Abstract
Background
Previous work indicates that dilatation of the pulmonary artery (PA) itself or in relation to the ascending aorta (PA:Ao ratio) predicts pulmonary hypertension (PH). Whether these results also apply for heart failure with preserved ejection fraction (HFpEF) is unknown.
In the present study we evaluated the diagnostic and prognostic power of PA diameter and PA:Ao ratio on top of right ventricular (RV) size, function, and septomarginal trabeculation (SMT) thickness by cardiovascular magnetic resonance (CMR) in HFpEF.
Methods and Results
159 consecutive HFpEF patients were prospectively enrolled. Of these, 111 underwent CMR and invasive hemodynamic evaluation.
By invasive assessment 64 % of patients suffered from moderate/severe PH (mean pulmonary artery pressure (mPAP) ≥30 mmHg). Significant differences between groups with and without moderate/severe PH were observed with respect to PA diameter (30.9 ± 5.1 mm versus 26 ± 5.1 mm, p < 0.001), PA:Ao ratio (0.93 ± 0.16 versus 0.78 ± 0.14, p < 0.001), and SMT diameter (4.6 ± 1.5 mm versus 3.8 ± 1.2 mm; p = 0.008). The strongest correlation with mPAP was found for PA:Ao ratio (r = 0.421, p < 0.001). By ROC analysis the best cut-off for the detection of moderate/severe PH was found for a PA:Ao ratio of 0.83.
Patients were followed for 22.0 ± 14.9 months. By Kaplan Meier analysis event-free survival was significantly worse in patients with a PA:Ao ratio ≥0.83 (log rank, p = 0.004). By multivariable Cox-regression analysis PA:Ao ratio was independently associated with event-free survival (p = 0.003).
Conclusion
PA:Ao ratio is an easily measureable noninvasive indicator for the presence and severity of PH in HFpEF, and it is related with outcome.
Similar content being viewed by others
Background
About 50 % of patients presenting with symptoms of heart failure are diagnosed with heart failure with preserved ejection fraction (HFpEF). HFpEF is characterized by impaired diastolic function due to abnormal relaxation of the left ventricle (LV) as well as increased chamber stiffness [1]. The pathophysiology underlying HFpEF is still incompletely understood. Arterial hypertension, coronary artery disease and diabetes mellitus seem to play an important role [2]. The development of post-capillary pulmonary hypertension (PH) is a common feature of HFpEF and associated with substantial morbidity and mortality, frequent hospitalizations and an impaired quality of life [3–6].
Accurate assessment of PH requires invasive measurement of pulmonary artery (PA) pressure and pulmonary arterial wedge pressure (PAWP) [7]. Because of the invasive nature of this procedure the majority of HFpEF patients today are not evaluated by right heart catheter (RHC). Thus, diagnosis of HFpEF-PH mostly relies upon echocardiography. The estimation of PA pressure by echocardiography is a routine measurement, however, the accuracy of this method is limited [8] and the echocardiographic estimation of pulmonary vascular resistance is unreliable [9]. Therefore, an alternative straightforward non-invasive technique in addition to echocardiography is desirable to predict the likelihood of PH in HFpEF patients.
Cardiovascular magnetic resonance (CMR) is the current gold-standard technique to assess right heart dimensions, function, wall thickness, as well as the dimensions of the great arteries [10]. Previous work shows that dilatation of the pulmonary artery (PA) itself or in relation to the diameter of the ascending aorta (PA:Ao ratio) predicts PH [11–15]. Furthermore, right ventricular septomarginal trabeculation (SMT) mass has been identified as a CMR-derived marker of PH [16].
The present study was intended to determine the usefulness and prognostic significance of easily measureable variables such as PA diameter and PA:Ao ratio on top of CMR derived right ventricular (RV) dimension, ejection fraction, SMT dimensions, and RV free wall thickness (RVFW) in a prospective, well defined HFpEF cohort.
Methods
Study design
This was a prospective observational study performed at the Medical University of Vienna. All participants gave written informed consent. The institutional review board approved the study protocol (EK #796/2010).
Patients
Consecutive patients with suspected HFpEF-PH were invited to participate. HFpEF was diagnosed in the presence of: (1) symptoms or signs of heart failure; (2) normal or mildly reduced left ventricular (LV) systolic function (LV ejection fraction (EF) >50 %); (3) evidence of abnormal LV relaxation or diastolic stiffness (E/e’ > 8 by echocardiography; and (4) NT-proBNP serum levels ≥220 pg/mL [17]. Reasons for exclusion included pacemaker or other conditions precluding patients from CMR, regional wall motion abnormalities, significant coronary artery disease, significant valvular or congenital heart disease, or a glomerular filtration rate (GFR) below 30 mL/min/1.73m2. All patients underwent ventilation-perfusion scans and lung function testing including diffusing capacity of the lung for carbon monoxide to rule out chronic thromboembolic PH and PH due to significant lung disease.
Right and left heart catheterization
PAWP, mean pulmonary artery pressure (mPAP), and cardiac output (CO) were determined. CO was measured by both thermodilution and Fick method. Simultaneously, all patients underwent direct assessment of LV filling pressures, followed by coronary angiography. Derived hemodynamic parameters were calculated according to standard formulae [18]. PH was diagnosed according to recent guidelines as mPAP ≥ 25 mmHg at rest [19]. PH was classified as moderate or severe when the mPAP was ≥30 mmHg. The transpulmonary pressure gradient (TPG) was defined as difference between mPAP and PAWP and the diastolic pressure gradient (DPG) as difference between diastolic PA pressure and PAWP.
Cardiovascular magnetic resonance
All CMR studies were performed on a 1.5-T scanner (Avanto, Siemens Medical Solutions, Erlangen, Germany) within 30 days of RHC (mean 14.8 ± 9.0 days). Studies consisted of functional and Late Gadolinium Enhancement (LGE) imaging, according to standard protocols [20]. For cine imaging, steady state free precision (SSFP) images were used (repetition time 3.2 ms, echo time 1.2 ms, flip angle 64°, voxel size 1.4 × 1.4 × 6 mm, matrix 180×256). For LGE imaging of the left ventricle, segmented inversion recovery sequences (repetition time: 700 ms, echo time 1.22 ms, flip angle 50°, voxel size 1.4 × 1.4 ×. 8 mm, matrix 146×256) were acquired 10 min after injection of 0.1 mmol/kg gadolinium-DTPA (Gadovist®; Bayer Vital GmbH, Leverkusen, Germany).
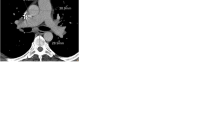
PA and Ao diameters were measured from inner-edge to inner-edge in axial half-Fourier single-shot turbo spin-echo (HASTE) black blood images (repetition time 810 ms, echo time 43 ms, flip angle 160°, voxel size 1.6 × 1.6 × 5 mm, matrix 192×256) at the level of the PA bifurcation (Fig. 1).
For SMT measurements, cine images were analyzed. The largest area and the longest medio-lateral diameter of the septal insertion part of SMT as identified in the second or third basal slice of the short axis cine stack were measured (Fig. 2). The RVFW was measured in end-diastole in the same short axis image used for SMT quantification.
All CMR studies were read by two independent observers blinded to clinical data (GK, SP and JB).
Outcome measures
Patients were prospectively followed by ambulatory visits and telephone calls at 6-month intervals. The main outcome measure was a combined endpoint consisting of hospitalization for heart failure or death from cardiac causes. Endpoints were ascertained by follow-up visits and phone calls.
Statistical analysis
Categorical data are presented as total numbers or percent, and continuous variables as mean ± standard deviation. Chi square test or Fisher´s exact test were applied for categorical variables and the Wilcoxon two-sample test for continuous variables. Kaplan-Meier estimates were used to calculate cardiac event rates. Differences between Kaplan-Meier curves were analyzed using the log rank test.
Binary logistic regression analysis was used to identify variables associated with moderate/severe PH (mPAP ≥30 mmHg). In addition, a univariable Cox regression analysis was used to identify parameters (SMT diameter, SMT area, RVFW, PA size and PA:Ao ratio) associated with event-free survival. In a second step these parameters were adjusted for potential cofounding clinical variables such as age, sex, atrial fibrillation, arterial hypertension, diabetes, chronic obstructive pulmonary disease, and coronary artery disease. All variables with a p-value <0.05 in the univariable model were entered into a multivariable model using a stepwise approach. Hazard and odds ratios for the PA:Ao ratio were reported as PA diameter divided by Ao diameter times 100. Receiver Operating Characteristic (ROC) curves were used to determine cut-off values for the detection of moderate/severe PH. Interobserver variability was described by using Intra Class Correlation Coefficients (ICCs). Statistical analyses were performed with SPSS Statistics version 18 (IBM, Armonk, New York). Results were considered statistically significant if p-values were <0.05.
Results
Baseline characteristics
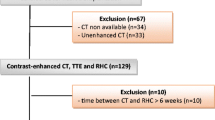
Between 2010 and 2013 159 consecutive patients were enrolled. Of these, 48 had to be excluded from the study because CMR was not possible due to pacemakers, GFR <30 mL/min/ 1.73 m2, or claustrophobia. Of the remaining 111 patients (32.4 % male, 70 ± 9 years old) who underwent CMR, 64 % suffered from moderate or severe PH (mPAP ≥ 30 mmHg by RHC). Clinical characteristics, hemodynamic measurements, and CMR parameters of registered patients are summarized in Table 1.
With respect to baseline clinical parameters, patients with moderate/severe PH more frequently presented with diabetes (50.0 % versus 23.1 %, p = 0.006), non-significant coronary artery disease/previous revascularization (27.1 % versus 5.1 %, p = 0.005); shorter 6-min walk distances (301 ± 110 m versus 397 ± 104 m, p < 0.001) and had more dilated left atria (LA; 66.7 ± 9.3 mm versus 62.9 ± 8.7 mm, p = 0.035 for diameters and 33.5 ± 10.2 cm2 versus 28.3 ± 6.8 cm2, p = 0.005 for areas).
Invasive assessment showed significant differences between patients with and without moderate/severe PH with respect to all measured variables except for CO, which was similar in both groups (5.2 ± 1.2 versus 5.2 ± 1.5 l/min, p = 0.829).
60 (54 %) patients had a TPG >12 mmHg (formerly classified as “out-of-proportion PH”) and 13 (12 %) had a DPG ≥7 mmHg, indicating combined pre- and postcapillary pulmonary hypertension [21].
Patients with a TPG >12 mmHg had a significantly higher PA:Ao ratio as compared to patients with a TPG ≤12 mmHg (0.9 ± 0.2 versus 0.8 ± 0.2, p = 0.001) whereas PA:Ao ratios did not differ between patients with a DPG of ≥ and <7 mmHg (0.9 ± 0.2 versus 0.9 ± 0.2, p = 0.230). However, this lack in statistical significance may be due to the small proportion of patients with DPGs ≥7 mmHg (12 % of the study cohort).
LGE was present in 31 % (n = 34) of our patients and involved 8.8 ± 4.3 % of left ventricular myocardial mass. In the majority (n = 22), LGE was present at the insertion point of the right ventricle; 8 patients had midmyocardial and 4 subendocardial enhancement. There were no significant differences in LGE pattern or degree between groups with and without moderate/severe PH.
Dimensions of the great arteries and indices of right ventricular hypertrophy for the prediction of moderate/severe pulmonary hypertension
Dimensions of the great arteries and indices of RV hypertrophy by CMR are displayed in Table 2. Patients with moderate/severe PH showed significantly larger PA diameters than the comparator (30.9 ± 5.1 mm versus 26 ± 5.1 mm, p < 0.001) and also had significantly higher PA:Ao ratios (0.93 ± 0.2 versus 0.79 ± 0.1, p < 0.001). SMT diameters (4.6 ± 1.2 mm versus 3.8 ± 1.2 mm, p = 0.008) were larger in patients with moderate/severe PH, while areas did not differ between groups (p = 0.136). RVFW showed a wide range from 2.0 to 7.2 mm (4.2 ± 1.1 mm) and did also not differ between patients with and without moderate/severe PH (p = 0.183). PA:Ao ratio, PA diameter and SMT diameter were significantly correlated with mPAP (r = 0.421, p < 0.001; r = 0.401, p < 0.001; r = 0.267, p = 0.005; respectively). Fig. 3 displays the correlation of PA:Ao ratio with mPAP.
Table 3 shows the binary logistic regression model for predicting the presence of moderate/severe PH. By univariable analysis, SMT diameter (p = 0.010), PA diameter (p < 0.001), and PA:Ao ratio (p < 0.001) were associated with moderate/severe PH. However, by multivariable analysis only PA:Ao ratio remained independently associated with the presence of moderate/severe PH.
By ROC analysis, a PA:Ao ratio of 0.83 was found to best detect mPAP ≥30 mmHg with a sensitivity of 73.2 % and a specificity of 67.5 %, and an area under the curve (AUC) of 0.759 (Fig. 4).
Measurements of PA:Ao ratio and PA diameter showed excellent inter-observer agreement with an Intra Class Correlation Coefficient (ICC) of 0.815 and 0.905 while ICCs for SMT diameters were only 0.563, and for RVFW 0.581.
Cardiac outcomes
Patients were followed for 22.0 ± 14.9 months. During follow-up 40 cardiac events (34 hospitalizations for heart failure, 6 deaths from cardiovascular causes) occurred.
Table 4 shows the results of the multivariable Cox-regression model. By univariable analysis, SMT diameter (p = 0.023), RVFW (p = 0.034), PA diameter (p = 0.002), and PA:Ao ratio (p = 0.003) were significantly associated with cardiovascular events. After adjustment for age, sex, atrial fibrillation, diabetes, chronic obstructive pulmonary disease and coronary artery disease, PA diameter (p = 0.003), and PA:Ao ratio (p = 0.002) remained significant predictors of outcome. However, after multivariable adjustment, only PA:Ao ratio remained independently associated with event-free survival.
By Kaplan Meier analysis (Fig. 5), event-free survival was significantly worse in patients with a PA:Ao ratio ≥0.83 (log rank, p = 0.004).
Discussion
The present study demonstrates that the PA:Ao ratio can be used as a simple and straight-forward indicator of PH associated with HFpEF. Moreover, the PA:Ao ratio is of prognostic value with respect to cardiac events.
In patients suffering from HFpEF right heart variables are of particular interest as they play an important role in terms of prognosis [5, 22–27]. Echocardiography is the first, and often only imaging modality used in HFpEF patients due to its wide availability. However, the RV is not easy to examine by echo and considerable inter- and intraobserver variability exists [9]. Furthermore, estimation of pulmonary hemodynamics may be inaccurate [8]. The definite diagnosis of PH demands invasive RHC to directly measure PA and wedge pressure, as well as trans-pulmonary and diastolic pulmonary pressure gradients. Although considered reasonably safe in experienced centers, RHC still carries a risk of morbidity and mortality [28]. Given the close link between pressures in the pulmonary circulation and RV dimensions and performance, several right heart imaging parameters have been shown to indicate the presence of PH [10, 13, 16, 26, 29–35]. CMR has been established as the gold-standard technique for the assessment of the right heart [10, 36]. It has the advantage that in addition to accurate and reproducible evaluation of heart size, function, and myocardial tissue characterization, CMR allows the assessment of the dimensions of the great arteries.
Indices of right ventricular hypertrophy for the prediction of pulmonary hypertension
RV mass and RV mass to LV mass ratio (VMI) have been shown to correlate reasonably well with mPAP in previous studies [33, 34], but the estimation of RV mass from CMR images is time-consuming and limited by significant inter- and intra-observer variability [16, 32]. Furthermore, it requires dedicated software for off-line analysis.
RVFW thickness is much more easy to assess than RV mass or VMI. In a historical series this parameter appeared useful as surrogate marker of PH [30] and was also shown to be of prognostic value [31]. However, due to the hypertrabeculated nature of the RV considerable rater-dependent variation exists for this measurement, which significantly limits its usefulness [30]. In the present series, RVFW measurements showed weak reproducibility and did not differentiate between patients with and without moderate/severe PH.
The SMT is a prominent muscle band that originates from the basal ventricular septum and runs apical-laterally towards the RV free wall [37]. A recent retrospective CMR study on a small number of patients (n = 49) found that SMT mass could be a useful marker for the identification of PH [16]. However, in the present study SMT was hard to delineate in many patients, leading to significant rater-dependent variation. Its diagnostic power for the identification of patients with moderate/severe PH was clearly inferior as compared to PA:Ao ratio and PA diameter.
Dimensions of the great arteries for the prediction of pulmonary hypertension
In contrast to the aforementioned parameters (RV myocardial mass, RVFW, SMT), the size of PA and Ao are easy to measure. Such measurements do not require dedicated software or advanced expertise. In the present study PA:Ao ratio was identified as the most potent predictor for the presence of moderate/severe PH. It correlated better with mPAP than PA diameter or indices of RV hypertrophy and showed excellent reproducibility. In addition, PA:Ao ratio was independently associated with event-free survival. From a clinical perspective the differentiation between isolated post-capillary PH and combined forms is crucial, in particular with regard to prognosis [21] and therapeutic consequences. The TPG and DPG have been introduced to quantify pulmonary vascular disease occurring as a consequence of PAWP elevation. In the present study, a greater PA:Ao ratio was predictive for TPG >12 mmHg.
Several previous studies have evaluated the usefulness of the PA:Ao ratio for the detection of PH [11–15]. However, only Chan and coworkers report data from a population with PH due to left heart disease [11]. In that retrospective analysis of 101 patients the authors defined a PA:Ao ratio cut-off of 0.84 for the detection of PH (mPAP >25 mmHg), which is in line with our results.
Other studies have used a PA:Ao ratio cut-off of >1.0 for the suspicion of PH. These included 60 patients with chronic obstructive pulmonary disease [13], 81 patients with connective tissue disease [15], 50 patients with mainly pulmonary disease [14], and a mixed population of 175 patients [12]. The differences with respect to the PA:Ao cut-off for the diagnosis of PH may be due to 1) retrospective design of all aforementioned studies, 2) heterogeneity of cut-offs for the diagnosis of PH (mPAP >20 mmHg [14] versus mPAP >25 mmHg [11–13, 15]), 3) heterogeneity of diagnoses underlying PH, and 4) small patient numbers [13–15]. Taken together, it remains to be determined whether the results of the present study are applicable in patient populations other than HFpEF.
Limitations
Presented data have been collected in a single center setting. Therefore, a center-specific bias cannot be excluded. However, the major advantages of limiting data collection to a single center are 1. inclusion of a homogenous patient population, 2. adherence to a constant clinical routine, 3. constant quality of echocardiographic and CMR work-up and 4. constant follow-up.
PA and Ao diameters were acquired from axial HASTE black blood images and were not planned exactly in line with the vessel orientation. This may have led to some over- or underestimation of the vessel size.
The majority of patients included in this study were suffering from PH (83.8 % had mPAP ≥25 mmHg). Sixty four percent had at least moderate PH (mPAP ≥30 mmHg). Due to the small number of patients in the group without PH, patients were dichotomized in groups with mPAPs above and below 30 mmHg, defined as groups with no/mild PH versus moderate/severe PH. However, previous work shows that a PA:Ao ratio of 0.84 also discriminates between patients with mPAPs above and below 25 mmHg [11]. In addition to morphological indicators of right heart pressure overload, recent data indicate the feasibility of CMR to estimate pulmonary hemodynamics, such as mPAP and pulmonary vascular resistance by using numerical models [35]. Such estimates, however, include various assumptions. Furthermore, they require extensive experience of the investigator. Thus, the accuracy and widespread applicability of these measurements still need to be proven. Furthermore, we did not analyse RV mass, which might have augmented the diagnostic performance of the PA:Ao ratio. However, in the present work we aimed to identify parameters that are easy to measure and do not require dedicated software or extensive training.
Conclusions
PA:Ao ratio is an easily assessable, straight-forward marker of PH in HFpEF patients. Moreover, it is strongly associated with outcome. Other CMR derived indices of RV pressure overload such as RVFW or SMT thickness are less accurate and reproducible.
References
Guazzi M. Pulmonary hypertension in heart failure preserved ejection fraction: Prevalence, pathophysiology, and clinical perspectives. Circ Heart Fail. 2014;7:367–77.
Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: Insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–7.
Vachiery JL, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62:D100–8.
Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: A community-based study. J Am Coll Cardiol. 2009;53:1119–26.
Zotter-Tufaro C, Mascherbauer J, Duca F, Koell B, Aschauer S, Kammerlander AA, et al. Prognostic significance and determinants of the six-minute walk test in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2014;[accepted for publication, #JHF082514-0348-TR]
Mascherbauer J, Marzluf BA, Tufaro C, Pfaffenberger S, Graf A, Wexberg P, et al. Cardiac magnetic resonance postcontrast t1 time is associated with outcome in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging. 2013;6:1056–65.
Perez VA, Haddad F, Zamanian RT. Diagnosis and management of pulmonary hypertension associated with left ventricular diastolic dysfunction. Pulm Circ. 2012;2:163–9.
Fisher MR, Forfia PR, Chamera E, Housten-Harris T, Champion HC, Girgis RE, et al. Accuracy of doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:615–21.
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the american society of echocardiography endorsed by the european association of echocardiography, a registered branch of the european society of cardiology, and the canadian society of echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. quiz 786–688.
McLure LE, Peacock AJ. Cardiac magnetic resonance imaging for the assessment of the heart and pulmonary circulation in pulmonary hypertension. Eur Respir J. 2009;33:1454–66.
Chan AL, Juarez MM, Shelton DK, MacDonald T, Li CS, Lin TC, et al. Novel computed tomographic chest metrics to detect pulmonary hypertension. BMC Med Imaging. 2011;11:7.
Corson N, Armato 3rd SG, Labby ZE, Straus C, Starkey A, Gomberg-Maitland M. Ct-based pulmonary artery measurements for the assessment of pulmonary hypertension. Acad Radiol. 2014;21:523–30.
Iyer AS, Wells JM, Vishin S, Bhatt SP, Wille KM, Dransfield MT. Ct scan-measured pulmonary artery to aorta ratio and echocardiography for detecting pulmonary hypertension in severe copd. Chest. 2014;145:824–32.
Ng CS, Wells AU, Padley SP. A ct sign of chronic pulmonary arterial hypertension: The ratio of main pulmonary artery to aortic diameter. J Thorac Imaging. 1999;14:270–8.
Rajaram S, Swift AJ, Capener D, Elliot CA, Condliffe R, Davies C, et al. Comparison of the diagnostic utility of cardiac magnetic resonance imaging, computed tomography, and echocardiography in assessment of suspected pulmonary arterial hypertension in patients with connective tissue disease. J Rheumatol. 2012;39:1265–74.
Vogel-Claussen J, Shehata ML, Lossnitzer D, Skrok J, Singh S, Boyce D, et al. Increased right ventricular septomarginal trabeculation mass is a novel marker for pulmonary hypertension: Comparison with ventricular mass index and right ventricular mass. Invest Radiol. 2011;46:567–75.
Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the heart failure and echocardiography associations of the european society of cardiology. Eur Heart J. 2007;28:2539–50.
Baim D. Grossman´s cardiac catheterization, angiography, and intervention. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the task force for the diagnosis and treatment of pulmonary hypertension of the european society of cardiology (esc) and the european respiratory society (ers), endorsed by the international society of heart and lung transplantation (ishlt). Eur Heart J. 2009;30:2493–537.
Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance (cmr) protocols 2013 update. J Cardiovasc Magn Reson. 2013;15:91.
Zotter-Tufaro C, Duca F, Kammerlander A, Koell B, Aschauer S, Dalos D, et al. Diastolic pressure gradient predicts outcome in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2015;[accepted for publication. JACC030215-0720RR]
Morris DA, Gailani M, Vaz Perez A, Blaschke F, Dietz R, Haverkamp W, et al. Right ventricular myocardial systolic and diastolic dysfunction in heart failure with normal left ventricular ejection fraction. J Am Soc Echocardiogr. 2011;24:886–97.
Mohammed SF, Hussain I, Abou Ezzeddine OF, Takahama H, Kwon SH, Forfia P, et al. Right ventricular function in heart failure with preserved ejection fraction: A community based study. Circulation. 2014;130:2310.
Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta DK, Fox J, et al. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014;7:288–99.
Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–62.
Aschauer S, Zotter-Tufaro C, Kammerlander AA, Pfaffenberger S, Marzluf BA, Bonderman D, et al. The right heart in hfpef, insights from a cardiac magnetic resonance study. Eur Heart J. 2014;35(Abstract Supplement):532.
Goliasch G, Tufaro C, Aschauer S, Kammerlander A, Pfaffenberger S, Mascherbauer J, et al. Outcome in heart failure with preserved ejection fraction strongly depends on right ventricular performance. Eur Heart J. 2014;35(Abstract Supplement):1036.
Hoeper MM, Lee SH, Voswinckel R, Palazzini M, Jais X, Marinelli A, et al. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol. 2006;48:2546–52.
Linguraru MG, Pura JA, Gladwin MT, Koroulakis AI, Minniti C, Machado RF, et al. Computed tomography correlates with cardiopulmonary hemodynamics in pulmonary hypertension in adults with sickle cell disease. Pulm Circ. 2014;4:319–29.
Bouchard A, Higgins CB, Byrd 3rd BF, Amparo EG, Osaki L, Axelrod R. Magnetic resonance imaging in pulmonary arterial hypertension. Am J Cardiol. 1985;56:938–42.
Ghio S, Pazzano AS, Klersy C, Scelsi L, Raineri C, Camporotondo R, et al. Clinical and prognostic relevance of echocardiographic evaluation of right ventricular geometry in patients with idiopathic pulmonary arterial hypertension. Am J Cardiol. 2011;107:628–32.
Pattynama PM, Lamb HJ, Van der Velde EA, Van der Geest RJ, Van der Wall EE, De Roos A. Reproducibility of mri-derived measurements of right ventricular volumes and myocardial mass. Magn Reson Imaging. 1995;13:53–63.
Roeleveld RJ, Marcus JT, Boonstra A, Postmus PE, Marques KM, Bronzwaer JG, et al. A comparison of noninvasive mri-based methods of estimating pulmonary artery pressure in pulmonary hypertension. J Magn Reson Imaging. 2005;22:67–72.
Saba TS, Foster J, Cockburn M, Cowan M, Peacock AJ. Ventricular mass index using magnetic resonance imaging accurately estimates pulmonary artery pressure. Eur Respir J. 2002;20:1519–24.
Swift AJ, Rajaram S, Hurdman J, Hill C, Davies C, Sproson TW, et al. Noninvasive estimation of pa pressure, flow, and resistance with cmr imaging: Derivation and prospective validation study from the aspire registry. JACC Cardiovasc Imaging. 2013;6:1036–47.
Grapsa J, O'Regan DP, Pavlopoulos H, Durighel G, Dawson D, Nihoyannopoulos P. Right ventricular remodelling in pulmonary arterial hypertension with three-dimensional echocardiography: Comparison with cardiac magnetic resonance imaging. Eur J Echocardiogr. 2010;11:64–73.
Kosinski A, Kozlowski D, Nowinski J, Lewicka E, Dabrowska-Kugacka A, Raczak G, et al. Morphogenetic aspects of the septomarginal trabecula in the human heart. Arch Med Sci. 2010;6:733–43.
Funding sources
This study received support from the Austrian Society of Cardiology (to JM), the Österreichischer Herzfonds (to JM) and the Austrian fellowship grants KLI 246 (to DB), and KLI 245 (to JM).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
GK, JB and SP were responsible for data acquisition. AAK and JM were responsible for data analysis and interpretation. JM and DB were responsible for the study design. AAK, GK and JM drafted the manuscript. SA, BAM, CZ, AB, AD, FD, JB, SP and DB critically revised the manuscript. All authors read and approved the final manuscript.
Gültekin Karakus and Andreas A. Kammerlander contributed equally to this work.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Karakus, G., Kammerlander, A.A., Aschauer, S. et al. Pulmonary artery to aorta ratio for the detection of pulmonary hypertension: cardiovascular magnetic resonance and invasive hemodynamics in heart failure with preserved ejection fraction. J Cardiovasc Magn Reson 17, 79 (2015). https://doi.org/10.1186/s12968-015-0184-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12968-015-0184-3