailed insight into the diagnostic accuracy of the test, with the last type of question measuring the ultimate value of a diagnostic test; the health outcomes resulting from the interventions that the diagnostic test results precipitate.
To understand the limited use of diagnostic robotic devices assessing viscoelastic joint properties and spasticity in clinical practice, this systematic review was performed to evaluate the diagnostic level of evidence of studies investigating these devices in patients with stroke or cerebral palsy (CP).
Methods
Search strategy
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [14].
We included mechanically driven robotic devices which use objective measurement outcomes (e.g., force, position, additional electromyography (EMG)) to estimate contributions of viscoelastic joint properties and/or spasticity of an extremity. Viscoelastic joint properties [15, 16] encompass a reduction in range of motion and changes in joint mechanical properties such as stiffness and damping and are assumed to be the result of muscle shortening (muscle contractures) or intrinsic changes in muscle tissue due to an increase in collagen fibers in the cellular joint matrix [1, 5, 17]. Spasticity is defined by Lance et al. (1980) [18] as "…a motor disorder, characterised by a velocity-dependent increase in tonic stretch reflexes (muscle tone) with exaggerated tendon jerks, resulting from hyper-excitability of the stretch reflex as one component of the upper motor neuron (UMN) syndrome" or by Sanger et al. (2003) [19] as "…hypertonia in which 1 or both of the following signs are present: 1) resistance to externally imposed movement increases with increasing speed of stretch and varies with the direction of joint movement, and/or 2) resistance to externally imposed movement rises rapidly above a threshold speed or joint angle". For this paper, we included studies that used either of these definitions.
We included English-language articles published between January 1946 and October 2020. The search strategy was developed in cooperation with a university librarian and comprised the following databases: Embase, Medline ALL, Web of Science Core Collection, Cochrane Central Register of Controlled Trials and Google Scholar. The following keywords were included: Stroke, Cerebral palsy, Diagnostic, Robotics, and Measurement.
In addition, we used CoCites to find additional articles [20]. In CoCites, we used the included paper, which had the highest number of citations (one paper for stroke patients and one for CP) from the above-mentioned search strategy as the primary papers (query articles). Then, CoCites finds articles based on their co-citation frequency to identify articles that address the same content as the query article. Next, CoCites organizes the identified articles based on the frequency of all citations that cite or are cited by the query articles.
Furthermore, a manual search was performed in Pubmed to retrieve missing papers because some papers used specific names for their measurement devices in their title/abstract that were not included in the results of the previously-mentioned search strategies. The complete search strategy and its outcomes can be found in Additional file 1.
Selection criteria
To be included, a study needed to meet both of two inclusion criteria: (i) involving human subjects with stroke or cerebral palsy; (ii) quantifying viscoelastic joint properties and/or spasticity of human extremities with a robotic device. As mentioned before, any method of identifying viscoelastic joint properties or spasticity was accepted, as long as it was measured with a mechanically driven device and the quantitative data was used for analysis. Systematic reviews and meta-analyses were excluded. Also, conference papers, unpublished articles, and studies that only used EMG as an outcome measurement without using force or position parameters were excluded.
Method of level classification
The model of Sackett and Haynes [13] describes four phases of diagnostic research with corresponding questions to help convert a clinical diagnostic question into the appropriate research design. In the current review, we adapted this model to identify five different research study designs (Levels 0-IV, see Table 1) corresponding to the phases identified by Sackett and Haynes.
Studies with less than ten participants are classified as Level 0, as these small sample sizes do not allow generalization. The cutoff point of ten participants was a choice of the authors. Studies that determine the range of values for viscoelastic joint properties or spasticity in patients or healthy controls are scored as Level I. Studies that compare patients and controls with descriptive statistics or with significance testing (e.g. t-test for group differences) are also scored as Level I. Studies that correlate the outcomes with a reference test (e.g., the MAS), compare outcomes between patients and controls, or analyze change over time (e.g., pre-intervention and post-intervention), are quantified as Level II. Level III studies determine the ability of the test to discriminate subjects with and without abnormal viscoelastic properties or spasticity, measured with a reference test, and report diagnostic accuracy with statistical values such as sensitivity or specificity. Note that the difference between Level II and III is that III analyses the ability to distinguish between subjects (either patients and controls or within a patient population), while II only compares the values of groups. Level IV studies evaluate the clinical consequences of using the diagnostic device (e.g. clinical added value) by evaluating the outcomes of the patients measured with the device and patients not measured with the device. This should establish whether using these tests in clinical care lead to better outcomes.
Data Extraction
All articles were reviewed for title/abstract, and the articles that met the inclusion criteria were also screened for full text. For data extraction and analysis purposes, we followed the Cochrane research methodology [21]. Two authors (MK, LV) with backgrounds in clinical rehabilitation medicine independently screened all articles for title/abstract and full-text using EndNote X9. The two reviewers discussed disagreements in scoring the included articles. When the reviewers still disagreed after a second evaluation, a third author (RS) decided. With the same screening process, the level of evidence (Level selection) was assigned to all included articles. From all included articles, we extracted the studied population, the joint measured by the robotic device, the clinical measurement used as a reference, and if a control group was present.
Since our goal was to describe the level of evidence of the available literature and not to describe the accuracy itself (e.g., the sensitivity and specificity), we did not apply a quality assessment of the included studies.
Results
Study selection
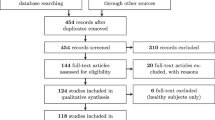
The initial search strategy in databases yielded 3584 articles. With CoCites, an additional 144 articles were included, and the manual search added an additional 16 articles. After the entire selection process, a total of 59 articles (1.6%) were included in the systematic review (Fig. 1).
Study characteristics
The characteristics of the included studies are shown in Additional file 2: Table S2, ordered by level of evidence. The total number of included patients was 1080; the mean sample size per study was 18 (± 18SD). In 42% of the studies, a control group was included. The total number of included controls was 504; the mean sample size per study was 9 (± 20SD). The majority of the studies measured adults with chronic stroke (68%); The other studies measured adults with subacute stroke (7%) and children with cerebral palsy (19%).
Most studies measured the upper extremities (64%); the ankle (30%), wrist (29%) and elbow (27%) were the most often measured joints. The most commonly-used reference test to quantify spasticity was the MAS (70%). For viscoelastic joint properties, all articles used the passive range of motion as a reference test. Many different robotic devices were used; the NeuroFlexor (15%) and the Wristalyzer (3%) were most commonly used. Both devices performed Level I and II.
The majority of the studies (51%) measured both the viscoelastic joint properties and spasticity. As outcomes, most studies quantified the torque–angle curve during movement (66%) and the passive range of motion (37%). A test–retest analysis to evaluate the reliability of the measurements was performed in 19% of the studies. It should, however, be noted that reliability may have been performed already in an earlier study, as was the case in some of the Neuroflexor studies (for example, see Andringa 2020 or Plantin 2019).
Level characteristics
Figure 2 shows the distribution of the different levels of evidence, together with the patient population (Fig. 2A), measured joints (Fig. 2B), reference tests (Fig. 2C) and control groups (Fig. 2D). The highest level was Level II (53% of the studies). Of the remaining studies, 30% were classified as Level 0, and 17% as Level I. None of the studies met the criteria for Level III or higher.
Distribution of the levels of evidence. The inner circles show the distribution in Levels. The donut around the pie in A illustrates the patient population that was measured, showing that most articles in all Levels measured adult stroke patients. The donut around the pie in B illustrates the measured joints, showing that most articles measured the shoulder in combination with the elbow in Level 0, the wrist or fingers in Level I and the ankle Level II. The donut around the pie in C illustrates the reference test, showing little variety in reference tests. The donut around the pie in D illustrates the number of articles that used control groups in certain levels. AS: Ashworth Scale, MAS: Modified Ashworth Scale, MTS: Modified Tardieu Scale, PROM: Passive Range of Motion, CP: Cerebral Palsy
Studies classified as Level II had the largest sample size, with a mean of 25 (± 22SD) and measured most of the included children with cerebral palsy (23%). In studies classified as Level 0 and Level I an upper limb joint (67% respectively 90%) was most frequently measured, in comparison with studies with Level II in which the ankle (45%) was the most commonly measured joint. Control groups were mostly included in studies classified as Level I and Level II. In the studies classified as Level II, 81% used a reference test, compared to 30% in Level I and 56% in Level 0 studies.
Discussion
This review shows that the diagnostic evidence needed for implementing robotic devices in clinical practice is lacking. We only found articles using research methods in diagnostic levels of evidence Level 0, I or II, for measuring viscoelastic joint properties and/or spasticity of patients with cerebral stroke, cerebral hemorrhage or cerebral palsy. None of the included studies tested their diagnostic robotic device for diagnostic accuracy (Level III) or added value (Level IV). To retrieve adequate evidence for the clinical implementation of diagnostic robotic devices, the higher levels of evidence need to be investigated.
One reason why the included studies were all classified as lower evidence levels could be that these are the first studies to perform in developing a new robotic device. Studies in Level 0, I or II designs have a role in initially showing the validity of the measurements. However, further studies are needed to determine the diagnostic accuracy of the device (Level III) or the added value (Level IV). Related to that, it was striking that the included studies mainly described newly developed robotic devices (81%) and only two devices (the NeuroFlexor [22] (Aggero MedTech AB, Älta, Sweden) and Wristalyzer [23]) were tested in multiple diagnostic levels. This may also reflect that many research groups in this field have a strong engineering background, focusing on developing hardware and software rather than on clinical testing. Translational research teams with both engineers and clinicians may be needed to overcome this problem.
A problem in clinical testing in this field is a lacking gold standard [4,5,6,7]. As stated in the introduction, clinical tools to measure viscoelastic joint properties and spasticity are subjected to the biases inherent to human perception. This is mainly a challenge for Level III study designs since these compare patients with and without the targeted disorder confirmed with a reference test. However, studying the impact of a diagnostic device (e.g. Level IV design) is possible without using a reference test, since it measures patient outcomes when using the device in clinical care by, for example, comparing patients measured with and without the device.
None of the included studies evaluated the impact of the diagnostic devices on clinical care (Level IV). An example of such a study, in this case comparing two different diagnostic workups for coronary artery disease, is the prospective diagnostic randomized clinical trial by M. Lubbers et al. [24] that compared the effectiveness and safety of a cardiac computed tomography (CT) algorithm with functional testing. An equivalent of such a study design for instrumented devices for measuring viscoelastic joint properties and spasticity could be a study where patients with a paretic limb with spasticity or abnormal viscoelastic properties seeking treatment are randomized to the diagnostic test or not. When the diagnostic test had added value then the test results could help the clinician to select the optimal treatment in individual patients, which should lead to better treatment outcomes than patients who were not tested.
While our aim was to describe the diagnostic level of evidence of the included studies, our review does provide an interesting overview of the characteristics of the robotic devices, joints most commonly studied and reference tests that were used. For example, we found that most studies measured a single joint; the majority of the articles measured the ankle or elbow. This might be explained by the fact that building a robotic device to measure one specific joint is less complex than a device to measure multiple joints [25]. Also, hinge joints such as the ankle or elbow joint are limited to two axes of movement and are less complex to measure than a multi-axial joint, such as the shoulder joint [26]. For clinical practice, this single-joint approach can be a problem since patients usually present with problems in different joints, which would currently require different robotic devices.
Study limitations
A possible limitation of this study is that the level of evidence classification we used was not tested or validated. We based it on literature [13] and adjusted it to the type of studies performed, as described in the Methods. Also, we focused on studies that used kinetic measurements (e.g. force and angle positions), to specifically measure the concept of spasticity or viscoelastic joint properties, and not the functional measurements (e.g. Fugl-Meyer assessment, Action Research Arm Test or Box and Block test). A limitation is also that we aimed to classify the literature based on the level of evidence of the study design to identify potentially lacking types of studies; in this review, we did not set out to review the psychometric properties of the instruments (e.g. test–retest reliability, validity, responsiveness, sensitivity, specificity) nor to score the quality of the included studies answering the questions these studies aimed to answer.
Conclusion/implications and recommendations for future research
This review shows a lack of diagnostic evidence to implement diagnostic robotic devices in clinical practice, since the highest level of evidence found was Level II. Therefore, this review emphasizes the importance of conducting further research with higher levels of diagnostic evidence to provide adequate evidence for reliable and valid implementation of the diagnostic robotic devices in clinical practice. To make a step towards implication in daily clinical care, existing devices with positive results in Level 0, I and II studies need to be extended in higher levels studies, which may require translational collaboration between clinicians, researchers, engineers and manufacturers to match the study methods with the diagnostic question optimally.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CP:
-
Cerebral palsy
- MAS:
-
Modified Ashworth Scale
- MTS:
-
Modified Tardieu Scale
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- EMG:
-
Electromyography
- UMN:
-
Upper Motor Neuron
- MK:
-
Maaike de Koff
- LV:
-
Levinia van der Velden
- RS:
-
Ruud Selles
- CT:
-
Computed tomography
References
van den Noort JC, Bar-On L, Aertbelien E, Bonikowski M, Braendvik SM, Brostrom EW, et al. European consensus on the concepts and measurement of the pathophysiological neuromuscular responses to passive muscle stretch. Eur J Neurol. 2017;24(7):981-e38.
Kwakkel G, Veerbeek JM, van Wegen EEH, Wolf SL. Constraint-induced movement therapy after stroke. The Lancet Neurology. 2015;14(2):224–34.
Taub E, Uswatte G, Mark V, Morris D. The learl1ed nonuse phenomenon: implications for rehabilitation. Eura Medicophys. 2006;42:241–55.
Meseguer-Henarejos AB, Sánchez-Meca J, López-Pina JA, Carles-Hernández R. Inter- and intra-rater reliability of the Modified Ashworth Scale: a systematic review and meta-analysis. Eur J Phys Rehabil Med. 2018;54(4):576–90.
Pandyan AD, Johnson GR, Price CI, Curless RH, Barnes MP, Rodgers H. A review of the properties and limitations of the Ashworth and modified Ashworth Scales as measures of spasticity. Clin Rehabil. 1999;13(5):373–83.
Fleuren JF, Voerman GE, Erren-Wolters CV, Snoek GJ, Rietman JS, Hermens HJ, et al. Stop using the Ashworth Scale for the assessment of spasticity. J Neurol Neurosurg Psychiatry. 2010;81(1):46–52.
Bar-On L, Aertbelien E, Molenaers G, Dan B, Desloovere K. Manually controlled instrumented spasticity assessments: a systematic review of psychometric properties. Dev Med Child Neurol. 2014;56(10):932–50.
Sloot LH, van der Krogt MM, de Groep KL, van Eesbeek S, de Groot J, Buizer AI, et al. The validity and reliability of modelled neural and tissue properties of the ankle muscles in children with cerebral palsy. Gait Posture. 2015;42(1):7–15.
Centen A, Lowrey CR, Scott SH, Yeh TT, Mochizuki G. KAPS (kinematic assessment of passive stretch): a tool to assess elbow flexor and extensor spasticity after stroke using a robotic exoskeleton. J Neuroeng Rehabil. 2017;14(1):59.
McPherson JG, Stienen AHA, Schmit BD, Dewald JPA. Biomechanical parameters of the elbow stretch reflex in chronic hemiparetic stroke. Exp Brain Res. 2019;237(1):121–35.
Firoozbakhsh KK, Kunkel CF, Scremin AM, Moneim MS. Isokinetic dynamometric technique for spasticity assessment. Am J Phys Med Rehabil. 1993;72(6):379–85.
Maggioni S, Melendez-Calderon A, van Asseldonk E, Klamroth-Marganska V, Lünenburger L, Riener R, et al. Robot-aided assessment of lower extremity functions: a review. J Neuroeng Rehabil. 2016;13(1):72.
Sackett DL, Haynes RB. The architecture of diagnostic research. BMJ. 2002;324(7336):539–41.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Hayes KC, Hatze H. Passive visco-elastic properties of the structures spanning the human elbow joint. Eur J Appl Physiol. 1977;37(4):265–74.
McFaull SR, Lamontagne M. In vivo measurement of the passive viscoelastic properties of the human knee joint. Hum Mov Sci. 1998;17(2):139–65.
Mathewson MA, Lieber RL. Pathophysiology of muscle contractures in cerebral palsy. Phys Med Rehabil Clin N Am. 2015;26(1):57–67.
Lance JW, Feldman RG, Young RR. Spasticity, disordered motor control. Chicago Year book Medical. 1980.
Sanger TD, Delgado MR, Gaebler-Spira D, Hallett M, Mink JW. Classification and definition of disorders causing hypertonia in childhood. Pediatrics. 2003;111(1):e89–97.
Janssens ACJW, Gwinn M, Brockman JE, Powell K, Goodman M. Novel citation-based search method for scientific literature: a validation study. BMC Med Res Methodol. 2020;20(1):25.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. New York: Wiley; 2019.
Plantin J, Pennati GV, Roca P, Baron JC, Laurencikas E, Weber K, et al. Quantitative assessment of hand spasticity after stroke: imaging correlates and impact on motor recovery. Front Neurol. 2019;10:836.
Grimaldi G, Lammertse P, Van Den Braber N, Meuleman J, Manto M, editors. A New Myohaptic Device to Assess Wrist Function in the Lab and in the Clinic – The Wristalyzer2008; Berlin, Heidelberg: Springer Berlin Heidelberg.
Lubbers M, Dedic A, Coenen A, Galema T, Akkerhuis J, Bruning T, et al. Calcium imaging and selective computed tomography angiography in comparison to functional testing for suspected coronary artery disease: the multicentre, randomized CRESCENT trial. Eur Heart J. 2016;37(15):1232–43.
Noda Y, Kimura T, Abiko S, Tsujita T, Sato D, Nenchev DN, editors. Development of a Hardware-in-the-Loop Simulator for Analyzing Motion of Multi-DoF Robots Without Modeling Complex Joint Parts. 2019 SICE International Symposium on Control Systems (SICE ISCS); 2019 7–9 March 2019.
Zhang L, Park H, Ren Y, editors. Shoulder, elbow and wrist stiffness in passive movement and their independent control in voluntary movement post stroke. 2009 IEEE International Conference on Rehabilitation Robotics; 2009 23–26 June 2009.
Acknowledgements
The authors wish to thank S.T.G Meertens from the Erasmus MC Medical Library for developing and updating the search strategies.
Funding
This collaboration project was supported by Rijndam Rehabilitation and a PPP Allowance made available by Health ~ Holland, Top Sector Life Sciences & Health, to stimulate public–private partnerships (Grant No: LSHM16030-H002).
Author information
Authors and Affiliations
Contributions
LV and MK performed the literature research, study selection, data extraction and data analysis. Both authors have written the manuscript. GR assisted in the study process and contributed writing the manuscript. RS was consulted when reviewers disagreed with articles to include. In addition, RS assisted in designing the study, and interpreted the data and contributed to writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Search string revision.
Additional file 2: Table S2.
Study characteristics.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
van der Velden, L.L., de Koff, M.A.C., Ribbers, G.M. et al. The diagnostic levels of evidence of instrumented devices for measuring viscoelastic joint properties and spasticity; a systematic review. J NeuroEngineering Rehabil 19, 16 (2022). https://doi.org/10.1186/s12984-022-00996-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12984-022-00996-7