Abstract
Background
There is limited evidence regarding the impact of pregnancy loss on the subsequent risk of metabolic disorders. We aimed to investigate whether history of pregnancy loss is associated with the subsequent risk of prediabetes (pre-DM), diabetes (DM), and metabolic syndrome (METs) among couples.
Method
In this population-based cohort study, 2765 couples with and without history of pregnancy loss and free of DM, pre-DM, and METs at baseline were included and followed for incidents of DM, pre-DM, and METs by 3-year intervals visits from 1999 to 2018. Detailed data of variables was collected using standard questionnaires, interviews, clinical and laboratory assessments. A modified Poisson regression for binary outcome data with a log link function and robust error variance was used to estimate relative risks (RRs) in couples with and without history of pregnancy loss. Both unadjusted and adjusted models were fitted, and effect measures were calculated.
Result
During a median follow-up of 15 years, females with history of pregnancy loss were experienced more pre-DM (50% vs. 45.5%), DM (28.9% vs. 21.3%), and METs (70% vs. 60.1%) than females without such history. Moreover, history of pregnancy loss increased the risk of METs by 8% among females. The incidence of DM in males with history of pregnancy loss in their spouses was higher than in males without it (28.8% vs. 23.5%). Among males, having a spouse with history of pregnancy loss was positively associated with the risk of pre-DM (RR = 1.12; 95%CI: 1.02, 1.23, p = 0.02); furthermore, they were more prone to the risk of METs than females with a history of pregnancy loss (RR = 1.13; 95%CI: 1.07, 1.20, p < 0.001).
Conclusion
Although pregnancy loss is a female-specific factor, may foreshadow the subsequent METs, our study identified a higher risk of subsequent pre-DM and METs in males with history of pregnancy loss in their spouses. Pregnancy loss could be considered a possible future risk factor for metabolic disorders in couples.
Similar content being viewed by others
Introduction
Metabolic syndrome (METs), diabetes mellitus (DM), and prediabetes (pre-DM) are the leading health problems around the world [1,2,3] and could lead to poor cardiovascular outcomes [4,5,6]. Despite the introduction of numerous established risk factors for DM and METs (such as physical inactivity, smoking, and unhealthy diet) [7, 8], there are still unknown factors that contribute to developing metabolic disorders in both males and females. Recently, gender-specific differences between females and males in terms of cardio-metabolic risk factors have been suggested [9, 10]. For example, pregnancy complications are known as unique risk factors for cardio-metabolic disorders in women [11].
It is well established that each pregnancy poses a diabetogenic effect on maternal metabolism [12]. Indeed, normal pregnancy is cardio-metabolic stress, and any pregnancy complication might be preceded by a metabolic abnormality [13, 14]. Pregnancy loss, both miscarriage and stillbirth, is a common pregnancy complication [15]. Several studies have shown that history of pregnancy loss (PL) in women is linked to subsequent chronic conditions like cardiovascular disease and renal disease [16,17,18,19]; however, there are limited and inconsistent reports of the association between pregnancy loss and the risk of metabolic disorders in mothers later in life [18, 20, 21].
The spousal concordance of cardio-metabolic risk factors received more attention in recent literature [22, 23]. A cohort study among middle-aged adults has demonstrated that males whose wives have a history of CVD were more prone to CVD [24]. Another study supported the concordance of glycaemic and cardio-metabolic parameters among females with a history of gestational diabetes and their spouses [25]. Moreover, several studies revealed the increased risk of developing metabolic disorders in spouses of women who experience pregnancy complications such as hyperglycemia during pregnancy, gestational diabetes, and gestational hypertension [26,27,28].
The exact mechanisms related to the adverse pregnancy outcomes and disease risk in couples later in life remain incompletely understood. It has been proposed that rather than biological factors related to pregnancy, some non-biological factors contribute to an increased risk of chronic disease in males and females [29]. Concordance regarding lifestyle factors may lead to the development of the same chronic disease in couples [30]. It seems that behaviors and lifestyle which implemented years after complicated pregnancy might affect the risk of metabolic disorders among couples.
Pregnancy loss is among the most common complication of pregnancy and might be a stressor event for both parents [31]; as a result, it acts as a trigger for metabolic abnormality [32]. A limited number of studies have investigated the risk of diabetes in women with a history of pregnancy loss [18, 20, 21], but to our knowledge, there is still no study addressing the role of pregnancy loss on the risk of METs and pre-DM; additionally, the adverse effect of pregnancy loss on spousal risk of metabolic disorders has not been investigated yet.
In the present study, we aimed to investigate the risk of pre-DM, DM, and METs in couples with pregnancy loss history in a population-based cohort study with, on average, 15 years of follow-up.
Method
This study has been undertaken using data from an ongoing population-based longitudinal Tehran Lipid and Glucose Study. The protocol of TLGS was developed according to the World Health Organization approach for surveillance of risk factors for non-communicable diseases [33]. The baseline phase of this ongoing cohort was conducted between 1999 and 2001, and follow-up phases were performed at 3-year intervals. The population of this study was selected from residents of district number 13 of Tehran (a representative sample of Tehran), the capital of Iran. Detailed information on the study design and the rationale behind the methodology has been addressed elsewhere [34].
Study population
In the present study, among a total of 20,145 subjects in TLGS, there were 3,650 matched couples. Regarding the female participants, we chose individuals who met the following criteria: married females aged > 18 at baseline, females whose spouses had complete records of variables, and females without DM, pre-DM, and METs at baseline and before exposure to pregnancy loss. Also, the inclusion criteria for males include married males aged > 18 at baseline, males whose spouses had complete records of variables, and males without DM, pre-DM, and METs at baseline and before exposure to pregnancy loss in their spouses.
After exclusion of those with history of pre-DM (n = 4), DM (n = 4), or METs (n = 9), and couples with no pregnancy (n = 79), finally, 3,554 couples remained eligible for the present analysis. Of the remaining participants, 1585 have history of pregnancy loss. In order for the percentage of women with history of pregnancy loss to represent the community (around 25% to 30%), we selected a random sample of these women (n = 796). Eventually, 2765 couples, including 1969 women who had never experienced pregnancy loss and 796 women who had experienced at least one pregnancy loss over the follow-up, remained in this study (Fig. 1). Couples were included from the first (n = 2362), second (n = 381), and third (n = 22) phases and followed until the end of the study (20 March 2018).
Measures
Trained staff and physicians studied the participants of this study according to the standard protocol of TLGS. Also, a standard and validated questionnaire was used to gather demographic and medical history and variables [34]. Anthropometric, laboratory, and clinical assessments were performed based on the TLGS measurement protocol. All blood analyses were carried out at the TLGS research laboratory. Details of measurement of systolic and diastolic blood pressure, anthropometric parameters, and laboratory measurements, including fasting blood glucose (FBS) levels, lipid profile triglyceride (TG), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), and total cholesterol (TC), have been reported previously [34].
Overall, variables including age, marital status, smoking status, education level, physical activity, number of gravidity or parity, and medical and drug history were obtained through self-reported questionnaires. The details of a female’s obstetric history, including pregnancy outcomes, were collected through a review of relevant medical documents and face-to-face interviews. Other variables which measured in this study were collected from both females and males using standard questionnaires, interviews, clinical and laboratory assessments.
In this study’s exposure variable, pregnancy loss was defined as history of any type of abortion or miscarriage, or stillbirth [15, 35]. Outcomes interest variables were as DM, METs, and pre-DM. Detailed information about these outcomes has been published elsewhere [36].
The Modifiable Activity Questionnaire (MAQ) [37], which was reliable and validated in the Iranian population, was used for assessing physical activity. This questionnaire measures the physical activities related to leisure time, household, and occupational activities. The metabolic equivalent (MET) was calculated based on min/week. 1500 min/week and appropriate physical activity defined as MET ≥ 600 min/week.
Statistical analysis
Continuous variables were checked for normality using the Shapiro–Wilk test; those with normal distribution were expressed as mean (standard deviation), and non-normal distributed variables were expressed as median (interquartile range). Categorical variables were expressed as percentages. Characteristics of participants were compared between the pregnancy loss categories by applying the independent t-test or Pearson’s Chi-squared test for continuous and categorical data, respectively. The Mann–Whitney U test was applied to compare variables with skewed distribution.
For this study, we do a post hoc analysis, which involves looking at the data after a study has been concluded and trying to find patterns that were not the primary objectives of the study. TLGS was initiated in 1999 to investigate non-communicable disease (NCD) and its associated risk factors or determinants among a representative family-based population of Tehran, the capital of Iran; however, in this study, we aimed to discover the impact of pregnancy loss on the subsequent risk of metabolic disorders among couples.
A modified Poisson regression for binary outcome data with a log link function and robust error variance was used to estimate relative risks (RRs) and 95% confidence intervals (CIs) for the associations between pregnancy loss and incidence of pre-DM, DM, and METs in males and females over the follow-up [38]. We considered three models for this analysis; model 1: unadjusted model, model 2: age-BMI adjusted model, and model 3 was adjusted for age, WHtR, BMI, education, parity, number of pregnancy loss, SBP, FBS, TG, TC, LDL, and family history of diabetes. Adjusting variables were determined based on the significant differences between those participants who experienced pregnancy loss and those who did not. Moreover, to adjust the results for matching cases and achieve a robust variance, we considered the couples as the cluster observations in the model. Finally, the plots of the relative risks were depicted for three outcomes and sex groups by pregnancy loss status. Statistical analysis was performed using the software package STATA (version 13; STATA Inc., College station, TX, USA); the significance level was set at P < 0.05.
Result
In the present study, 2765 couples were included. Among those pairs, 1969 (71.2%) had no pregnancy loss, and 796 (28.8%) experienced at least one pregnancy loss. Among couples with history of pregnancy loss, those with only abortion history were 618, only stillbirth history were 42, and those who experienced both types were 136 couples.
The total median (IQR) follow-up time was 15 (10–16) years which was 14 (8–16) years, and 15 (11–16) years for males and females, respectively.
Tables 1 and 2 summarize the characteristics of participants at the baseline and last follow-up for females and males, respectively. The characteristics are categorized by the pregnancy loss status. According to the baseline part of Table 1, the mean entry age was higher in both males and females in group with history of pregnancy loss (49.0 (11.8) for males and 42 (11.0) for females) compared to no group without history of pregnancy loss (44.5 (11.4) for males and 37.9 (10.4) for females) (p < 0.001). Couples with history of pregnancy loss also had the highest waist to hip ratio (males: mean (SD): 0.54(0.06) vs. 0.53(0.06)) and females: (mean (SD): 0.57(0.08) vs. 0.56 (0.08)) SBP (males: mean (SD): 122.5(19.9) vs. 119.5(17.6)) and females: (mean (SD): 118.6(19.3) vs. 115.1 (16.8)), FBS (males: mean (SD): 5.6(1.9) vs. 5.5(1.7)) and females: (mean (SD): 5.4(1.8) vs. 5.3 (1.6)), TC (males: mean (SD): 5.4(1.1) vs. 5.3(1.0)) and females: (mean (SD): 5.5(1.2) vs. 5.3 (1.2)), and lower educational status (males: number (percentage): 352(44.3) vs. 1019(51.8) and females: number (percentage): 296(37.3) vs. 911 (46.3)). According to pregnancy history, the median of number of pregnancy losses in couples with pregnancy loss was 1 (IQR: 1, 2) (Table 1).
The incidence of outcome variables pre-DM, DM and METs in females with history of pregnancy loss increases compared to females with no pregnancy loss (50% vs. 45.5% for pre-DM (p = 0.03), 28.9% vs. 21.3% for DM (p < 0.001) and 70% vs. 60.1% for METs (p < 0.001)). Moreover, there was a significantly higher incidence of DM outcome in males with history of pregnancy loss in their spouses compared to males with no history of pregnancy loss in their spouses (28.8% vs. 23.5% (p = 0.004)) (Table 2).
Table 3 shows the unadjusted and adjusted relative risks of pre-DM, DM, and METs based on poisson regression models when the effect of sex, pregnancy loss, and the interaction term of these two are in the model. Model 1 (unadjusted model) reveals that having pregnancy loss history was associated with 22% higher risk of DM in males (RR = 1.22; 95%CI: (1.07, 1.40), p = 0.003) (Table 2); however, this association was disappeared after adjusting variables (model 2 & 3). Furthermore, Model 3 shows overall males experienced higher risk of pre-DM (RR = 1.14; 95%CI: (1.06, 1.22), p < 0.001), DM (RR = 1.13; 95%CI: (1.01, 1.28), p = 0.04), and METs (RR = 1.22; 95%CI: (1.16, 1.27), p < 0.001) compared to females.
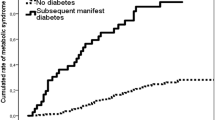
Figure 2 represents unadjusted and adjusted relative risks and corresponding 95% confidence intervals of metabolic disorders based on the interaction term sex*pregnancy loss status. In couples with history of pregnancy loss, males were more prone to the risk of pre-DM (RR = 1.12; 95%CI: (1.02,1.23), p = 0.02), and METs (RR = 1.13; 95%CI: (1.07, 1.20), p < 0.001) than females, however, males with history of pregnancy loss revealed no higher risk of metabolic disorders compared to the males without it. Moreover, females with history of pregnancy loss increased the risk of METs by 8% than females without history of pregnancy loss (RR = 1.08; 95%CI: (1.02, 1.14), p = 0.01) (Fig. 2). Additionally, the interaction effects of each type of pregnancy loss (abortion or stillbirth) with sex in relation to the risk of metabolic disorders were presented in Figs. 3 and 4. In couples with history of abortion, males demonstrated higher risk of pre-DM (RR = 1.12; 95%CI: (1.02, 1.23), p = 0.02) and METs (RR = 1.13; 95%CI: (1.07, 1.20), p < 0.001) compared to their spouses. However, couples with a history of stillbirth did not show such an association. Besides, among females, those who had a history abortion were 7% more likely for METs risk (RR = 1.07; 95% CI: (1.01, 1.13); p = 0.02), while females with history of stillbirth had no higher risk of METs compared to females without history of stillbirth. Figure 5 shows cartoon representation of results.
Unadjusted (a) and adjusted (b) Relative Risks and 95% CIs for pre-DM, DM, and METs outcomes comparing couples with and without history of pregnancy loss. RRs are adjusted for age, WHtR, BMI, education, parity, number of pregnancy losses, family history of DM, SBP, FBS, TG, TC, and LDL. WHtR: Waist-to-Height Ratio; BMI: body mass index; FBS: fasting blood glucose; pre-DM: prediabetes; DM: diabetes mellitus; METs: metabolic syndrome SBP; systolic blood pressure; DBP: Diastolic blood pressure; TG: triglyceride; LDL: low-density lipoprotein cholesterol; TC: total
Unadjusted (a) and adjusted (b) Relative Risks and 95% CIs for pre-DM, DM, and METs outcomes comparing couples with and without history of abortion. RRs are adjusted for age, WHtR, BMI, education, parity, number of pregnancy losses, family history of DM, SBP, FBS, TG, TC, and LDL. WHtR: Waist-to-Height Ratio; BMI: body mass index; FBS: fasting blood glucose; pre-DM: prediabetes; DM: diabetes mellitus; METs: metabolic syndrome SBP; systolic blood pressure; DBP: Diastolic blood pressure; TG: triglyceride; LDL: low-density lipoprotein cholesterol; TC: total
Unadjusted (a) and adjusted (b) Relative Risks and 95% CIs for pre-DM, DM, and METs outcomes comparing couples with and without history of stillbirth. RRs are adjusted for age, WHtR, BMI, education, parity, number of pregnancy losses, family history of DM, SBP, FBS, TG, TC, and LDL. WHtR: Waist-to-Height Ratio; BMI: body mass index; FBS: fasting blood glucose; pre-DM: prediabetes; DM: diabetes mellitus; METs: metabolic syndrome SBP; systolic blood pressure; DBP: Diastolic blood pressure; TG: triglyceride; LDL: low-density lipoprotein cholesterol; TC: total
Discussion
This population-based cohort study was conducted to determine whether couples with a history of pregnancy loss are at an elevated risk of pre-DM, DM, and METs in the long term. The main findings of this study were that males with history of pregnancy loss in their spouses were at increased risk of pre-DM and METs compared to females after adjustment for confounders, while for DM, no significant association was noticed. Moreover, females with history of pregnancy loss just experienced an elevated risk of METs compared with females without such history.
Today, the growing epidemic of METs and DM can be observed worldwide [39]; these disorders are considered as two main major risk factors for CVD [40, 41]. Pregnancy is considered as a potential risk factor for further cardio-metabolic events due to some physiological adaptations that occur during pregnancy [13, 14]. This adverse effect is exaggerated by pregnancy complications including gestational diabetes, preterm delivery, and pregnancy-induced hypertension [42]. This complication not only increases the probability of developing CVD, DM, and HTN in females, but also increases the cardio-metabolic disturbances among their spouses [27, 28]; the exact mechanisms related to this concordance in couples later in life remain incompletely understood. It is assumed that rather than biological factors related to pregnancy, some non-biological factors contribute to an increased risk of chronic disease in males and females [43, 44]. Prior studies demonstrate that women with a history of pregnancy loss are at an increased risk of DM, HTN, and hypercholesterolemia [18, 45]. Furthermore, while pregnancy loss increases the probability of developing CVD risk factors in females [18, 45], by our knowledge, its adverse effect on their spouse’s cardio-metabolic situation has not been reported yet. We found that in addition to females, DM and METs were occurred more often in males with history of pregnancy loss in their spouses; even these males were more prone to the risk of METs than females with history of pregnancy loss.
Among females who participated in the present study, while having a history of at least one pregnancy loss increased the risk of various adverse metabolic disorders (pre-DM, DM, and METs); however, after adjustment for confounders, this association just remained significant for METs. There is limited study in terms of determining the subsequent risk of DM in women with history of pregnancy loss [18, 21, 46] and the subsequent risk of pre-DM and METs in women with history of pregnancy loss had not been reported before, by our knowledge. In line with our study, a cohort study (the Women’s Health Initiative) demonstrated that history of pregnancy loss was associated with a higher rate of DM [18]. It has been shown in another large population-based study that having history of pregnancy loss increases the risk of female CVD [46]. Moreover, a Danish nationwide case–control study among 24,774 women with DM and 247,740 controls revealed that women with a history of pregnancy loss are at increased risk of DM [21]. By contrast, Kharazmi et al. in a prospective cohort study, found that history of abortion and stillbirth was not significantly associated with the risk of DM in women [20]. When we evaluated the possible excessive risk of metabolic disorders in terms of abortion and stillbirth separately, we found that having history of abortion was associated with an increased risk of METs among females. However, couples with history of stillbirth were not more prone to metabolic disorders; however it may be due to the lack of adequate number of stillbirth in present study. Another studies which proposed that any form of pregnancy loss (including stillbirth and miscarriage) may increase women’s future risk of cardio-metabolic disorders [18, 42, 45, 47].
Moreover, our study showed that males with a history of pregnancy loss or abortion in their spouses were more likely to experience METs and per-DM than females. This may be explained by the impact of the paternal metabolic conditions on pregnancy loss. Kasman et al. reported that in men with increasing components of METs in the preconception period, the risk of pregnancy loss was significantly increased [48]. As a result, it is assumed that those males with history of pregnancy loss in their spouses may have a greater baseline risk of metabolic disorders. Moreover, a systematic review revealed that following a pregnancy loss, males might be faced with double-disenfranchised grief [49]. Indeed, lack of support and facing diverse challenges due to pregnancy loss could manifold the disenfranchised grief for males [50]. These men might be at risk for psychological disorders which might share a common pathway with metabolic disorders [32, 51, 52]. Indeed, it is evident that gender differences in sex hormones, energy balance, and body composition may partly explain the susceptibility of males to metabolic disorders [53, 54]. A recent review shows that due to biological sex differences, men are more likely to develop DM in middle age groups [55]. Additionally, spouses are concordant in lifestyle habits [56]. So spouses who have an unhealthy lifestyle are more likely to develop cardiovascular risk factors [30]. Therefore sharing lifestyle/environmental factors might affect the couple's risk for metabolic disorders.
The exact underlying pathophysiology of an association between pregnancy loss and DM and METs is unknown. It is established that gut microbiota triggers metabolic inflammation and subsequent metabolic disorders [57,58,59]. All the proposed mechanisms were also mentioned as the main players for the occurrence of pregnancy loss [60]. Collectively, sharing common pathways for metabolic dysfunction may provoke the onset of DM or METs in women with history of pregnancy loss. It is worth noting that, recently, Dill-McFarland et al. revealed that cohabiting couples had more similar microbiota composition [61]. In addition, pregnancy loss per se is a traumatic event [62]. Pregnancy loss and following disenfranchised grief negatively impact parents’ health; this also may be prolonged and jeopardize the mental health of couples [63, 64]. The mechanism which links depression and METs is mainly related to low-grade chronic inflammatory conditions [65]. Apart from this, individuals with a history of psychological disorders are more prone to unhealthy behaviors [65]. Recent evidence highlighted inflammatory pathways' role in the pathophysiology of DM and METs [66,67,68]. It is proposed that paternal lifestyle factors in the preconception period per se are associated with the risk of pregnancy loss [6, 7]. Fossé et al. (2020), in their recent meta-analysis, concluded that paternal smoking > 10 cigarettes per day in the preconception period is linked with an increased risk of pregnancy loss [7]. It is also reported that paternal obesity could affect pregnancy outcomes [8, 9]. Moreover, paternal unhealthy diet may adversely affect pregnancy outcomes [10]. These situations could also induce pro-inflammatory pathways in the exposed men [10]. As a result, a paternal unhealthy lifestyle may directly cause inflammation in exposed men and indirectly associated with an increase in pregnancy loss in their spouses with subsequent further rising metabolic disorder risk.
The findings of this study should be interpreted in the context of weakness and strength. This study was conducted on a longitudinal cohort study with a large number of participants that were followed on average for 15 years. All variables were measured based on a standard protocol with several follow-up visits with 3-years intervals. We adjusted the results based on most potential confounders. Our study has several limitations as well. There is a number of biases concerning study design in terms of indication bias. This is a cohort study which was conducted on an urban population, so the observed effect may be exaggerated, and findings from the research population may not apply to the rural population. The visit intervals were 3-years, and we could not capture shorter variability estimates of metabolic conditions. The length of the time after losing a pregnancy may affect the outcome; since we have no data on the exact date, we assumed the outcomes occurred in the mid-time interval. Diet and nutrition status might influence the metabolic profile, which was not considered in the present study. Moreover, in our study, data related to physical activity were drawn from a questionnaire, which tends to be overestimated by individuals (social desirability bias). In this study, we have no data on the genetic background of participants, which may affect the risk of metabolic abnormality. There was no data on lifestyle and psychological situation during and before pregnancy as potential influential factors.
Conclusion
Although pregnancy loss is a female-specific factor, may foreshadow the subsequent METs, our study identified a higher risk of subsequent pre-DM and METs in males with a history of pregnancy loss in their spouses. Pregnancy loss could be considered as a future risk factor for metabolic disorders in couples. Despite well-documented impact of some pregnancy complications in developing chronic disease in later life, long-term preventive care for those couples with such history of adverse pregnancy outcomes is lacking [69]. This is the first study that explored the risk of subsequent metabolic disturbances in couples with history of pregnancy loss, more investigations are highly needed to confirm these findings.
Abbreviations
- PL:
-
Pregnancy loss
- pre-DM:
-
Prediabetes
- DM:
-
Diabetes mellitus
- METs:
-
Metabolic syndrome
- WHtR:
-
Waist-to-height ratio
- BMI:
-
Body mass index
- FBS:
-
Fasting blood glucose
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- TG:
-
Triglyceride
- LDL:
-
Low-density lipoprotein cholesterol
- HDL:
-
High-density lipoprotein cholesterol
- TC:
-
Total cholesterol
References
Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, Song X, Ren Y, Shan P-F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10:1–11.
Ostovar R, Kiani F, Sayehmiri F, Yasemi M, Mohsenzadeh Y, Mohsenzadeh Y. Prevalence of metabolic syndrome in Iran: A meta-analysis. Electron Physician. 2017;9:5402–18.
Misra R, Patel T, Kotha P, Raji A, Ganda O, Banerji M, Shah V, Vijay K, Mudaliar S, Iyer D. Prevalence of diabetes, metabolic syndrome, and cardiovascular risk factors in US Asian Indians: results from a national study. J Diabetes Complications. 2010;24:145–53.
Tune JD, Goodwill AG, Sassoon DJ, Mather KJ. Cardiovascular consequences of metabolic syndrome. Transl Res. 2017;183:57–70.
DeFronzo RA, Abdul-Ghani M. Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am J Cardiol. 2011;108:3b–24b.
Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17:83.
Bellou V, Belbasis L, Tzoulaki I, Evangelou E. Risk factors for type 2 diabetes mellitus: An exposure-wide umbrella review of meta-analyses. PLoS ONE. 2018;13:e0194127–e0194127.
Park Y-W, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–36.
Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016;37:278–316.
Beigh SH, Jain S. Prevalence of metabolic syndrome and gender differences. Bioinformation. 2012;8:613–6.
Graves M, Howse K, Pudwell J, Smith GN. Pregnancy-related cardiovascular risk indicators: Primary care approach to postpartum management and prevention of future disease. Can Fam Phys Med Fam. 2019;65:883–9.
Parrettini S, Caroli A, Torlone E. Nutrition and metabolic adaptations in physiological and complicated pregnancy focus on obesity and gestational diabetes. Front Endocrinol. 2020;11:89.
Rich-Edwards JW, Fraser A, Lawlor DA, Catov JM. Pregnancy characteristics and women’s future cardiovascular health: an underused opportunity to improve women’s health? Epidemiol Rev. 2013;36:57–70.
Lei Q, Niu J, Lv L, Duan D, Wen J, Lin X, Mai C, Zhou Y. Clustering of metabolic risk factors and adverse pregnancy outcomes: a prospective cohort study. Diabetes Metab Res Rev. 2016;32:835–42.
Robinson GE. Pregnancy loss. Best Pract Res Clin Obstet Gynaecol. 2014;28:169–78.
Barrett PM, McCarthy FP, Evans M, Kublickas M, Perry IJ, Stenvinkel P, Khashan AS, Kublickiene K. Stillbirth is associated with increased risk of long-term maternal renal disease: a nationwide cohort study. Am J Obstet Gynecol. 2020;223:427.
Oliver-Williams CT, Heydon EE, Smith GC, Wood AM. Miscarriage and future maternal cardiovascular disease: a systematic review and meta-analysis. Heart. 2013;99:1636–44.
Hall PS, Nah G, Vittinghoff E, Parker DR, Manson JE, Howard BV, Sarto GE, Gass ML, Sealy-Jefferson SM, Salmoirago-Blotcher E. Relation of pregnancy loss to risk of cardiovascular disease in parous postmenopausal women (from the Women’s Health Initiative). Am J Cardiol. 2019;123:1620–5.
Peters SA, Yang L, Guo Y, Chen Y, Bian Z, Tian X, Chang L, Zhang S, Liu J, Wang T. Pregnancy, pregnancy loss, and the risk of cardiovascular disease in Chinese women: findings from the China Kadoorie Biobank. BMC Med. 2017;15:1–10.
Kharazmi E, Lukanova A, Teucher B, Groß M-L, Kaaks R. Does pregnancy or pregnancy loss increase later maternal risk of diabetes? Eur J Epidemiol. 2012;27:357–66.
Egerup P, Mikkelsen AP, Kolte AM, Westergaard D, Rasmussen S, Knop FK, Lidegaard Ø, Nielsen HS. Pregnancy loss is associated with type 2 diabetes: a nationwide case–control study. Diabetologia. 2020;63:1521–9.
Retnakaran R, Wen SW, Tan H, Zhou S, Ye C, Shen M, Smith GN, Walker MC. Spousal Concordance of Cardiovascular Risk Factors in Newly Married Couples in China. JAMA Netw Open. 2021;4:e2140578–e2140578.
Di Castelnuovo A, Quacquaruccio G, Donati MB, De Gaetano G, Iacoviello L. Spousal concordance for major coronary risk factors: a systematic review and meta-analysis. Am J Epidemiol. 2009;169:1–8.
Ohbe H, Yasunaga H. Spouse’s cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a matched-pair cohort study. Circ Cardiovasc Qual Outcomes. 2021;14: e007649.
Goyal A, Gupta Y, Kalaivani M, Sankar MJ, Kachhawa G, Bhatla N, Gupta N, Tandon N. Concordance of glycaemic and cardiometabolic traits between Indian women with history of gestational diabetes mellitus and their spouses: an opportunity to target the household. Diabetologia. 2019;62:1357–65.
Gupta Y, Goyal A, Kalaivani M, Singhal S, Bhatla N, Gupta N, Tandon N. High burden of cardiometabolic risk factors in spouses of Indian women with hyperglycaemia in pregnancy. Diabet Med. 2020;37:1058–65.
Kabootari M, Hasheminia M, Guity K, Ramezankhani A, Azizi F, Hadaegh F. Gestational diabetes mellitus in mothers and long term cardiovascular disease in both parents: results of over a decade follow-up of the Iranian population. Atherosclerosis. 2019;288:94–100.
Pace R, Rahme E, Dasgupta K. Considering parents as a unit: Associations of gestational diabetes and gestational hypertension with postpartum diabetes and hypertension in couples. Pregnancy Hypertens. 2019;16:32–7.
Peters SAE, Yang L, Guo Y, Chen Y, Bian Z, Millwood IY, Bragg F, Zhou X, Ge P, Chen B, et al. Parenthood and the risk of diabetes in men and women a 7 year prospective study of 0.5 million individuals. Diabetologia. 2016;59:1675–82.
Jun SY, Kang M, Kang SY, Lee JA, Kim YS. Spousal concordance regarding lifestyle factors and chronic diseases among couples visiting primary care providers in Korea. Korean J Fam Med. 2020;41:183.
Voss P, Schick M, Langer L, Ainsworth A, Ditzen B, Strowitzki T, Wischmann T, Kuon RJ. Recurrent pregnancy loss: a shared stressor–-couple-orientated psychological research findings. Fertil Steril. 2020;114:1288–96.
Moradi Y, Albatineh AN, Mahmoodi H, Gheshlagh RG. The relationship between depression and risk of metabolic syndrome: a meta-analysis of observational studies. Clin Diabet Endocrinol. 2021;7:4.
Bonita R, De Courten M, Dwyer T, Jamrozik K, Winkelmann R. Surveillance of risk factors for noncommunicable diseases: the WHO STEPwise approach: summary. Noncommunicable Diseases and Mental Health: World Health Organization; 2001.
Azizi F, Zadeh-Vakili A, Takyar M. Review of rationale, design, and initial findings Tehran Lipid and Glucose Study. Int J Endocrinol Metab. 2018;16:8.
Huchon C, Deffieux X, Beucher G, Capmas P, Carcopino X, Costedoat-Chalumeau N, Delabaere A, Gallot V, Iraola E, Lavoue V. Pregnancy loss French clinical practice guidelines. Elsevier. 2016;201:18–26.
Hadaegh F, Shafiee G, Ghasemi A, Sarbakhsh P, Azizi F. Impact of metabolic syndrome, diabetes and prediabetes on cardiovascular events: Tehran lipid and glucose study. Diabetes Res Clin Pract. 2010;87:342–7.
Delshad M, Ghanbarian A, Ghaleh NR, Amirshekari G, Askari S, Azizi F. Reliability and validity of the modifiable activity questionnaire for an Iranian urban adolescent population. Int J Prev Med. 2015;6:3.
Chen W, Qian L, Shi J, Franklin M. Comparing performance between log-binomial and robust Poisson regression models for estimating risk ratios under model misspecification. BMC Med Res Methodol. 2018;18:1–12.
Tabish SA. Is Diabetes Becoming the Biggest Epidemic of the Twenty-first Century. Int J Health Sci. 2007;1:8.
Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–32.
Dal Canto E, Ceriello A, Rydén L, Ferrini M, Hansen TB, Schnell O, Standl E, Beulens JW. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol. 2019;26:25–32.
Parikh NI, Gonzalez JM, Anderson CA, Judd SE, Rexrode KM, Hlatky MA, Gunderson EP, Stuart JJ, Vaidya D, Epidemiology AHACo, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. 2021;143:e902–16.
Vlassoff C. Gender differences in determinants and consequences of health and illness. J Health Popul Nutr. 2007;25:47.
Varì R, Scazzocchio B, D’Amore A, Giovannini C, Gessani S, Masella R. Gender-related differences in lifestyle may affect health status. Annali dell’Istituto superiore di sanita. 2016;52:158–66.
Horn J, Tanz LJ, Stuart JJ, Markovitz AR, Skurnik G, Rimm EB, Missmer SA, Rich-Edwards JW. Early or late pregnancy loss and development of clinical cardiovascular disease risk factors: a prospective cohort study. BJOG An Int J Obstet Gynaecol. 2019;126:33–42.
Wang Y-X, Mínguez-Alarcón L, Gaskins AJ, Wang L, Ding M, Missmer SA, Rich-Edwards JW, Manson JE, Chavarro JE. Pregnancy loss and risk of cardiovascular disease: the Nurses’ Health Study II. Eur Heart J. 2022;43:190–9.
Ranthe MF, Andersen EA, Wohlfahrt J, Bundgaard H, Melbye M, Boyd HA. Pregnancy loss and later risk of atherosclerotic disease. Circulation. 2013;127:1775–82.
Kasman AM, Zhang CA, Li S, Lu Y, Lathi RB, Stevenson DK, Shaw GM, Eisenberg ML. Association between preconception paternal health and pregnancy loss in the USA: an analysis of US claims data. Hum Reprod. 2021;36:785–93.
Obst KL, Due C, Oxlad M, Middleton P. Men’s grief following pregnancy loss and neonatal loss: a systematic review and emerging theoretical model. BMC Pregnancy Childbirth. 2020;20:1–17.
Obst KL, Due C, Oxlad M, Middleton P. Men’s grief following pregnancy loss and neonatal loss: a systematic review and emerging theoretical model. BMC Pregnancy Childbirth. 2020;20:11.
Ghanei Gheshlagh R, Parizad N, Sayehmiri K. The Relationship Between Depression and Metabolic Syndrome: Systematic Review and Meta-Analysis Study. Iran Red Crescent Med J. 2016;18:e26523–e26523.
Balhara YPS. Diabetes and psychiatric disorders. Indian journal of endocrinology and metabolism. 2011;15:274–83.
Regitz-Zagrosek V, Lehmkuhl E, Weickert MO. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol. 2006;95:147–147.
Pradhan AD. Sex differences in the metabolic syndrome: implications for cardiovascular health in women. Clin Chem. 2014;60:44–52.
Tramunt B, Smati S, Grandgeorge N, Lenfant F, Arnal J-F, Montagner A, Gourdy P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63:453–61.
Shih D-P, Wen C-T, Kuo H-W, Liang W-M, Liu L-F, Su C-T, Wang J-Y. Spousal Concordance in Dietary Behaviors and Metabolic Components, and Their Association: A Cross-Sectional Study. Nutrients. 2020;12:3332.
Wang P-X, Deng X-R, Zhang C-H, Yuan H-J. Gut microbiota and metabolic syndrome. Chin Med J. 2020;133:808.
Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19:55–71.
Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas M-E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome medicine. 2016;8:1–12.
Amir M, Brown JA, Rager SL, Sanidad KZ, Ananthanarayanan A, Zeng MY. Maternal Microbiome and Infections in Pregnancy. Microorganisms. 1996;2020:8.
Dill-McFarland KA, Tang Z-Z, Kemis JH, Kerby RL, Chen G, Palloni A, Sorenson T, Rey FE, Herd P. Close social relationships correlate with human gut microbiota composition. Sci Rep. 2019;9:1–10.
Westby CL, Erlandsen AR, Nilsen SA, Visted E, Thimm JC. Depression, anxiety, PTSD, and OCD after stillbirth: a systematic review. BMC Pregnancy Childbirth. 2021;21:782.
Lang A, Fleiszer AR, Duhamel F, Sword W, Gilbert KR, Corsini-Munt S. Perinatal loss and parental grief: the challenge of ambiguity and disenfranchised grief. Omega (Westport). 2011;63:183–96.
Grauerholz KR, Berry SN, Capuano RM, Early JM. Uncovering Prolonged Grief Reactions Subsequent to a Reproductive Loss: Implications for the Primary Care Provider. Front Psychol. 2021;12:673050–673050.
Marazziti D, Rutigliano G, Baroni S, Landi P, Dell’Osso L. Metabolic syndrome and major depression. CNS Spectr. 2014;19:293–304.
Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis G-A, Vogiatzi G, Papaioannou S, Deftereos S, Tousoulis D. The role of inflammation in diabetes: current concepts and future perspectives. European Cardiology Review. 2019;14:50.
Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. 2017;11:215–25.
Akash MSH, Rehman K, Chen S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J Cell Biochem. 2013;114:525–31.
Timm A, Nielsen KK, Christensen U, Maindal HT. Healthcare Professionals’ Perspectives on the Cross-Sectoral Treatment Pathway for Women with Gestational Diabetes during and after Pregnancy—A Qualitative Study. J Clin Med. 2021;10:843.
Acknowledgements
We thank all staff of TLGS for data collection and entering data into the database.
Funding
No.
Author information
Authors and Affiliations
Contributions
MR and MSG, conceived the study plan. MR, performed the analyses. MSG, MR, FRT, FA wrote the manuscript. MR, MSG, FRT, FA contributed to the final editing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participants provided written informed consent. Ethical approval was obtained from the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Consent for publication
All authors assured that the manuscript is an original work, has not been previously published whole or in part, and is not under consideration for publication elsewhere.
Competing interests
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rahmati, M., Saei Ghare Naz, M., Azizi, F. et al. Pregnancy loss and subsequent risk of prediabetes, diabetes and metabolic syndrome in couples: Tehran lipid and glucose study. J Transl Med 20, 372 (2022). https://doi.org/10.1186/s12967-022-03578-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-022-03578-2