Abstract
Background
Moringa oleifera Lam., an herb commonly consumed by HIV-infected people on antiretroviral therapy, inhibits cytochrome P450 3A4, 1A2 and 2D6 activity in vitro; and may alter the pharmacokinetics (PK) of antiretroviral drugs metabolized via the same pathways. However, in vitro drug interaction activity may not translate to a clinically significant effect. Therefore, the effect of moringa leaf powder on the PK of nevirapine in HIV-infected people was investigated.
Methods
Adult patients at steady-state dosing with nevirapine were admitted for 12-h intensive PK sampling following a 21-day herbal medicine washout. Blood sampling was repeated after 14 days of nevirapine and moringa (1.85 g leaf powder/day) co-administration. Nevirapine plasma concentrations were determined by liquid chromatography-tandem mass spectrometry. To assess the effect of moringa on nevirapine PK, the change in nevirapine area under the plasma concentration–time curve (AUC) was determined. The mean difference in pre- and post-moringa nevirapine, maximum concentration (Cmax) and concentration at 12 h (C12h) were also calculated. The PK parameters were compared by assessing the post/pre geometric mean ratios (GMRs) and associated 90% confidence intervals (CIs).
Results
Pharmacokinetics analyses were performed on the results from 11 participants for whom complete data were obtained. The post/pre GMRs and associated 90% CIs for nevirapine were 1.07 (1.00–1.14) for the AUC; 1.06 (0.98–1.16) for Cmax and 1.03 (0.92–1.16) for C12h.
Conclusion
Co-administration of Moringa oleifera Lam. leaf powder at the traditional dose did not significantly alter the steady-state PK of nevirapine.
Trial registration number NCT01410058 (ClinicalTrials.gov)
Similar content being viewed by others
Background
Concomitant use of herbs with drugs may result in herb-drug interactions through various pharmacokinetic and pharmacodynamic mechanisms [1, 2]. The risk of interaction increases with the number of co-administered agents. As a result, HIV patients in developing countries are at high risk of herb-drug interactions because of the widespread self-directed herbal medicine use; and the polypharmacy associated with HIV treatment [3].
A growing number of studies are being conducted to evaluate the pharmacokinetic effects of herbs on drugs when taken together. However, the majority of studies use preclinical in vitro and animal models, which generate data that often do not translate to a clinical effect [4, 5]. In addition, currently available data are skewed towards Chinese and Western herbs. Given the high prevalence of herbal medicine use and the rapid scaling up of treatment for HIV infection in developing countries, rigorous clinical studies are urgently required to assess the effects of commonly used herbal medicines on antiretroviral drugs. This will provide evidence to accurately guide herbal medicine use among HIV-infected people who choose to take herbs and drugs together.
In developing countries, the leaf powder of Moringa oleifera Lam. is commonly used as a medicinal herb, rather than food as is the case in Asian populations. It is often taken as a supplement by HIV-infected people to enhance immunity and manage opportunistic infections [6, 7]. In-vitro data suggest that moringa inhibits cytochrome P450 (CYP) 3A4, 1A2 and 2D6 activity which could potentially lead to metabolic interactions with antiretroviral drugs metabolized via the same pathways [5, 8, 9]. The non-nucleoside reverse transcriptase inhibitor nevirapine (200 mg twice daily) is widely used as a component of first line antiretroviral therapy in many developing countries. It is metabolized mainly by CYP3A4 and CYP2B6 and to a lesser extent CYP3A5, CYP2C9, and CYP2D6 [10, 11]. Previous studies have demonstrated that concomitant administration of fluconazole, a potent inhibitor of CYPs, results in markedly increased trough plasma nevirapine concentrations when compared to the administration of nevirapine alone [12]. Concomitant dosing with the herb St. John’s wort, a CYP3A4 and P-glycoprotein inducer, reduced exposure to nevirapine [13]. Such interactions with CYP inhibitors or inducers could potentially increase toxicity [11] or reduce efficacy, respectively. Prior to this study, it was not known whether the in vitro inhibition potential of Moringa oleifera Lam. is of clinical significance when co-administered with nevirapine. It was hypothesized that inhibition of CYP3A4 by M. oleifera leaf powder may result in a clinically significant increase in nevirapine exposure. This study was conducted to assess the effect of 14 days Moringa oleifera leaf powder supplementation on the steady-state pharmacokinetics of nevirapine in HIV-infected patients.
Methods
Study participants and setting
HIV-infected male and female adults reporting for routine HIV clinic visits at a referral hospital in Zimbabwe were identified through an interviewer-administered questionnaire. To be enrolled in the study, participants had to be >18 years old, on an nevirapine based regimen for at least 2 weeks and to have supplemented their antiretroviral therapy with herbal medicine of their own accord in the past. Exclusion criteria included anemia (hemoglobin <10 g/dL), impaired renal function (serum creatinine >300 µmol/L) or abnormal hepatic function (serum alanine transaminase >5 times the upper limit of normal) at enrolment, and current treatment with rifampicin, isoniazid or imidazoles. All concomitant medications were also evaluated on a case by case basis and current literature reviewed to exclude any drugs with potential to interact with nevirapine. In addition, female participants were required to have a negative pregnancy test and not to be breastfeeding.
Preparation and analysis of moringa
Fresh Moringa oleifera Lam. leaves positively identified by a botanist from the National Botanical Gardens were harvested from a rural district in Zimbabwe. A voucher specimen was retained and another deposited at the National Herbarium and Botanical Gardens of Zimbabwe (ref. GPS 13504-C). The leaves were then room dried and coarsely ground into powder using a kitchen blender according to the method published by the Moringa Association of Ghana (batch MO001 [14]. The dried leaf powder was assessed for microbial and heavy metal content and a chemical fingerprint of the methanol extract was generated by ultra-performance liquid chromatography (UPLC) coupled to a time of flight mass spectrometer (TOF MS ES+). The fingerprint was archived at the University of Zimbabwe College of Health Sciences.
Dosage regimen and standardization
In the authors’ experiences and findings from previous surveys, moringa leaf powder is consumed in a wide range of doses [6, 15]. The dose of moringa for the trial was planned at approximately two teaspoons (10 mL) once a day because it was within the reported range and could also be encapsulated into a reasonable number of capsules that would not be a barrier to adherence. This was equivalent to about 1.85 g of the dry leaf powder. To standardize the dose, each 1.85 g dose was weighed out and filled into hard gelatin capsules. This was equivalent to 4 size ‘000’ capsules. Each participant was supplied with enough capsules for the 2-week period.
Study design and treatments
An open label, two phase, one sequence, cross-over, pharmacokinetic study was conducted over 35 days. The study had a minimum 3-week herbal medication wash out period prior to the first dosing to reduce the possibility of carryover from any type of previously used moringa or other herbal medication, so the participants would have the same baseline. On the first visit (day one), all enrolled participants were instructed not to take any herbal supplements, including moringa. They were also counseled to continue taking their regular antiretroviral drug regimen supplied at the HIV outpatient clinic which included 200 mg nevirapine twice daily. On the second visit (day 22), serial whole blood (5 mL) samples and spot urine sample were collected for determination of baseline plasma nevirapine concentration and urinalysis, respectively. Pre-dose urine and blood samples were collected immediately before an observed nevirapine morning dose. Additional pharmacokinetic blood samples were also collected via cannula at 0.5, 1, 1.5, 2, 3, 4, 6, 8 and 12 h post nevirapine dose. Participants were given further counseling and instructions to continue taking the regular antiretroviral drug regimen supplied at the HIV outpatient clinic together with moringa supplied by the study staff. On the third visit (day 35), a second set of serial whole blood (5 mL) samples and a spot urine sample were collected for determination of post-moringa plasma nevirapine concentration and urinalysis. Pre-dose urine and blood samples were again collected immediately before an observed nevirapine morning dose, and additional pharmacokinetic blood samples collected via cannula at 0.5, 1, 1.5, 2, 3, 4, 6, 8 and 12 h post nevirapine dose. The observed dose of nevirapine was administered in the fed state on both visits. On blood sampling days, a standard diet was provided and participants were required to remain on-site over the course of both 12 h intensive sampling periods.
Documentation of adherence to medication and supplementation restrictions between visits was achieved through the use of a food/herb/medication diary. Exposure to moringa was based on self-reported adherence and assessed through personal interviews, pill counts and evaluation of the food/herb/medication diary.
Assessment of adverse events
All participants were monitored for adverse events throughout the study. The study medical officer documented any adverse events observed from clinical examinations or self-reported by the participants at each visit.
Sample preparation
Blood samples were collected into 10-mL tubes containing ethylenediaminetetraacetic acid (EDTA) for determination of nevirapine concentration. All blood specimens were centrifuged at 2800×g for 5 min at 4 °C. The plasma was harvested and stored at −80 °C until time of analysis.
Determination of nevirapine
Nevirapine concentrations were determined by LC–MS/MS in the laboratory of the Division of Clinical Pharmacology, University of Cape Town. The assay method has been reviewed by the AIDS Clinical Trials Group Quality Assurance and Quality Control program (ACTG CPQA). It was validated according to the United States Food and Drug Administration and European Medicines Agency guidelines using externally prepared proficiency testing samples supplied by the ACTG CPQA.
Plasma samples were extracted from 100 µL human plasma by protein precipitation using acetonitrile (Honeywell, Burdick & Jackson®). The extraction procedure was followed by liquid chromatography with MS/MS detection. Chromatographic separation was achieved on a Luna 5 μm PFP (2), 100 A, 50 mm × 2 mm analytical column column (Phenomenex, Torrance, CA, U.S.). An AB Sciex API 4000 mass spectrometer (SCIEX, Framingham, MA, U.S.) was operated at unit resolution in the multiple reaction monitoring (MRM) mode, monitoring the m/z 266.9 (MH+) → 198.2 transition for nevirapine, and the m/z 270.1 → 229.1 transition for the deuterated nevirapine internal standard. The calibration curve was fitted by quadratic regression (weighted by 1/concentration2) over the range 0.0195–20.0 µg/mL. The combined accuracy and precision statistics of the low, medium and high quality control samples during sample analysis were between 89.8 and 93.4%, and 4.2 and 9.1%, respectively.
Statistical and pharmacokinetic analysis
In designing the study, a difference in pre- and post-moringa nevirapine area under the plasma concentration–time curve from 0 to 12 h (AUC0–12h) of at least 20% was considered to be clinically relevant for the purpose of establishing sample size. An intra-individual coefficient of variation of 25% was assumed for nevirapine AUC based on previously published data [16]. With α = 0.05, it was determined that a total of 12 participants would provide 80% power in the case of an equivalence range 0.8–1.25 [17]. After making adjustments for loss to follow-up (10%) and the cross-over design, a sample size of 13 was targeted.
Statistical analyses were performed using StataMP® version 13 (StataCorp, College Station, TX). Demographic characteristics were summarized as means and standard deviations or frequencies. Nevirapine pharmacokinetic parameters [i.e. AUC0–12h, peak concentration (Cmax,ss) and concentration at 12 h post dose (C12h)] were calculated for each individual using a non-compartmental approach by means of the Phoenix WinNonlin software application (Version 6.0; Certara, Princeton, NJ). AUC was calculated using the linear trapezoidal method. The individual differences in the pharmacokinetic parameters were calculated on log transformed data. The confidence interval approach was used to assess the effect of moringa on the pharmacokinetics of nevirapine. To evaluate if pharmacokinetic changes could have clinical relevance, it is recommended to treat pharmacokinetic interactions as equivalence problems [18]. Absence of a moringa effect was assumed if the 90% confidence interval (CI) of the geometric mean ratio (GMR) of AUC0–12h, Cmax,ss and C12h with and without moringa were contained within the limits 80–125%. The significance of the GMR results were assessed by paired Student t test.
Post-moringa/pre-moringa geometric mean ratios (GMRs) and their confidence intervals were calculated as follows: GM[x] = 10^mean[log[x]]; GMR[y/x] = GM[y]/GM[x]. The 90% confidence interval (CI) of GMR[t2/t1] was calculated from the difference in the logs (DL) of the t2 and t1 values: DL[t2,t1] = log[t2]–log[t1], where t2 and t1 are corresponding values for the same subject post-moringa and pre-moringa, for 11 subjects. If m = mean[DL[t2,t1]], sd = standard deviation [DL[t2,t1]], and (a,b) = CI[m,sd,n], then the CI of GMR[t2/t1] = (10^a,10^b) and GMR[t2/t1] = 10^m. The confidence intervals of the DL values were also verified with that obtained by the statistical package.
Results
Participant enrolment
Thirty-three HIV-infected adult patients were screened. Thirteen participants met the eligibility criteria and were enrolled after giving oral and written informed consent. All participants completed the study. Based on pill counts and a review of entries in the food/herb/medication diaries, all participants were fully compliant with medication and supplementation restrictions. The baseline demographic characteristics are summarized in Table 1.
Adverse events
The combination of moringa with nevirapine was well tolerated. Participants were in good health upon physical examination at the post moringa visit. There were no serious adverse events and all participants completed the study. A total of two adverse events were reported by the participants; one complained of transient mild pain at the site of cannula insertion and the other, a headache. During the observation period, only grade 1 Common Terminology Criteria for Adverse Events (CTCAE) toxicities were observed in the clinical assessments. The incidence rate was 5% (n = 202 readings), and there were no significant differences in the number observed pre-moringa compared to post-moringa. The medical officer and consultant physician monitoring adverse events in the study decided these were not related to the moringa.
Pharmacokinetics
Pharmacokinetic analyses were performed on the results from 11 participants who had complete data. Of the two participants that were excluded, one had difficulties with venous access at points during visit 2. The other took the day 35 nevirapine dose at home instead of at the clinic such that only samples for the latter time points could be collected.
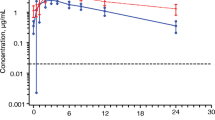
Figure 1 shows the mean plasma concentration–time profiles for nevirapine with and without Moringa oleifera leaf powder supplementation (Figs. 2, 3, 4).
Individual AUC 0–12 of nevirapine with and without moringa. All patients maintained therapeutic plasma concentrations (>3.0 µg/mL) 12 h post-dose [19]
Individual Cmax,ss of nevirapine with and without moringa. All patients maintained therapeutic plasma concentrations (>3.0 µg/mL) 12 h post-dose [19]
Individual C12 of nevirapine with and without moringa. All patients maintained therapeutic plasma concentrations (>3.0 µg/mL) 12 h post-dose [19]
The plasma pharmacokinetics parameters and comparisons for nevirapine are presented in Table 2.
Discussion
Based on previous in vitro studies that have noted significant inhibitory effects of moringa on the nevirapine-metabolizing CYP3A4 and CYP2D6 isoforms, the effect of Moringa oleifera leaf supplementation on nevirapine pharmacokinetics was investigated. While the nevirapine pharmacokinetic profiles from HIV-infected adults at the dosage of moringa used show an inhibitory trend, consistent with that observed in vitro [5, 8, 9], the change in steady-state nevirapine pharmacokinetic parameters is neither clinically nor statistically significant. The 90% confidence intervals for the nevirapine geometric mean ratios for AUC0–12h, Cmax,ss and C12h following concomitant administration of moringa over 14 days were within the 80–125% limit, indicating lack of a clinically significant interaction.
The findings are supported by observations from previous studies that demonstrated that the inhibitory effects of moringa on CYP3A4 and CYP2D6 are significantly less as compared to their positive control inhibitors ketoconazole and quinidine [20]. Data from other studies show that nevirapine induces its metabolism by both CYP3A4 and CYP2B6, which may compensate to some degree for CYP3A4 inhibition [21] Any inhibition of CYP3A4 may also have been compensated for by the alternative metabolic pathways (CYP2B6, CYP3A5, CYP2C9, and CYP2D6) reducing the resulting pharmacokinetic changes [22, 23]. This may not be the case for other drugs such as saquinavir, ritonavir, indinavir, darunavir and atazanavir whose main or sole route of elimination is via CYP3A4.
In general, moringa has a good safety profile consistent with its long history of use as food and medicine [24]. The combination of moringa and nevirapine in this study was also well tolerated since moringa did not alter the safety profile of nevirapine when co-administered. A minimum concentration of 3.0 µg/mL is recommended as a therapeutic cut-off for nevirapine trough concentrations [19]. All patients maintained nevirapine 12-h concentrations above 3.0 µg/mL with moringa supplementation. Because the study did not assess clinical outcomes, this observation cannot be discussed in terms viral load.
The toxicity of nevirapine as a function of concentration is not well established. Some studies could not find evidence of a concentration—toxicity relationship while others report an association between hepatotoxicity and higher nevirapine concentrations [25–28]. In this study, no nevirapine related adverse effects were reported despite the C12h and Cmax,ss values observed being mostly higher than the 6 µg/mL value previously associated with increased toxicity. The findings are consistent with Thai and Ugandan data that did not detect increased toxicity, despite increases in nevirapine Ctrough.
One limitation of the study is that for operational economy, a single sequence of moringa administration was used; hence, potential period effects cannot be ruled out.
Conclusions
We conclude that co-administration of moringa leaf powder at traditionally used doses has no clinically significant effect on the steady-state pharmacokinetics of nevirapine.
References
Meng Q, Liu K. Pharmacokinetic interactions between herbal medicines and prescribed drugs: focus on drug metabolic enzymes and transporters. Curr Drug Metab. 2014;15:791–807.
Cho HJ, Yoon IS. Pharmacokinetic interactions of herbs with cytochrome p450 and p-glycoprotein. Evid Based Complement Altern Med. 2015. doi:10.1155/2015/736431.
Müller AC, Kanfer I. Potential pharmacokinetic interactions between antiretrovirals and medicinal plants used as complementary and African traditional medicines. Biopharm Drug Dispos. 2011;32:458–70.
van den Bout-van den Beukel CJ, Koopmans PP, van der Ven AJ, De Smet PA, Burger DM. Possible drug-metabolism interactions of medicinal herbs with antiretroviral agents. Drug Metab Rev. 2006;38:477–514.
Monera TG, Wolfe AR, Maponga CC, Benet LZ, Guglielmo J. Moringa oleifera leaf extracts inhibit 6 beta-hydroxylation of testosterone by CYP3A4. J Infect in Dev Ctries. 2008;2:379–83.
Monera TG, Maponga CC. Prevalence and patterns of Moringa oleifera use among HIV positive patients in Zimbabwe: a cross-sectional survey. J Public Health Afr. 2012;3:22–4.
Popoola JO, Obembe OO. Local knowledge, use pattern and geographical distribution of Moringa oleifera Lam. (Moringaceae) in Nigeria. J Ethnopharmacol. 2013;150:682–91.
Taesotikul T, Navinpipatana V, Tassaneeyakul W. Selective inhibition of human cytochrome P450 1A2 by Moringa oleifera. Thai J Pharmacol. 2010;32:256–8.
Awortwe C, Bouic PJ, Masimirembwa CM, Rosenkranz B. Inhibition of major drug metabolizing CYPs by common herbal medicines used by HIV/AIDS patients in Africa—implications for herb-drug interactions. Drug Metab Lett. 2014;7:83–95.
Riska P, Lamson M, MacGregor T, Sabo J, Hattox S, Pav J, Keirns J. Disposition and biotransformation of the antiretroviral drug nevirapine in humans. Drug Metab Dispos. 1999;27:895–901.
Wen B, Chen Y, Fitch WL. Metabolic activation of nevirapine in human liver microsomes: dehydrogenation and inactivation of cytochrome P450 3A4. Drug Metab Dispos. 2009;37:1557–62.
Manosuthi W, Athichathanabadi C, Uttayamakul S, Phoorisri T, Sungkanuparph S. Plasma nevirapine levels, adverse events and efficacy of antiretroviral therapy among HIV-infected patients concurrently receiving nevirapine-based antiretroviral therapy and fluconazole. BMC Infect Dis. 2007;7:1–8.
de Maat MMR, Hoetelmans RMW, Mathôt RAA, van Gorp ECM, Meenhorst PL, Mulder JW, Beijnen JH. Drug interaction between St John’s wort and nevirapine. AIDS. 2001;15:420–1.
Sauveur A, Broin M. Growing and processing moringa leaves. Moringa and Plant Resources Network 2010; http://hdl.handle.net/10568/76865.
Monera-Penduka TG, Jani ZT, Maponga CC, Mudzengi J, Morse GD, Nhachi CFB. Quality and labeling information of Moringa oleifera products marketed for HIV-infected people in Zimbabwe. J Public Health Afr. 2016;7:84–8.
Byakika-Tusiime J, Chinn LW, Oyugi JH, Obua C, Bangsberg DR, Kroetz DL. Steady state bioequivalence of generic and innovator formulations of stavudine, lamivudine, and nevirapine in HIV-infected Ugandan adults. PLoS ONE. 2008. doi:10.1371/journal.pone.0003981.
Hauschke D, Steinijans VW, Diletti E, Burke M. Sample size determination for bioequivalence assessment using a multiplicative model. J Pharmacokin Biopharm. 1992;20:557–61.
Prueksaritanont T, Chu X, Gibson C, Cui D, Yee KL, Ballard J, Cabalu T, Hochman J. Drug-drug interaction studies: regulatory guidance and an industry perspective. AAPS J. 2013;15:629–45.
la Porte CJL, Back D, Blaschke T, Boucher CAB, Fletcher CV, Flexner C, et al. Updated guideline to perform therapeutic drug monitoring for antiretroviral agents. Rev Antivir Ther. 2006;3:4–14.
Ahmmed SKM, Mukherjee PK, Bahadur S, Kar A, Al-Dhabi NA, Duraipandiyan V. Inhibition potential of Moringa oleifera Lam. on drug metabolizing enzymes. Indian J Tradit Know. 2015;14:614–9.
Davit B, Reynolds K, Yuan R, Ajayi F, Conner D, Fadiran E, et al. FDA Evaluations using in vitro metabolism to predict and interpret in vivo metabolic drug-drug interactions: impact on labeling. J Clin Pharmacol. 1999;39:899–910.
Erickson DA, Mather G, Trager WF, Levy RH, Kearns JJ. Characterization of the in vitro biotransformation of the HIV-1 reverse transcriptase inhibitor nevirapine by human hepatic cytochromes P-450. Drug Metab Dispos. 1999;27:1488–95.
Isoherranen N, Lutz JD, Chung SP, Hachad H, Levy RH, Ragueneau-Majlessi I. Importance of multi-P450 inhibition in drug-drug interactions: evaluation of incidence, inhibition magnitude and prediction from in vitro data. Chem Res Toxicol. 2012;25:2285–300.
Stohs SJ, Hartman MJ. Review of the safety and efficacy of Moringa oleifera. Phytother Res. 2015;29:796–804.
Kappelhoff BS, van Leth F, Robinson PA, MacGregor TR, Baraldi E, Montella F, et al. Are adverse events of nevirapine and efavirenz related to plasma concentrations? Antivir Ther. 2005;10:489–98.
Wakeham K, Parkes-Ratanshi R, Watson V, Ggayi AB, Khoo S, Lalloo DG. Co-administration of fluconazole increases nevirapine concentrations in HIV-infected Ugandans. J Antimicrob Chemother. 2010;65:316–9.
Wang J, Kou H, Fu Q, Han Y, Qiu Z, Zuo L, et al. Nevirapine plasma concentrations are associated with virologic response and hepatotoxicity in Chinese patients with HIV infection. PLoS ONE. 2011. doi:10.1371/journal.pone.0026739.
Ratanasuwan W, Jariyasethpong T, Anekthananon T, Intalapaporn P, Kongpatanakul S, Pongnarin P, et al. Association of nevirapine levels with rash or hepatotoxicity among HIV-infected Thai women. Open AIDS J. 2012;6:266–73.
Authors’ contributions
TM was responsible for the conception of the study, its design, data collection, drafting of the manuscript and analysis of data. CM supervised the research process, assisted with interpretation of the data, revised the draft critically and gave final approval of the version to be published. GM and AW contributed to the design of the study, analyzed the data, assisted with the interpretation of the data and revised the draft critically. LW analyzed the data and revised the draft critically. CN assisted in the design of the study, supervised the work, contributed to the analysis of data, assisted with the interpretation of the data, revised the draft critically and gave final approval of the version to be published. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Emmanuel Chigutsa who provided insight that greatly assisted the research; as well as Alfred Tarumbwa, Tariro Chawana and Noleen Chifamba who assisted with the data collection.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used during the current study available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was registered prospectively with clinicaltrials.gov (NCT01410058). Study approval was obtained from the local and collaborating institutional ethics committees (JREC 130/10, PHP1220911A), as well as from the national ethics committee (MRCZ/B/255) and national drug regulatory authority (MCAZ REF CT0093/2011). Oral and written consent to participate was given before enrolment procedures.
Funding
This publication was made possible by grant numbers 2U2RTW007367, D43TW007991-01A2 and D43TW010313-01 from the Office of Global AIDS Coordinator (U. S. Department of State) and the Fogarty International Centre (National Institutes of Health, U. S. Department of Health and Human Services). The drug assays were also supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (UM1 AI068634, UM1 AI068636 and UM1AI106701, U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Mental Health (AI068632). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Monera-Penduka, T.G., Maponga, C.C., Wolfe, A.R. et al. Effect of Moringa oleifera Lam. leaf powder on the pharmacokinetics of nevirapine in HIV-infected adults: a one sequence cross-over study. AIDS Res Ther 14, 12 (2017). https://doi.org/10.1186/s12981-017-0140-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12981-017-0140-4