Abstract
Background
This study aims to investigate whether the neurometabolites of the anterior cingulate cortex (ACC) were distinct in patients with active and inactive juvenile idiopathic arthritis (JIA) using the proton magnetic resonance spectroscopy.
Methods
We measured the levels of total N-acetylaspartate (tNAA), choline (Cho), myo-inositol (ml), glutamate (Glu) and the complex of glutamate and glutamine (Glx) relative to total creatine (tCr) in ACC of each participant.
Results
Compared with the healthy controls, a significant decrease of total Cho/tCr and Glx/tCr ratio in ACC occurred in active and inactive JIA group. The tCho/Cr level was negatively associated with the serum level of ESR in active JIA patients. There was no difference in NAA/tCr ratio among the three groups, which may imply that no neuron and axonal losses occurred in either active or inactive JIA patients.
Conclusions
The abnormal neurometabolites in tCho/tCr and Glx/tCr in ACC may indicate that persistent dysfunction of glial cell, while neither neuron nor axonal losses occurred in active and inactive JIA patients.
Similar content being viewed by others
Introduction
Juvenile idiopathic arthritis (JIA) is defined as chronic arthritis remaining unknown etiology, which begins before 16 years old and persists for a minimum of 6 weeks [1]. It comprises seven distinct categories with heterogeneous conditions defined by the International League of Associations for Rheumatology (ILAR) as follows: oligoarticular (persistent or extended), polyarticular (RF-negative or RF-positive), systemic JIA, psoriatic arthritis, and enthesitis-related arthritis (ERA) with each differing in genetic susceptibility and severity of arthritis. Any arthritis that does not fit into these categories or corresponds to >1 subtype is considered undifferentiated [2].
Empirically, a number of children and adolescents with JIA may suffer from hypersensitivity to pain, fatigue, or depression referred to as the “sick behaviors”. These changes occur may result from that inflammatory-molecular messengers of damage and infection signal the central nervous system (CNS) through afferent nerves, sentinel cells in the circumventricular organs, and transport across the blood-brain barrier (BBB) [3]. However, the exact correlations between neural activities and inflammation in chronic conditions such as JIA were scarcely explored by imaging modality, especially in the rapidly developing and perhaps vulnerable central neural system (CNS) of children and adolescents. Although previous studies have indicated that many patients with JIA can achieve inactivity or remission of the inflammation owing to the currently advanced treatment strategy (e.g., biologic agent) [4], pain and cognitive disfunction were still significant concerns for JIA patient [5]. These findings imply that centralized pain sensitization and synaptic plasticity may occur in acute and chronic inflammatory conditions [6, 7].
Localized proton magnetic resonance spectroscopy (1H-MRS) is a noninvasive tool for measuring neurochemical information in vivo [8]. Using MRS to explore the abnormal neurometabolites may be helpful for understanding the specific neuropathology and metabolic dysfunctions in the brain of young JIA patients. The anterior cingulate cortex (ACC) is a part of the limbic system related to the affective and attentional process of affection, cognition, as well as pain and empathy, and can be divided into three subregions, including the subgenual, rostral, and dorsal ACC [9]. These subregions interacting with multiple cortical regions usually function as relay hubs by transmitting various input signals after evaluating requirements from other regions to guide adaptive behaviors. Since some metabolite levels in the ACC are reported altered in patients suffering from chronic pain (such as osteoarthritis, chronic low back pain, and inflammatory bowel disease) [10,11,12], there is a high probability that the ACC is involved in young patients with JIA.
In this study, we measured the natural fluctuation in the condition of peripheral inflammation that are featured as JIA to determine how inflammation affects the neurometobolites in ACC. The JIA cohort is ideal for addressing this issue because that the condition is archetypically inflammatory and chronic, with some suffers experiencing disease flares wherein both circulating inflammatory marker and symptoms become elevated [2]. Among a variety of MRS-detectable metabolites, we focused on the levels of total N-acetylaspartate (tNAA), glutamate (Glu), the complex of glutamate and glutamine (Glx), myo-inositol (mI), and total choline (tCho) relative to total creatine (tCr) including Cr and phosphocreatine (pCr) in the ACC. This study aimed to investigate the sensitive and specific neurochemical biomarkers and neuromatabolites associating with active and inactive JIA patients. Furthermore, we explored the respective relationship of abnormal levels of neurometabolites with peripheral serum levels of pro-inflammatory cytokines and clinical assessments on patients with JIA.
Methods
Participants
A total of 65 JIA patients were recruited from the Pediatric Clinic of The First Affiliated Hospital of Xiamen University, China, PR. We recruited thirty-eight drug-naïve patients who is newly diagnosed as JIA according to the ILAR classification. At the same time, twenty-seven inactive JIA patients were also included in this study. In addition, we were also informed of self-report complaints, medical imaging evidence, as well as the laboratory blood tests of both JIA groups. Our sample of children and adolescents with active JIA included following subtypes: 52.6% (n = 20) oligoarticular, 28.9% (n = 11) enthesitis-related arthritis, 10.5% (n = 4) polyarticular, 5.2% (n = 2) systemic arthritis, 5% (n = 2) undifferentiated arthritis, while 2.6% (n = 1) those with obvious immune system disorders should be excluded. Twenty-seven inactive JIA patients included the following subtypes: 32.1% (n = 9) oligoarticular, 35.7% (n = 10) enthesitis-related arthritis, 22.2% (n = 6) polyarticular, and 7.1% (n = 2) undifferentiated arthritis. The patient groups were compared with 21 age- and sex-matched healthy controls (HCs, 6 females/15males, age 8.95 ± 2.01 years old (mean ± SD)). The detailed demographic information was shown in Table 1.
Further exclusive criteria were as followed: 1) either younger than eight or older than sixteen years old; 2) left-handedness; 3) brain abnormality on routine MRI scans; 4) with history of brain surgery and 5) with significant physical or psychiatric conditions; or 6) with MRI contraindications. This study was carried out according to the declaration of Helsinki and approved by the ethics committee of The First Hospital Affiliated to Xiamen University. All participants gave written Informed consent before the neurological tests and MRI scanning.
Marker of inflammation, disease activity, and neuropsychological evaluation
Before MRI scanning, participants were assessed by a series of validated neuropsychological and clinical scales: 1) Juvenile Arthritis Disease Activity Score (JADAS-27) [13] and Childhood Health Assessment Questionnaire (C-HAQ) was chosen to assess the diseased severity and the life impact of the disability [14]. The JADAS-27 was used to evaluate the disease activity of certain JIA subgroups (oligoarticular JIA, polyarticular JIA and undifferentiated arthritis). Moreover, the Juvenile Spondyloarthritis Disease Activity Index (JSpADA) and the systemic Juvenile Arthritis Disease Activity Score (sJADAS) were used for enthesitis-related arthritis and systemic JIA, respectively [15]. Notably, the inactivity or remission of JIA was defined as JADAS ≤ 1 [15]. 2) The screen for child anxiety Related Emotional Disorders (SCARED) was selected to evaluate the emotional status of both children and adolescents with active and inactive JIA and healthy controls [16]. 3) The Visual Analogue Scale (VAS) was also selected to measure the intensity of the pain involving joint discomfort for JIA patients. Additionally, the venous blood of each JIA patient was drawn by a trained phlebotomist, and then the level of serum erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) immediately were immediately processed and measured.
MR Scanning Acquisition
All the subjects were scanned on a 3.0-T MR scanner (Siemens Tim Verio, Erlangen, Germany), and an 8-channel phased-array coil was used to provide an optimum signal-to-noise ratio for MRI images and spectroscopy data. Sagittal T1-weighted images were acquired using 3-dimensional magnetization prepared rapid acquisitions gradient echo (MP-RAGE) sequence [2150 ms, 1100 ms, 4.38 ms, and 1 (TR, inversion time (TI), TE, and signal average)], with in-plane resolution of 256 x 256, slice thickness of 1 mm, field of view of 256 mm, and 162 slices, this provided isotropic voxel resolution of 1 mm3. The sagittal images were used to generate axial and coronal images using a multiplanar reconstruction (MPR) routine provided by the manufacturer. These images had the same resolution as the sagittal images. Axial T2-weighted turbo spin-echo images were acquired [8000 ms, 107, and 2 (TR, effective TE, signal averages)] with turbo factor of 15, and using 256 x 256 image matrix and slices thickness of 5 mm.
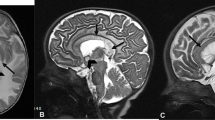
A single voxel point RESolved Spectroscopy (PRESS) sequence with and without water suppression pulse [2000 ms, 30 ms (TR, TE)], with voxel size of 3.0 cm (left-right), 2.0 cm (anterior-posterior), 1.0 cm (head-foot), 512 sampling points, and 2 kHz bandwidth, was used to acquire data from the ACC (Fig. 1) and for water-unsuppressed data, 5 signal averages were acquired [17].
1H-MRS Data processing
Spectral analysis was performed by using the linear combination model (LC model, version. 6.3-1J) software, a user-independent time-domain fitting routine that uses a basis set of concentration-calibrated model spectra of individual metabolites to estimate the relative concentrations of similar brain metabolites from in vivo spectral data. The metabolite content was normalized to that of tCr levels. As commanded by the LC model software, concentrations and ratios with Cramer-Rao lower bounds (CRLB) of more than 20% were not used for analysis. Of note, glutamine resonance was fit poorly with CRLB (> 20%) and hence were not used in the analysis. The final analysis included only those metabolites with a Cramer-Rao lower bound (CRLB) <20% [18].
Statistical analysis
Statistical analyses were performed using SPSS statistics 21.0 (SPSS, Chicago, IL). The sex difference between the groups was tested by Chi-Square analysis. The Kolmogorov-Smirnov test was applied to measure the normality of the quantitative data. Subsequently, the group differences in normally distributed data were assessed using a two-tailed student’s t-test; whereas a non-parametric test, the Mann-Whitney U test, was used for data that were not normally distributed. The group differences of the levels of neurometabolites among the three groups were analyzed using both parametric test (analysis of variance) and non-parametric test (Kruskal-Walls H-test). The Spearman correlation analysis was conducted between every two indices, which were derived from the significant difference of the neurometabolites, inflammation factors, clinical measurements, and psychological test score. The P values < 0.05 were considered statistically significant.
Results
Demographics
A total of sixty-five active and inactive JIA and twenty-one healthy control subjects completed the data collection. The demographic and clinical characteristics of the subjects are present in Table 1. Age, gender ratios were not significantly different between the two groups. The JADAS-27 scores (sJADAS and JSpADA for sJIA and ERA) for active and inactive JIA patients were 13.91 ± 6.22 (Mean ± SD) and 0, respectively. The C-HAQ and VAS scores of active JIA patients were significantly higher than that of inactive JIA patients (p <0.001).
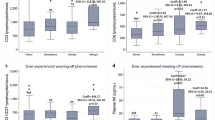
The levels of NAA/tCr, tCho/tCr, mI/tCr, Glu/tCr, Glx/tCr in active and inactive JIA patients and healthy controls were listed in Table 2 and Fig. 2. The level of tCho/tCr and Glx/tCr in active and inactive JIA patients were significantly lower than those in health controls (p <0.05). The NAA/tCr, Glu/tCr, and mI/tCr levels did not significantly differ from active JIA, inactive JIA and healthy controls. Neither tCho/tCr nor Glx/tCr levels for active and inactive JIA patients showed no significant difference. We also reported the non-parametric test p-levels in Supplementary Table 1.
There were negative correlations between tCho/tCr and peripheral levels of ESR in active JIA patients (r = -0.411, p = 0.010) (Fig. 3). There was no correlation between tCho/tCr levels and peripheral levels of ESR for inactive JIA patients. No correlation was found between Glx/tCr and peripheral serum levels of ESR and CRP in active and inactive JIA patients. JADAS (sJADAS and JSpADA for sJIA and ERA) and VAS scores for active and inactive JIA patients were not correlated with tCho/tCr (r = 0.051, p = 0.761; r = 0.103, p = 0.540, respectively) and Glx/tCr levels (r = -0.038, p = 0.821; r = -0.212, p = 0.202, respectively) in ACC.
Discussion
This study revealed the abnormality of neurometabolites in JIA patients, which may indirectly reflect the vulnerability of microstructural in the condition of chronic inflammation. Firstly, we observed the abnormality in tCho/tCr and Glx/tCr of ACC indicating the dysfunction of glial cells in both active and inactive JIA patients. whereas no significant difference was found between the two patient groups. Furthermore, the levels of tCho/tCr were negatively associated with peripheral levels of ESR in active JIA patients. Secondly, there was no significant difference in the level of NAA/tCr when respectively compared the active and inactive JIA patients with healthy controls, which might imply that no neuron and axonal losses occurred in either active or inactive JIA patients. To the best of our knowledge, this is the first study to demonstrate a decrease of tCho/tCr and Glx/tCr ratios in ACC of JIA patients by using 1H-MRS.
Our findings of the decreased tCho/tCr ratio in ACC of both active and inactive JIA indicated the occurrence of biochemical perturbation such as a decelerated turnover of the membrane. The Cho is a marker to detect the turnover of glial cell membrane in CNS and is also an essential precursor of acetylcholine and membrane lipids, phosphatidylcholine, and sphingomyelin [19,20,21,22]. The reduction of tCho/tCr levels in the ACC of JIA was consistent with previously reported alterations in the brain phospholipid metabolism, which may lead to altered lipid membrane turnover and neuroplasticity [23, 24]. The decreased tCho/tCr ratio also supported the impaired astrocytic functioning and altered neuroplasticity in JIA patients. Furthermore, increases in ESR and CRP together with other clinical assessments such as swollen joints could represent an important metric for measuring disease severity and benchmarks for the success of treatment [25,26,27]. In our study, the tCho/Cr level was negatively associated with peripheral levels of ESR, indicating that dysfunction of glial cells especially astrocyte cells occurred during acute inflammation in JIA. The tCho/Cr level showed no significant difference between active and inactive JIA patients, implying that dysfunction of glial cells in active inflammatory conditions may not be reversible, and glial cells may be vulnerable to insult of inflammatory damage during the developing CNS for children and adolescents, and this can persist in the inactive phase of JIA. These findings may explain why many inactive JIA patients have persistent pain and fatigue symptoms. It should be noted that the result of decreased tCho/tCr levels in JIA was not consistent with the previous studies on adult rheumatic autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus and systemic sclerosis [8]. The increase of Cho/Cr ratio was usually observed in different adult rheumatic autoimmune diseases, implying that glial reactivity occurred in response to inflammation and involvement of cerebral myelin [8]. The period of childhood and adolescence is considered as an important and yet vulnerable course of immense development that is distinct from adulthood, and therefore should not be directly compared with.
In current study, our main observations on glutamatergic neurometabolites are threefold: (1) no significant difference was found in Glu/tCr ratio level in ACC between active and inactive JIA patients and controls; (2) Glx/tCr ratio level was lower within the ACC in active and inactive JIA patients compared with controls, and no significant difference showed in active and inactive JIA patients; (3) no relationships were found between the Glx /tCr level of ACC any clinical variables in JIA patients. The glial cells such as astrocytes are key regulators of glutamine-glutamate homeostasis. A growing body of evidence shows impaired astrocytic functioning and abnormal glutamate/glutamine homeostasis in a series of CNS diseases such as major mood disorders and neurodegenerative disorders [28,29,30]. Recent evidence indicates that inflammatory mediators might regulate extracellular glutamatergic concentrations by exerting a profound effect on the functioning of glial cells including astrocytes, oligodendrocytes, and microglia that regulate glutamatergic levels under both physiological and pathological conditions [31]. The progression of glutamatergic dysfunction in chronic inflammation may result in atrophic changes of astrocytic and oligodendrocyte cells [28]. The decreased Glx/tCr ratio in our study may also indicate that dysfunction of glial cells in ACC for JIA patients.
Compared with healthy controls, we failed to detect the differences in the levels of NAA between JIA patients and healthy cohort. The NAA is synthesized in the mitochondria of neurons, then transported into neuronal cytoplasm and along axons [32]. The NAA is exclusively found in the peripheral and central nervous system and is detected in both gray matter and white matter. The rates of NAA synthesis provide an indication of the rate of glucose consumption by neurons, containing information on the level of neuron activity [8]. The NAA peaks were used as a marker of density, functionality, and neuronal integrity, and a decrease in concentration may reflect the loss of neurons or neuronal dysfunction. Accordingly, this negative finding was in good agreement with that no neuronal and axonal losses occurred in JIA patients.
Moreover, we also did not find any aberrances of ml in JIA. The mI is one of the most abundant metabolites in the brain, with the peak at 3.56 ppm on short TE 1H-MRS [33]. It is a sugar-like molecule that acts as an osmolyte and is involved in the various key biochemical process, such as signal transduction, phosphorylation of target protein, chromatin remodeling, and gene expression [33, 34]. The mI can be found mainly within astrocytes and cannot cross the blood-brain barrier, and the increase of mI/Cr level is thought to reflect glial proliferation or an increase in glial cell size [35]. The stable ml level in ACC might indicated that no gliosis and the increasement of glial cell size occurred in JIA.
This study has some limitations. Firstly, the sample in this study was relatively small. JIA unifies all forms of chronic childhood arthritis, and not all subtypes of JIA were included in this study. Secondly, we performed a cross-sectional study and only analyzed a single area of the brain, while other brain regions such as the insula and putamen may be included in the future. To address above-mentioned disadvantages, the further studies with a larger sample size and longitudinal follow-up are needed before a definitive conclusion can be drawn. Thirdly, although ESR and CRP are metrics of inflammatory activity, they could not show the same level of mechanistic specificity as pro-inflammatory cytokines, such as IL-6, TNF-α, and IL-1β, which should be also measured in the future work. Last but not the least, the sex effect should be also considered in the future study as there is evidence for sexual-specific differences in acute inflammatory paradigms.
Conclusion
In conclusion, our study revealed the abnormal neurometabolites including tCho/tCr and Glx/tCr of ACC in JIA patients, which indicated the persistent dysfunction of glial cell. However, no neuron and axonal losses occurred in active and inactive JIA patients.
Availability of data and materials
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
References
Parkken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. 2011;377(9783):2138–49.
Zaripova LN, Midgley A, Christmas SE, Bereford MW, Baildam EM, Oldershaw RA. Juvenile idiopathic arthritis: from aetiopathogenesis to therapeutic approaches. Periatric Rheumatology. 2021;19:135.
Schrepf A, Kaplan CM, Ichesco E, Larkin T, Harte SE, Harris RE, et al. A multi-modal MRI study of the central response to inflammation in rheumatoid arthritis. Nat commu. 2018;9(1):2243.
Rahman MM, Laila K, Rahman SA. Efficacy and safety of tofacitinib in the treatment of refractory cases of polyarticular course juvenile idiopathic arthritis: A study from Bangladesh. Int J Rheum Dis. 2022. https://doi.org/10.1111/1756-185X.14324 Online ahead of print.
Kwok CHT, Learoyd AE, Canet-Pons J, Trang T, Fitzgerald M. Spinal interleukin-6 contributes to central sensitisation and persistent pain hypersensitivity in a model of juvenile idiopathic arthritis. Brain Behav Immun. 2020;90:145–54.
Consolaro A, Ravelli A. Paediatric rheumatology: juvenile idiopathic arthritis-are biologic agents effective for pain? Nat Rev Rheumatol. 2013;9(8):447–8.
Cornelissen L, Donado C, Kim J, Chiel L, Zurakowski D, Logan DE, et al. Pain hypersensitivity in juvenile idiopathic arthritis: a quantitative sensory testing study. Pediat Rheumatol. 2014;12(1):39.
Fritoli RB, Pereira DR, Rittner L, Appenzeller S. Proton magnetic resonance spectroscopy (1H-MRS) in rheumatic autoimmune disease: A systematic review. Lupus. 2020;29(14):1873–84.
Stevens FL, Hurley RA, Taber KH. Anterior cingulate cortex: unique role in cognition and emotion. J Neuropsychiatry Clin Neurosci. 2011;23(2):121–5.
Reckziegel D, Raschke F, Cottam WJ, Auer DP. Cingulate GABA levels inversely correlate with the intensity of ongoing chronic knee osteoarthritis pain. Mol Pain. 2016;20(12):1744806916650690.
Kameda T, Fukui S, Tominaga R, Sekiguchi M, Iwashita N, Ito K, et al. Brain metabolite changes in the anterior cingulate cortex of chronic low back pain patients and correlations between metabolites and psychological state. Clin J Pain. 2018;34(7):657–63.
Kong N, Gao C, Xu MS, Gao XN. Changes in the anterior cingulate cortex in Crohn’s disease: A neuroimaging perspective. Brain Behav. 2021;11(3):e02003.
McErlane F, Bereford MW, Baildam EM, Thomson W, Hyrich KL. Recent development in disease activity indices and outcome measures for juvenile idiopathic arthritis. Rheumatology (Oxford). 2013;52(11):1941–51.
Ruperto N, Ravelli A, Pistorio A, Malattia C, Cavuto S, Gado-West L, et al. Paediatric Rheumatology International Trials Organisation. Cross-culture adaptation and psychometric evaluation of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ) in 32 countries. Review of the general methodology. Clin Exp Rheumatol. 2001;19(4 Suppl 23):S1–9.
Xu D, Zhang Y, Zhang ZY, Tang XM. Association between high mobility group box 1 protein and juvenile idiopathic arthritis: a prospective longitudinal study. Pediatr Rheumatol Online J. 2021;19(1):112.
Fair DC, Rodriguez M, Knight AM, Rubinstein TB. Depression and anxiety in patients with juvenile idiopathic arthritis: current insights and impact on quality of life. A systematic review Open Access Rheumatol. 2019;11:237–52.
Nerurkar L, Siebert S, McInnes IB, Cavanagh J. Rheumatoid arthritis and depression: an inflammatory perspective. Lancet. Psychiatry. 2019;6(2):164–73.
Plitman E, de la Fuente-Sandoval C, Reyes-Madrigal F, Chavez S, Gómez-Cruz G, León-Ortiz P. Elevated myo-inositol, choline, and glutamate levels in the associative striatum of antipsychotic-naive patients with first-episode psychosis: a proton magnetic resonance spectroscopy study with implication for glial dysfunction. Schizophr Bull. 2016;42(2):415–24.
Kusumakar V, MacMaster FP, Gates L, Sparkes SJ, Khan SC. Left medial temporal cytosolic choline in early onset depression. Can J Psychiatry. 2001;46:959–64.
Hong ST, Chol CB, Park C, Moon HY, Song KS, Cheong C, et al. Specific hippocampal choline decrease in an animal model of depression. Br J Radiol. 2009;82(979):549–53.
Kahl KG, Atalay S, Maudsley AA, Sheriff S, Cummings A, Frieling H, et al. Altered neurometabolism in major depressive disorder: a whole brain 1H-magneitc resonance spectroscopic imaging study at 3T. Prog Neuropsychopharmacol Biol Psychiatry. 2020;13(101):109916.
Kusumakar V, MacMaster FP, Gates L, Sparkes SJ, Khan SC. Left medial temporal cytosolic choline in early onset depression. Can J Psychiatry. 2001;46(10):959–64.
Henigsberg N, Savić A, Radoš M, Radoš M, Šarac H, Šečić A, et al. Choline elevation in amygdala region at recovery indicates longer survival without depressive episode: a magnetic resonance spectroscopy study. Psychopharmacology (Berl). 2021;238(5):1303–14.
Lener MS, Iosifescu DV. In pursuit of neuroimaging biomarkers to guide treatment selection in major depressive disorder: a review of the literature. Ann N Y Acad Sci. 2015;1344:50–65.
Rezaei E, Hogan D, Trost B, Kusalik AJ, Boine G, Cabral DA, et al. Associations of clinical and inflamatory biomarker clusters with juvenile idiopathic arthritis categories. Rheumatology. 2020;59(5):1066–75.
Süß P, Rothe T, Hoffmann A, Schlachetzki JCM, Winkle J. The joint-brain axis: insights from rheumatoid arthritis on the crosstalk between chronic peripheral inflammation and the brain. Front Immun. 2020;11:612104.
Fritz M, Klawonn AM, Zhao QY, Sullivan EV, Zahr NM. Structural and biochemical imaging reveals systemic LPS-induced changes in the rat brain. J Neuroimmunol. 2020;348(15):577367.
Harnoon E, Miller AH, Sanacora G. Inflammation, glutamate, and glia: a trio of trouble in mood disorders. Neuropsychopharmacology. 2017;42(1):193–215.
Blanco-Su´arez E, Caldwell AL, Allen NJ. Role of astrocyte-synapse interactions in CNS disorders. J Physiol. 2017;595(6):1903–16.
Kwon HS, Koh SH. Neuroinfammation in neurodegenerative disorders: the role of microglia and astrocytes. Transl Neurodegener. 2020;9(1):42.
Colombo E, Farina C. Atrocyte: key regulators of neuroinflammation. Trends Immunol. 2016;37(9):608–20.
Bertholdo D, Watcharakorn A, Castillo M. Brain proton magnetic resonance spectroscopy: introduction and overview. Neuroimag Clin. 2013;23:359–80.
Nagashima H, Sasayama T, Tanaka K, Kyotani K, Sato N, Maeyama M, et al. Myo-inositol concentration in MR spectroscopy for differentiating high grade glioma from primary central nervous system lymphoma. J Neurooncol. 2018;136(2):317–26.
Jupp B, Sawiak SJ, Ven der Veen B, Lemstra S, Toschi C, Barlow RL, et al. Diminished myoinositol in ventromedial perfrontal cortex modulates the endophenotype of impulsivity. Cereb Cortex. 2020;30(5):3392–402.
Bernabeu A, Alfaro A, García M, Fernández E. Proton magnetic resonance spectroscopy (1H-MRS) reveals the presence of elevated myo-inositol in the occipital cortex of blind subjects. Neuroimage. 2009;47(4):1172–6.
Acknowledgements
The authors gratefully acknowledge the altruism of all participants and contributors for this research.
Funding
This work was supported by the grant from the Clinical Transformation and Incubation Foundation of the First Affiliated Hospital of Xiamen University (XFY2020001).
Author information
Authors and Affiliations
Contributions
All individuals listed as authors are qualified for authorship according to the ICMJE criteria, and all of them have read and approved the final manuscript. H.H and J.X. were involved in the literature review, neuroimaging data analysis, and manuscript writing. Y.W. was in charge of the clinical and laboratory data analysis, the literature review, and the modification of the manuscript. H.L. edited the manuscript. C.H. and C.Y performed clinical data analysis and aided in writing the manuscript. H.W. designed the study and assisted in writing the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study complies with the Declaration of Helsinki, and that the ethics committee of The First Affiliated Hospital of Xiamen University has approved the research protocol (No. XMYY-2021KYSB011). The written informed consent has been obtained from all the participants.
Consent for publication
The authors assign all rights and privileges and give consent for publication of this work to Pediatric Rheumatology.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Han, H., Xiao, J.H., Weng, Y. et al. Evidence of persistent glial cell dysfunction in the anterior cingulate cortex of juvenile idiopathic arthritis children: a proton MRS study. Pediatr Rheumatol 20, 53 (2022). https://doi.org/10.1186/s12969-022-00711-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12969-022-00711-9