Abstract
Background
Schizophrenia is a widespread and debilitating mental disorder. However, the underlying molecular mechanism of schizophrenia remains largely unknown and no objective laboratory tests are available to diagnose this disorder. The aim of the present study was to characterize the alternations of glucose metabolites and identify potential diagnostic biomarkers for schizophrenia.
Methods
Gas chromatography/mass spectrometry based targeted metabolomic method was used to quantify the levels of 13 glucose metabolites in peripheral blood mononuclear cells (PBMCs) derived from healthy controls, schizophrenia and major depression subjects (n = 55 for each group).
Results
The majority (84.6%) of glucose metabolites were significantly disturbed in schizophrenia subjects, while only two (15.4%) glucose metabolites were differently expressed in depression subjects relative to healthy controls in both training set (n = 35/group) and test set (n = 20/group). Antipsychotics had only a subtle effect on glucose metabolism pathway. Moreover, ribose 5-phosphate in PBMCs showed a high diagnostic performance for first-episode drug-naïve schizophrenia subjects.
Conclusion
These findings suggested disturbance of glucose metabolism may be implicated in onset of schizophrenia and could aid in development of diagnostic tool for this disorder.
Similar content being viewed by others
Background
Schizophrenia is a multifaceted and devastating mental disorder affecting up to 1% of the general population [1]. It causes progressive functional decline, lifelong disability and tremendous socioeconomic burden for patients [2, 3]. Despite extensive researches into schizophrenia, the definite pathogenesis of schizophrenia remains largely unknown. Currently, diagnosis of schizophrenia primarily relies on subjective identification of symptom clusters by psychiatrist. However, this subjective diagnostic modality results in a considerable error rate due to the complex spectrum of symptoms and their similarities to other mental disorders [4]. In light of these facts, it is greatly needed to explore the molecular mechanism and develop objective diagnostic biomarkers for schizophrenia.
Metabolites represent the final product of interactions among various factors including genetic, physiological and environmental factors, and may better reflect the functional status of individuals. Quantifiable differences in metabolite patterns provide valuable clues to uncover the mechanism and develop diagnostic biomarkers for psychiatry disorders [5, 6]. Recently, our group has characterized metabolite signatures associated with stress resilience and suicide [7, 8]. Besides, we have identified diagnostic metabolite biomarkers for major depression and bipolar disorder [9, 10].
As to schizophrenia, early studies have identified disturbances of several metabolic pathways, such as oxidative stress, lipid and amino acid metabolism [11–13]. Specially, it was repeatedly reported that schizophrenia patients showed disturbance of several glucose metabolite intermediates in periphery and brain [14–16]. Transcriptomics and proteomics studies also displayed abnormal expression of glucose metabolism-related enzymes [17, 18]. Moreover, glucose regulation is related to verbal declarative memory performance in schizophrenia patients [19]. These scattered evidences indicated glucose metabolic pathway may be disturbed in schizophrenia. However, currently there has not been a comprehensive global evaluation of glucose metabolic pathway in schizophrenia. More importantly, it remains unclear whether and how specific changes of glucose metabolism might account for schizophrenia.
Brain tissues and cerebrospinal fluid seem to be the most suitable biological samples for schizophrenia research. However, brain tissue biopsy and lumbar puncture cannot be practically applied in schizophrenia patients due to ethical and safety constraints. In contrast, peripheral blood mononuclear cells (PBMCs) can be easily collected at minimal risk and cost and allow to capture the freshly isolated systemic cellular reactivity in the early phases of the disorder [20]. Besides, PBMCs may reflect molecular processes in the central nervous system as the brain and PBMCs show a number of parallel responses [21–23]. Moreover, peripheral immune dysfunction was widely implicated in the pathogenesis of schizophrenia [24, 25]. Significantly, previous studies have indicated that such cells are useful peripheral sources to identify the underlying pathogenic mechanisms and diagnostic markers for other psychiatric disorders such as autism, depression and borderline personality disorder [26–28]. Considering the above information, PBMCs were thus chosen as the sample source in this study.
Here, using a gas chromatography/mass spectrometry (GC/MS) based targeted metabolomic method, we simultaneously quantified the levels of 13 glucose metabolites in PBMCs derived from schizophrenia, major depression and healthy control groups (n = 55 for each group). Our aim was to characterize how the glucose metabolites change in the early stage of schizophrenia, and identify the potential diagnostic PBMC metabolite biomarkers for schizophrenia. Besides, we sought to determine whether antipsychotic drugs effect on glucose metabolism pathway.
Methods
Ethics statement
Prior to sample collection, written informed consents were obtained from all recruited subjects. The protocols of this study were reviewed and approved by the Ethical Committee of Chongqing Medical University. All procedures were conducted according to the principles expressed in the Declaration of Helsinki.
Participants
Totally, 55 schizophrenia and 55 major depression subjects were recruited from the psychiatric center in the First Affiliated Hospital of Chongqing Medical University. All diagnoses were performed according to the Structured Psychiatric Interview using the DSM-IV-TR criteria [29]. Candidates with pre-existing physical or other mental disorders, or illicit drug use were excluded. During the same time period, 55 healthy controls were enrolled from the medical examination center in the First Affiliated Hospital of Chongqing Medical University. Healthy controls were required to have no current or previous lifetime history of neurological, DSM-IV Axis I/II, or systemic medical illness.
The recruited samples were divided into a training set and a test set. The training set, comprising healthy controls, first-episode drug-naïve schizophrenia and major depression subjects (n = 35/group), was used to characterize the unique glucose metabolic pattern and identify the potential diagnostic markers for schizophrenia. The test set, comprising 20 schizophrenia subjects, 20 major depression subjects and 20 healthy controls, was used to independently validate the identified glucose metabolic pattern of schizophrenia and assess the effect of antipsychotics on glucose metabolites.
Sample preparation and GC/MS acquisition
Fasting blood samples were collected into EDTA-coated tubes and were centrifuged at 3,000 rpm for 15 min at 4°C to obtain plasma. Subsequently plasm was overlaid onto Ficoll-Paque Plus (GE Healthcare Bio-sciences AB, Sweden), and centrifuged at 2,000 rpm for 20 min at room temperature. The purified PBMCs were harvested and washed three times in phosphate buffer saline. After that, each PBMC sample was divided into equal aliquots, transferred into liquid nitrogen for 3 min and then stored at −80°C until undergoing later analysis.
For GC–MS analysis, each aliquot of PBMCs, which was isolated from 5 ml blood sample, was added with a 400 μL solution of water–methanol–chloroform (2:5:2, v/v/v). After vortexing for 30 s and standing overnight at −20°C, the mixture was sonicated for 3 min and subsequently centrifuged at 14,000g for 10 min at 4°C. 300 μL supernatant was collected, and the residue was resuspended in 200 μL methanol. The residue was then vortexed and centrifugated as before. 200 μL supernatant of the residue was extracted and mixed with the first. A 250 μL aliquot of mixed supernatant was evaporated to dryness under a stream of nitrogen gas. The dried metabolic extract was derivatized first with 30 µL of methoxamine (20 mg/mL) for 90 min at 37°C with continuous shaking. Subsequently, 30 µL of BSTFA with 1% TCMS was added to the mixture and heated for 1 h at 70°C to form trimethylsilyl (TMS) derivatives. After derivatization and cooling to room temperature, 1 μL of this derivative was injected in the GC/MS for analysis.
GC/MS analysis
GC/MS analysis was carried out according to this group’s previously published work [7, 30]. Briefly, each 1 μL of the derived sample was injected into an Agilent 7890A GC system (Agilent Technologies Inc., USA). An HP-5 MS fused silica capillary column (30 m × 0.25 mm × 0.25 μm, Agilent, USA) was used for metabolite separation with helium carrier gas at a flow rate of 1 mL/min. The injector temperature was set at 280°C. The column temperature was initially kept at 80°C for 2 min and then increased to 320°C at 10°C/min, where it was held for 6 min. The column effluent was introduced into the ion source of an Agilent 5975 mass selective detector (Agilent Technologies). The MS quadrupole temperature was set at 150°C, and the ion source temperature was set at 230°C. Data acquisition was performed first in the full-scan mode (scanning range from 50 to 550 m/z) and then in selected ion monitoring (SIM) mode for quantification. The characteristic fragment ions and retention times of metabolites were shown in Additional file 1: Table S1. All samples were analyzed consecutively at random. A quality control (QC) sample, pooled from a representative PBMCs sample of each group, was added in each batch of analyses in order to adjust the variations between batch variability.
Targeted metabolomic data analysis
Mass spectral data were converted to NetCDF format and then processed by XCMS software for peak finding, integration and alignment. The optimized XCMS parameters were set as follows: method = “matchedFilter”; full width at half maximum (fwhm) = 4.0; signal-to-noise cutoff (snthresh) = 10.0; retention time window (bw) = 3. Each metabolite concentration was expressed in relative abundance (metabolite peak area of study sample divided by that of QC sample) before the following statistical analysis.
Identification of PBMC metabolite biomarkers for schizophrenia
As clinical diagnosis based on the quantification of a small number of metabolites would be more practical, a stepwise optimization algorithm based on Akaike’s information criterion (AIC) was applied to optimize the metabolite biomarker combination [9, 10]. To evaluate the diagnostic generalizability of the schizophrenia biomarker, the ability of the simplified biomarker panel to discriminate schizophrenia subjects from non-schizophrenia subjects was quantified using a receiver-operating characteristic (ROC) curve analysis [31].
Statistical analysis
The Chi square test was applied to analyze categorical data (sex). All continuous variables such as age, BMI and metabolite concentrations were analyzed using one-way ANOVA followed by Bonferroni post hoc test. All continuous variables were expressed as means ± standard errors of the mean. The statistical analyses were carried out with SPSS software (version 17.0). A p value of less than 0.05 was considered to be statistically significant.
Results
Demographic and clinical characteristics
The detailed demographic and clinical characteristics of the recruited subjects are presented in Table 1. The demographic parameters such as age, gender and BMI were not distinguishable among these three groups in both training set and test set.
Characterization of differentially expressed metabolites between schizophrenia subjects and healthy controls
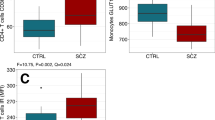
Using GC–MS approach, we measured 13 key metabolites of glucose metabolism pathways in PBMCs. In training set, 11 metabolites (84.6%) were differentially expressed in first-episode drug-naïve schizophrenia subjects relative to healthy controls (Table 2; Figure 1). Thereinto, seven metabolites were significantly increased in schizophrenia subjects relative to healthy controls, including glucose, glucose 6-phosphate, fructose, fructose 6-phosphate, glycerate 3-phosphate, succinic acid and ribose 5-phosphate. Moreover, four metabolites were significantly decreased in schizophrenia subjects relative to healthy controls, including glyceraldehyde-3-phosphate, dihydroxyacetone phosphate, glycerol 3-phosphate and citric acid. The remaining metabolites, pyruvate and lactic acid, did not differ between these two groups.
Differential metabolites and metabolic enzymes involved in glucose metabolism. All these metabolites were compared among three groups in training set. Red-colored boxes indicate increased metabolites in schizophrenia or major depression subjects compared to healthy controls, while green-colored boxes indicate decreased metabolites; Red letters indicate upregulated metabolic enzymes in PBMCs of schizophrenia subjects as previous proteomic study reported [18]. ALDOC aldolase C, TPIS triosephosphate isomerase, GAPDH glyceraldehydes-3-phosphate dehydrogenase, PGK1 phosphoglycerate kinase 1, PGAM2 phosphoglycerate mutase 2, PKL pyruvate kinase, LDHB lactate dehydrogenase B.
Additionally, the results aforementioned could be replicated in independent test set except for four metabolites, including glycerate 3-phosphate, pyruvate, lactic acid and ribose 5-phosphate (Table 2). In detail, compared to healthy controls, levels of glycerate 3-phosphate and ribose 5-phosphate in schizophrenia subjects were significantly higher in training set, but did not differ in test set. On the contrary, relative to healthy controls, levels of pyruvate and lactic acid in schizophrenia subjects were not distinguishable in training set, but were significantly differently expressed in test set.
To test whether antipsychotics influenced glucose metabolism, we compared levels of glucose metabolites between unmedicated and medicated schizophrenia subjects in test set (Additional file 1: Table S2). Of these 13 metabolites, only ribose 5-phosphate were differently expressed between unmedicated and medicated schizophrenia subjects. These findings demonstrated that antipsychotics may not significantly influence glucose metabolism in individuals with episode schizophrenia.
Characterization of differentially expressed metabolites between depression subjects and healthy controls
In opposite to schizophrenia, only 2 of 13 (15.4%) glucose metabolites were differently expressed between depression subjects and healthy controls in both training set and test set (Table 3; Figure 1). The majority of results were consistent in both sets. Specifically, fructose 6-phosphate was significantly increased in depression subjects relative to healthy controls in training set, but were not distinguishable between these two groups in test set. Compared to healthy controls, dihydroxyacetone phosphate in depression subjects were significantly increased in training set, but decreased in test set. Moreover, compared to healthy controls, levels of succinic acid in depression subjects were not distinguishable in training set, but were significantly increased in test set.
Characterization of differentially expressed metabolites between schizophrenia and depression subjects
In training set, 11 of 13 glucose metabolites were differently expressed between schizophrenia and depression subjects (Table 4). Coincidentally, these 11 altered metabolites were also differently expressed between schizophrenia subjects and healthy controls in training set.
In test set, only five metabolites were consistently differently expressed between schizophrenia and depression subjects (Table 4). However, the changes of other five metabolites (glucose, fructose, glyceraldehyde-3-phosphate, dihydroxyacetone phosphate and succinic acid) in test set showed the same trend as those in training set, although did not reach statistical significance.
These results demonstrated the glucose metabolic signature of schizophrenia subjects in PBMCs was significantly distinguishable from that of non-schizophrenia subjects.
A potential diagnostic biomarker for schizophrenia
Although unique glucose metabolic pattern provided great potential for diagnosis of schizophrenia, measurements of all glucose metabolites were not convenient and economical in clinical. To identify a simplified diagnostic biomarker for schizophrenia, a step-wise optimization algorithm based on AIC was applied. The statistical analysis demonstrated that ribose 5-phosphate which involved in pentose phosphate pathway showed the highest diagnostic performance in identifying first-episode drug-naïve schizophrenia subjects in training set. The area under ROC curve (AUC) was 1.00 in discriminating schizophrenia subjects from healthy controls or major depression subjects in training set. As an AUC of 1 indicates perfect discrimination, these results demonstrated that ribose 5-phosphate in PBMCs may be a potential biomarker for first-episode drug-naïve schizophrenia subjects.
We found that ribose 5-phosphate was consistently increased in unmedicated schizophrenia subjects relative to healthy controls in both training set and test set. However, levels of ribose 5-phosphate in medicated schizophrenia subjects was significantly lower than healthy controls in test set (Additional file 1: Table S2). This findings indicated that antipsychotics could reduce the diagnostic performance of ribose 5-phosphate for schizophrenia.
Discussion
Schizophrenia is a widespread and debilitating mental disorder. Current diagnosis of schizophrenia remains subjective, and the underlying molecular mechanisms remains largely unknown. Here, we found that majority of glucose metabolites in PBMCs were disturbed in schizophrenia subjects, which was in contrast to the findings of depression subjects. Furthermore, ribose 5-phosphate showed the highest diagnostic performance for first-episode drug-naïve schizophrenia subjects among the identified glucose metabolites. These results suggest that disturbance of glucose metabolism pathway may be implicated in the onset of schizophrenia, and may have utility in diagnosis of schizophrenia.
Antipsychotic medication and demographic factors (such as age, sex, obesity) were considered as possible confounders affecting the glucose metabolism [32, 33]. We circumvented this problem by carrying out analyses of PBMCs samples from first-episode drug-naïve schizophrenia subjects and demographic-matched controls. Hence, in this study, the glucose metabolism pattern of schizophrenia subjects might truly reflect the pathophysiologic changes inherent in the schizophrenia disease state. Consistent with these findings, several studies have also revealed poor glucose regulation and abnormal expressions of glucose metabolism related molecules in unmedicated schizophrenia subjects [12, 18, 20]. Different from previous studies, we found that antipsychotics had only a subtle effect on glucose metabolites in PBMCs except ribose 5-phosphate in this study. The discrepancy with our results may be due to the more stable microenvironment in PBMCs than peripheral body fluids and antipsychotic drug-sensitive tissues.
Glucose is mainly metabolized via the glycolytic pathway and TCA cycle to provide energy and biosynthetic intermediates. Glucose metabolism homeostasis is fundamental to maintain normal brain functions. In this study, of the 12 metabolites relevant to glycolysis and TCA cycle, 50% metabolites were increased while only 33.3% metabolites were decreased in first-episode drug-naïve schizophrenia subjects compared to healthy controls. In concordance with this finding, a recent proteomics study reported that majority (87.5%) of glucose metabolic enzymes in PBMCs were significantly elevated in first-episode drug-naïve schizophrenia subjects relative to healthy controls [18]. Besides, several glucose metabolic enzymes such as aldolase C, triosephosphate isomerase, glyceraldehydes-3-phosphate dehydrogenase, phosphoglycerate kinase 1, phosphoglycerate mutase were consistently elevated in both brain tissues and PBMCs of schizophrenia as described in previous studies [16, 34–37]. Combined with these evidences, we deduced that glycolysis pathway and TCA cycle may be activated in the early stage of schizophrenia onset. Other groups have also reported higher levels of ATP and increased gene/protein expression associated with glucose metabolism in schizophrenia patients [16, 38, 39], further supporting our findings.
Pentose phosphate pathway is another important branch of glucose metabolism that functions in the formation of NADPH for biosynthetic processes and cellular redox balance. Ribose 5-phosphate is the main product of this pathway. Here, ribose 5-phosphate was increased in PBMCs of first-episode drug-naïve schizophrenia subjects compared to healthy controls, suggesting disturbance of pentose phosphate pathway may play an important role in the onset of schizophrenia. In agreement with this speculation, previous studies have also reported increased expressions of pentose phosphate pathway related molecules in schizophrenia [36, 40].
As disturbances of glucose metabolism have also been observed in other psychiatry disorders, such as major depression. The question then arises whether disturbance of glucose metabolism observed in this study reflects schizophrenia-specific pathophysiological changes. To elucidate this question, we also recruited first-episode drug-naive major depression subjects that shared a number of factors in common with schizophrenia subjects. We found that only few kinds of glucose metabolites were differentially expressed in major depression subjects compared to healthy controls, reflecting subtle alterations of glucose metabolism pathways in this disorder. The results contrast with those of previous studies that reported significant disturbances of glucose metabolism in major depression subjects [41, 42]. The discrepancy may be caused by the differences in sample type, study population, antidepressant treatment and detected components. Here, we found that majority of glucose metabolites were distinguishable between major depression and schizophrenia subjects. The results reflected that distinct, rather than generalized, glucose metabolic changes exist in PBMCs of schizophrenia.
Certain limitations of this study should be noted. Firstly, the sample size of first-episode drug-naïve schizophrenia subjects used in this study was relatively small. This was limited by the fact that large clinical centers (~1,500 patients) recruit only 10–20 antipsychotic-naive patients who are free of substance abuse or comorbidities per year. Further studies with larger cohorts should be performed to validate these findings, especially with regard to the diagnostic performance of ribose 5-phosphate in schizophrenia. Secondly, all subjects were of the same ethnicity and were recruited from the same site. Thus, ethno- and site-specific biases cannot be ruled out. Further studies involving heterogeneous populations from multiple clinical sites are required.
Conclusion
In this study, we provide evidence that severe disturbance of glucose metabolism in PBMCs may be implicated in the onset of schizophrenia. Moreover, ribose 5-phosphate showed a high diagnostic performance for first-episode drug-naïve schizophrenia subjects. These findings will be valuable in uncovering the pathogenesis of schizophrenia and facilitate to develop diagnostic tools for this disorder.
References
Saha S, Chant D, Welham J, McGrath J (2005) A systematic review of the prevalence of schizophrenia. PLoS Med 2:e141
Gutiérrez-Maldonado J, Caqueo-Urízar A, Kavanagh DJ (2005) Burden of care and general health in families of patients with schizophrenia. Soc Psychiatry Psychiatr Epidemiol 40:899–904
Rössler W, Salize HJ, van Os J, Riecher-Rössler A (2005) Size of burden of schizophrenia and psychotic disorders. Eur Neuropsychopharmacol 15:399–409
Keller WR, Fischer BA, Carpenter WT Jr (2011) Revisiting the diagnosis of schizophrenia: where have we been and where are we going? CNS Neurosci Ther 17:83–88
Lan M, McLoughlin G, Griffin J, Tsang T, Huang J, Yuan P et al (2009) Metabonomic analysis identifies molecular changes associated with the pathophysiology and drug treatment of bipolar disorder. Mol Psychiatry 14:269–279
Quinones MP, Kaddurah-Daouk R (2009) Metabolomics tools for identifying biomarkers for neuropsychiatric diseases. Neurobiol Dis 35:165–176
Shao WH, Fan SH, Lei Y, Yao GE, Chen JJ, Zhou J et al (2013) Metabolomic identification of molecular changes associated with stress resilience in the chronic mild stress rat model of depression. Metabolomics 9:433–443
Zheng P, Gao HC, Qi ZG, Jia JM, Li FF, Chen JJ et al (2013) Peripheral metabolic abnormalities of lipids and amino acids implicated in increased risk of suicidal behavior in major depressive disorder. Metabolomics 9:688–696
Zheng P, Wang Y, Chen L, Yang D, Meng H, Zhou D et al (2013) Identification and validation of urinary metabolite biomarkers for major depressive disorder. Mol Cell Proteomics 12:207–214
Zheng P, Wei YD, Yao GE, Ren GP, Guo J, Zhou CJ et al (2013) Novel urinary biomarkers for diagnosing bipolar disorder. Metabolomics 9:800–808
Bitanihirwe BK, Woo TUW (2011) Oxidative stress in schizophrenia: an integrated approach. Neurosci Biobehav Rev 35:878–893
Wu X, Huang Z, Wu R, Zhong Z, Wei Q, Wang H et al (2013) The comparison of glycometabolism parameters and lipid profiles between drug-naive, first-episode schizophrenia patients and healthy controls. Schizophr Res 150:157–162
Lee M, Jayathilake K, Dai J, Meltzer HY (2011) Decreased plasma tryptophan and tryptophan/large neutral amino acid ratio in patients with neuroleptic-resistant schizophrenia: relationship to plasma cortisol concentration. Psychiatry Res 185:328–333
Holmes E, Tsang TM, Huang JTJ, Leweke FM, Koethe D, Gerth CW et al (2006) Metabolic profiling of CSF: evidence that early intervention may impact on disease progression and outcome in schizophrenia. PLoS Med 3:e327
Xuan J, Pan G, Qiu Y, Yang L, Su M, Liu Y et al (2011) Metabolomic profiling to identify potential serum biomarkers for schizophrenia and risperidone action. J Proteome Res 10:5433–5443
Martins-de-Souza D, Maccarrone G, Wobrock T, Zerr I, Gormanns P, Reckow S et al (2010) Proteome analysis of the thalamus and cerebrospinal fluid reveals glycolysis dysfunction and potential biomarkers candidates for schizophrenia. J Psychiatr Res 44:1176–1189
Olsen L, Hansen T, Jakobsen KD, Djurovic S, Melle I, Agartz I et al (2008) The estrogen hypothesis of schizophrenia implicates glucose metabolism: association study in three independent samples. BMC Med Genet 9:39
Herberth M, Koethe D, Cheng T, Krzyszton N, Schoeffmann S, Guest P et al (2011) Impaired glycolytic response in peripheral blood mononuclear cells of first-onset antipsychotic-naive schizophrenia patients. Mol Psychiatry 16:848–859
Fucetola R, Newcomer JW, Craft S, Melson AK (1999) Age-and dose-dependent glucose-induced increases in memory and attention in schizophrenia. Psychiatry Res 88:1–13
van Beveren NJ, Buitendijk GH, Swagemakers S, Krab LC, Röder C, de Haan L et al (2012) Marked reduction of AKT1 expression and deregulation of AKT1-associated pathways in peripheral blood mononuclear cells of schizophrenia patients. PLoS One 7:e32618
Rollins B, Martin MV, Morgan L, Vawter MP (2010) Analysis of whole genome biomarker expression in blood and brain. Am J Med Genet Part B Neuropsychiatr Genet 153:919–936
van Heerden JH, Conesa A, Stein DJ, Montaner D, Russell V, Illing N (2009) Parallel changes in gene expression in peripheral blood mononuclear cells and the brain after maternal separation in the mouse. BMC Res Notes 2:195
Li SX, Liu LJ, Jiang WG, Lu L (2009) Morphine withdrawal produces circadian rhythm alterations of clock genes in mesolimbic brain areas and peripheral blood mononuclear cells in rats. J Neurochem 109:1668–1679
Gardiner EJ, Cairns MJ, Liu B, Beveridge NJ, Carr V, Kelly B et al (2013) Gene expression analysis reveals schizophrenia-associated dysregulation of immune pathways in peripheral blood mononuclear cells. J Psychiatr Res 47:425–437
Maxeiner H-G, Rojewski MT, Schmitt A, Tumani H, Bechter K, Schmitt M (2009) Flow cytometric analysis of T cell subsets in paired samples of cerebrospinal fluid and peripheral blood from patients with neurological and psychiatric disorders. Brain Behav Immun 23:134–142
Jyonouchi H, Geng L, Streck DL, Toruner GA (2011) Children with autism spectrum disorders (ASD) who exhibit chronic gastrointestinal (GI) symptoms and marked fluctuation of behavioral symptoms exhibit distinct innate immune abnormalities and transcriptional profiles of peripheral blood (PB) monocytes. J Neuroimmunol 238:73–80
Segman R, Goltser-Dubner T, Weiner I, Canetti L, Galili-Weisstub E, Milwidsky A et al (2010) Blood mononuclear cell gene expression signature of postpartum depression. Mol Psychiatry 15:93–100
Díaz-Marsá M, MacDowell KS, Guemes I, Rubio V, Carrasco JL, Leza JC (2012) Activation of the cholinergic anti-inflammatory system in peripheral blood mononuclear cells from patients with borderline personality disorder. J Psychiatr Res 46:1610–1617
American Psychiatry Association (2000) Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatry Association, Washington, DC, p 371
Xu XJ, Zheng P, Ren G-P, Liu ML, Mu J, Guo J et al (2014) 2, 4-Dihydroxypyrimidine is a potential urinary metabolite biomarker for diagnosing bipolar disorder. Mol BioSyst 10:813–819
Bradley AP (1997) The use of the area under the ROC curve in the evaluation of machine learning algorithms. Pattern Recogn 30:1145–1159
Basu R, Dalla Man C, Campioni M, Basu A, Klee G, Toffolo G et al (2006) Effects of age and sex on postprandial glucose metabolism differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes 55:2001–2014
Power C, Thomas C (2011) Changes in BMI, duration of overweight and obesity, and glucose metabolism: 45 years of follow-up of a birth cohort. Diabetes Care 34:1986–1991
Johnston-Wilson N, Sims C, Hofmann J, Anderson L, Shore A, Torrey E et al (2000) Disease-specific alterations in frontal cortex brain proteins in schizophrenia, bipolar disorder, and major depressive disorder. Mol Psychiatry 5:142–149
Pennington K, Dicker P, Dunn MJ, Cotter DR (2008) Proteomic analysis reveals protein changes within layer 2 of the insular cortex in schizophrenia. Proteomics 8:5097–5107
Martins-de-Souza D, Gattaz WF, Schmitt A, Maccarrone G, Hunyadi-Gulyás E, Eberlin MN et al (2009) Proteomic analysis of dorsolateral prefrontal cortex indicates the involvement of cytoskeleton, oligodendrocyte, energy metabolism and new potential markers in schizophrenia. J Psychiatr Res 43:978–986
Martins-de-Souza D, Gattaz WF, Schmitt A, Novello JC, Marangoni S, Turck CW et al (2009) Proteome analysis of schizophrenia patients Wernicke’s area reveals an energy metabolism dysregulation. BMC Psychiatry 9:17
Jensen JE, Miller J, Williamson PC, Neufeld RW, Menon RS, Malla A et al (2006) Grey and white matter differences in brain energy metabolism in first episode schizophrenia: 31 P-MRS chemical shift imaging at 4 Tesla. Psychiatry Res Neuroimaging 146:127–135
Kuzman MR, Medved V, Terzic J, Krainc D (2009) Genome-wide expression analysis of peripheral blood identifies candidate biomarkers for schizophrenia. J Psychiatr Res 43:1073–1077
Kumarasinghe N, Beveridge NJ, Gardiner E, Scott RJ, Yasawardene S, Perera A et al (2013) Gene expression profiling in treatment-naive schizophrenia patients identifies abnormalities in biological pathways involving AKT1 that are corrected by antipsychotic medication. Int J Neuropsychopharmacol 16:1483–1503
Martins-de-Souza D, Guest P, Harris L, Vanattou-Saifoudine N, Webster M, Rahmoune H et al (2012) Identification of proteomic signatures associated with depression and psychotic depression in post-mortem brains from major depression patients. Transl Psychiatry 2:e87
Beasley CL, Pennington K, Behan A, Wait R, Dunn MJ, Cotter D (2006) Proteomic analysis of the anterior cingulate cortex in the major psychiatric disorders: evidence for disease-associated changes. Proteomics 6:3414–3425
Authors’ contributions
Authors PX, M-LL and X-TZ designed the study and revised this manuscript. Authors M-LL, X-YD, ZF and ZL performed the experiments and wrote the draft of manuscript. Authors YX, PZ, X-JX, P-FC, TH, S-JB, L-BZ, Z-GQ and W-HS collected the clinical samples. All authors read and approved the final manuscript.
Acknowledgements
Our sincere gratitude is extended to Delan Yang, and Hua Hu for their efforts in sample collection. We also thank Dr. N.D. Melgiri for editing and proofreading the manuscript. This work was supported by the Natural Science Foundation Project of China (CSTC, 31300881), the Chongqing Postdoctoral Research Project (Grant No. xm201101003) and the National Basic Research Program of China (973 Program) (Grant No. 2009CB918300).
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mei-Ling Liu, Xiao-Tong Zhang and Xiang-Yu Du contributed equally to this work
Additional file
Additional file 1.
The characteristics of glucose metabolites and its relation to antipsychotic use.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, ML., Zhang, XT., Du, XY. et al. Severe disturbance of glucose metabolism in peripheral blood mononuclear cells of schizophrenia patients: a targeted metabolomic study. J Transl Med 13, 226 (2015). https://doi.org/10.1186/s12967-015-0540-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-015-0540-y