Abstract
Background
There are no nationally representative population-based studies investigating the relationship between physical activity, chronic conditions and multimorbidity (i.e., two or more chronic conditions) in low- and middle-income countries (LMICs), and studies on a multi-national level are lacking. This is an important research gap, given the rapid increase in the prevalence of chronic diseases associated with lifestyle changes in these countries. This cross-sectional study aimed to assess the association between chronic conditions, multimorbidity and low physical activity (PA) among community-dwelling adults in 46 LMICs, and explore the mediators of these relationships.
Methods
World Health Survey data included 228,024 adults aged ≥18 years from 46 LMICs. PA was assessed by the International Physical Activity Questionnaire (IPAQ). Nine chronic physical conditions (chronic back pain, angina, arthritis, asthma, diabetes, hearing problems, tuberculosis, visual impairment and edentulism) were assessed. Multivariable logistic regression and mediation analyses were used to assess the association between chronic conditions or multimorbidity and low PA.
Results
Overall, in the multivariable analysis, arthritis (OR = 1.12), asthma (1.19), diabetes (OR = 1.33), edentulism (OR = 1.46), hearing problems (OR = 1.90), tuberculosis (OR = 1.24), visual impairment (OR = 2.29), multimorbidity (OR = 1.31; 95% CI = 1.21–1.42) were significantly associated with low PA. More significant associations were observed in individuals aged ≥50 years. In older adults, depression mediated between 5.1% (visual impairment) to 23.5% (angina) of the association between a chronic condition and low PA. Mobility difficulties explained more than 25% of the association for seven of the eight chronic conditions. Pain was a strong mediator for angina (65.9%) and arthritis (64.9%), while sleep problems mediated up to 43.7% (angina) of the association.
Conclusions
In LMICs, those with chronic conditions and multimorbidity are significantly less physically active (especially older adults). Research on the efficacy and effectiveness of PA in the management of chronic diseases in LMICs is urgently needed. Targeted promotion of physical activity to populations in LMICs experiencing chronic conditions may ameliorate associated depression, mobility difficulties and pain that are themselves important barriers for initiating or adopting an active lifestyle.
Similar content being viewed by others
Background
While the average life expectancy is increasing worldwide, the number of years lived with disability with various chronic conditions is also rising [1, 2]. Of particular concern is the increasing global burden of angina [3], arthritis [4], asthma [5], chronic back pain [6], diabetes [7], oral diseases, such as edentulism [8], hearing problems [9], tuberculosis [10], and visual impairments [11], mainly due to population growth and aging of the worldwide population. There is also an increasing recognition that in the years to come, this disease burden and the loss of economic output associated with chronic diseases will be greatest in low- and middle-income countries (LMICs) [12].
Recently, more research has noted the burden of multimorbidity (i.e., two or more chronic conditions) [13]. In a meta-analysis [14] of 70,057,611 primary care patients in 12 countries, the prevalence of multimorbidity ranged from 12.9 to 95.1%. The prevalence of multimorbidity is increasing, mainly due to the growing incidence of chronic conditions and increasing life-expectancy [15], and it is undoubtedly one of the most significant challenges faced by global health care providers [16]. Multimorbidity is associated with a lower quality of life [17], increased health-care utilization and costs [18], and ultimately, higher risk for premature mortality [19]. The worldwide evolving disease burden [1], along with a growing understanding of multimorbidity and its risk factors [20], necessitates a continuum of care.
Within the multifaceted care of individuals with chronic disease and multimorbidity, the promotion of physical activity is extensively supported in the published literature [21]. Regular physical activity contributes to the primary and secondary prevention of a wide range of chronic diseases [21], improves quality of life [22] and is associated with reduced risk of premature death [23]. However, to date, most of the research investigating associations between physical activity, chronic diseases and multimorbidity has focused on high-income countries. For example, in a Spanish study [24] involving 22,190 adults, an inverse association was found between multimorbidity and levels of physical activity participation in the youngest and oldest age groups. In addition, both low self-rated health status and functional limitations were related to lower physical activity in most of the examined population groups. In an English nationally representative cohort of people aged ≥50 years (n = 15,688) [25], compared to the physically inactive group, the odds ratio (OR) for multimorbidity was 0.84 (95% confidence interval (CI) = 0.78–0.91) in the mild, 0.61 (95% CI = 0.56–0.66) in the moderate, and 0.45 (95% CI = 0.41–0.49) in the vigorous physical activity groups.
However, to the best of our knowledge, there are no nationally representative population-based studies investigating the associations between physical activity behavior, chronic conditions and multimorbidity in LMICs. Moreover, to the best of our knowledge there are no studies investigating physical activity and multimorbidity on a multi-national level. This is an important research gap given the rapid increase in chronic diseases in these countries, mainly due to changes in lifestyle [1]. Furthermore, the association between chronic conditions or multimorbidity on physical activity behavior may differ in LMICs due to different disease profiles [26], suboptimal treatment of chronic conditions [27, 28], differences in knowledge regarding the benefits of physical activity [29], or other environmental factors such as work conditions [30]. In addition, at the population level, there is a paucity of information on factors that might influence the relationship between physical activity, chronic diseases and multimorbidity. Such information could guide the design and delivery of targeted interventions.
Given the aforementioned gaps within the literature, we aimed to assess the association between chronic conditions or multimorbidity and low physical activity (i.e., not achieving international physical activity recommendations) among community-dwelling adults in 46 LMICs, and to assess the factors that might influence this relationship. We hypothesize that low physical activity is associated with the presence of chronic conditions and multimorbidity.
Methods
Settings and protocol
The World Health Survey (WHS) was a cross-sectional study undertaken in 2002–2004 in 70 countries worldwide. Single-stage random sampling and stratified multi-stage random cluster sampling were conducted in 10 and 60 countries respectively. The details of the survey have been provided elsewhere (http://www.who.int/healthinfo/survey/en/). Briefly, all those aged ≥18 years with a valid home address were eligible to participate. Each member of the household had equal probability of being selected with the use of Kish tables. The data were collected in all countries using the same set of questionnaires with some countries however using a shorter version. The individual response rate ranged from 63% (Israel) to 99% (Philippines) [31]. Ethical approval was obtained from ethical boards at each study site. Sampling weights were generated to adjust for non-response and the population distribution reported by the United Nations Statistical Division. Informed consent was obtained from all participants.
Physical activity
In order to assess if participants achieved the recommended physical activity levels of 150 min of moderate to vigorous physical activity per week [32], we used items from the International Physical Activity Questionnaire. Specifically, participants were asked how much over the past week on average they engaged in moderate and vigorous physical activity. Those scoring ≥150 min were classified as meeting the recommended guidelines and those scoring <150 min (low physical activity) were classified as not meeting the recommended guidelines.
Physical health conditions
A total of nine physical conditions were assessed, representing all physical conditions available in the WHS. Arthritis, asthma and diabetes were based on self-reported lifetime diagnosis. For angina, in addition to a self-reported diagnosis, a symptom-based diagnosis based on the Rose questionnaire was also used [33]. Chronic back pain was defined as having had back pain (including disc problems) every day during the last 30 days. Visual impairment was defined as having extreme difficulty in seeing and recognizing a person that the participant knows across the road (i.e., from a distance about 20 m) [34]. A validity study showed that this response generally corresponds to World Health Organization definitions of visual impairment [34]. The participant was considered to have hearing problems if the interviewer observed this condition at the end of the survey. Edentulism was assessed by the question “Have you lost all your natural teeth?” Those who responded affirmatively were considered to have edentulism. Finally, a tuberculosis diagnosis was based on past 12-month symptoms and was defined as: 1) having had a cough that lasted for three weeks or longer; and 2) having had blood in phlegm or coughed up blood [35]. In line with a previous publication using the same dataset [36], we calculated the total number of these conditions while allowing for one missing variable in order to retain a larger sample size. Multimorbidity was defined as having at least two of the assessed chronic conditions.
Health status and depression
Participants’ health status was evaluated with six health-related questions pertaining to three health domains including (a) mobility; (b) pain and discomfort; (c) sleep and energy (Additional file 1). These domains have been used as indicators of functional health status in prior studies utilizing the WHS dataset [37–39]. Each domain consists of two questions that assessed health function in the past 30 days. Each item was scored on a five-point scale ranging from ‘none’ to ‘extreme/cannot do’. For each separate domain, we used a factor analysis to obtain a factor score which was later converted to scores ranging from 0 to 100 [37, 39] with higher values representing worse health function. In order to determine the presence of depression, the DSM-IV algorithm was used, based on the duration and persistence of depressive symptoms in the previous 12 months [40].
Control variables
The control variables included sex, age, highest educational level achieved (no formal education, primary education, secondary or high school completed, or tertiary education completed) and wealth. Principal component analysis based on 15–20 assets was performed to establish country-wise wealth quintiles.
Statistical analysis
Data from 69 countries were publically available. Of these countries, 10 countries (Austria, Belgium, Denmark, Germany, Greece, Guatemala, Italy, Netherlands, Slovenia, UK) were deleted as sampling information was missing. Furthermore, 10 high-income countries (Finland, France, Ireland, Israel, Luxembourg, Norway, Portugal, Sweden, Spain, United Arab Emirates) were omitted as the focus of the study was on LMICs. Of the remaining LMICs, Morocco and Latvia were not included as they lacked information on physical activity, and Turkey was also excluded due to lack of several variables pertaining to the analysis. Thus, a total of 46 countries, which were all LMICs according to the World Bank classification in 2003, were included in the analysis [41] (see Table 1). We stratified the analyses by age (18–34, 35–49, 50–64, ≥65 years) as chronic conditions are known to be much more prevalent in the older population. Differences in sample characteristics by age group were evaluated by Chi-squared tests. Across all countries, we conducted multivariable logistic regression analysis to assess the association between chronic conditions (angina, arthritis, asthma, chronic back pain, diabetes, edentulism, hearing problem, tuberculosis, visual impairment) or multimorbidity (exposure variables) and low physical activity (outcome variable) while adjusting for age, sex, wealth, education and country. Each chronic condition and multimorbidity were included separately in the models. Furthermore, based on the results of these analyses, we conducted mediational analysis to evaluate underlying factors that may explain the link between chronic conditions or multimorbidity and low physical activities among those aged ≥50 years. We only included the older age group for this analysis as most of the significant association between chronic conditions (or multimorbidity) and low physical activity were only observed in the older age groups. We did not conduct this analysis for chronic back pain as this condition was not significantly associated with low physical activity in either of the older age groups.
Given that depression, mobility difficulties, pain/discomfort and sleep problems may be linked with chronic conditions as part of the symptomatology per se or the consequences of the symptoms [42], we investigated the mediating effect of these factors with the use of Karlson-Holm-Breen command in Stata [43]. This method can be applied to logistic regression models and decomposes the total effect into direct and indirect effects. Using this method, the mediated percentage (percentage of the main association explained by the mediator) can also be calculated. Each potential mediator was included in the models separately, and the models were adjusted for age, sex, education, wealth and country. For all analyses, adjustment for country was done by including dummy variables for each country as in previous WHS publications [37, 44]. The percentage of missing values for all the variables used in this study were <10% with the exception of the number of chronic conditions (10.9%), edentulism (12.2%), and tuberculosis (15.0%). Complete-case analysis was done. The sample weighting and the complex study design were taken into account in all analyses. Results from the logistic regression models are presented as odds ratios (ORs) with 95% confidence intervals (CIs). The level of statistical significance was set at P < 0.05. The statistical analysis was performed with Stata 14.1 (Stata Corp LP, College station, Texas).
Results
The final sample consisted of 228,024 individuals aged ≥18 years. Of these individuals, 97,841 (47.9%), 68,657 (27.5%), 37,688 (16.0%), and 23,838 (8.6%) were aged 18–34, 35–49, 50–64 and ≥65 years respectively. The prevalence of low physical activity in the overall sample was 29.2% (95% CI = 28.3–30.0%). The corresponding figures by age group were: 18–34 years [25.1% (24.2–26.1%)]; 35–49 years [25.5% (24.6–26.5%)]; 50–64 years [34.9% (33.5–36.3%)]; and ≥65 years [53.3% (51.4–55.2%)]. The overall prevalence of low physical activity ranged from 5.4% (Ethiopia) to 81.8% (Mauritania) (Table 1).
More than half of the population also engaged in low physical activities in South Africa (66.6%), Uruguay (63.9%), the Dominican Republic (58.9%), and Pakistan (51.1%). Older individuals were significantly more likely to be females, have lower education and wealth, chronic conditions and a higher number of chronic conditions (Table 2).
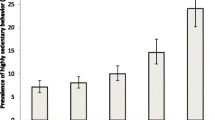
For most chronic conditions, the prevalence of low physical activity increased in a linear fashion with increasing age. There was a particularly high prevalence of low physical activity among those with visual impairment and diabetes especially among the older population (Fig. 1).
The results of the multivariable logistic regression analysis assessing the association between chronic conditions or multimorbidity and low physical activity are presented in Table 3. In the overall sample, arthritis, asthma, diabetes, edentulism, hearing problems, tuberculosis, visual impairment and multimorbidity were significantly associated with low physical activity. When the analysis was stratified by age groups, most significant associations only existed in the older age groups (i.e., ≥50 years). For example, the ORs (95% CIs) for the following conditions among those aged 50–64 years were: angina 1.17 (1.02–1.34); arthritis 1.28 (1.11–1.47); asthma 1.67 (1.33–2.10); diabetes 1.51 (1.24–1.85); hearing problem 1.69 (1.36–2.11); tuberculosis 1.57 (1.09–2.25); visual impairment 2.01 (1.40–2.88); and multimorbidity 1.38 (1.19–1.60).
The results of the mediation analysis among those aged ≥50 years are shown in Table 4. The indirect effect was significant in all analyses. For the individual chronic conditions, depression mediated 5.1% (visual impairment) to 23.5% (angina) of the association between the chronic condition and low physical activity. Mobility difficulties explained more than 25% of the association for seven out of the eight chronic conditions with particularly high mediated percentages observed for angina (95.3%) and arthritis (67.8%). Pain was a major mediator for angina (65.9%) and arthritis (64.9%), and sleep problems mediated between 5.9% (edentulism) to 43.7% (angina) of the association. In terms of multimorbidity, depression, mobility difficulties, pain and sleep mediated 12.5, 56.4, 38.7 and 21.6% of the association respectively.
Discussion
General findings
To the best of our knowledge, the current study is the first large-scale (n = 228,024), multinational (46 LMICs) analysis investigating chronic conditions, multimorbidity and low levels of physical activity. We found that most chronic conditions were associated with low physical activity in the overall sample, although this relationship was most notable among the older population. Our mediational analysis for the older age group showed that mobility difficulties was an important factor for most of the chronic conditions studied, while pain was a central factor for angina and arthritis. Depression and sleep problems also explained a large proportion of these associations, particularly for angina. As for multimorbidity, mobility difficulties and pain were important factors mediating low physical activity. The identification of mediators offers potential targets for future public health interventions and our study provides important information. For example, in the overall sample, those with arthritis were less likely to achieve the physical activity recommendations (OR = 1.12). This significant association was mediated by depressive feelings, pain, mobility and sleep problems. Arthritis is associated with pain which might cause mobility and sleep problems and ultimately feelings of depression, which in turn will be a barrier for physical activity participation [45]. The current data indicate that similar vicious cycles may also exist for other chronic conditions and multimorbidity.
Although there is no rigorous evidence that physical activity has a direct effect on the pathogenesis of arthritis [21], there is evidence that physical activity reduces pain [46] and depression [47] and improves sleep [48] and functionality [49] in people with arthritis. In terms of the training effect on mobility impairments, the immediate mechanism of action may be through improved balance, muscle strength and endurance [21]. If there is any sign of acute joint inflammation and/or a worsening of symptoms, the affected joint should rest until a drug treatment has taken effect [21]. Also the nature of the training can be varied to include, for example, aquatic exercises [50], although this will not always be possible in most low resourced settings. A similar line of action can be proposed for people with tuberculosis. Low physical activity in people with tuberculosis is mainly mediated through infection-related anemia and is associated with elevated acute phase response [51]. In the acute stages, pharmacotherapy is primordial [52]. To the best of our knowledge, there is no evidence for the beneficial effects of physical activity on the management of tuberculosis, and tuberculosis should rather be considered a barrier for physical activity participation. However, it could be hypothesized that physical activity might improve conditions associated with tuberculosis such as pain, depression and functional limitations, although research to confirm this is needed. In contrast, there is rigorous evidence for the beneficial effects of physical activity in chronic conditions such as diabetes, angina and low chronic back pain [21]. Health care professionals need to consider barriers, such as pain, sleep and mobility problems, in addition to condition-specific contra-indications.
In people with diabetes, physical activity interventions should be delayed until acute high blood sugar levels have been corrected [21]. Low-cost methods to assess blood sugar levels in low resourced settings need to be developed. In the case of active proliferative retinopathy, it is recommended that high-intensity training or training involving Valsalva maneuvers be avoided [21]. Strength training should be done with light weights and at low contraction velocity. In the case of neuropathy and the risk of foot ulcers, body-bearing activities should be avoided as repeated strain on neuropathic feet can lead to ulcers and fracture. Non-body-bearing exercise is recommendable such as cycling and swimming [21], but other strategies for resource-limited settings should be explored. Physical activity is not a contra-indication for stable (at least 5 days) angina [53] nor for chronic back pain [21] or asthma [21]. Effectiveness studies are needed to explore how international guidelines for these chronic conditions such as 12 weeks supervised training with individually organized training programs after an initial exercise test: two to five sessions a week of 30–60-min at an intensity of 50–80% of the maximum exercise capacity and/or daily low-intensity training walking over 30 min, increasing over time under the supervision of the rehabilitation team [21], can be implemented in resource-limited settings.
We also found significant associations between low physical activity and hearing problems and visual impairments. Hearing problems [54] and visual impairments [55] should therefore be considered as an important barrier for being physically active in LMICs. Stigma and discrimination associated with these chronic conditions and a lack of social support may further complicate physical activity participation in these populations. In the same way, negative self-perceptions associated with edentulism might, particularly in younger patients, be a barrier for participation in physical activity [56].
Practical implications and future research
The key to management of chronic conditions and multimorbidity is to strengthen a multidisciplinary approach simultaneously targeting both lifestyle factors and physical health outcomes (e.g., risk for chronic diseases, multimorbidity). In LMICs, in addition to economic restraints, other challenges may also undermine the development of effective and sustainable primary and secondary interventions. For example, implementing non-pharmacological interventions may be difficult in LMICs, due to the predominant biomedical model of practice within existing healthcare systems [57]. Results from a qualitative study that explored treatment adherence among patients with diabetes, hypertension or both, in a South-African community, suggest that factors that may influence adherence to behavior change interventions may be multifactorial, including the attribution of the origin of the illness, negative experiences with the public healthcare system, financial problems, transport problems and lack of social support [58]. Our analyses show that strategies to deal with lifestyle factors, such as physical inactivity, are urgently needed in LMICs, particularly targeting the earlier stages of disease.
First of all, there is a clear need to increase awareness of the importance of considering physical activity participation among care providers in LMICs. Continued medical education should be used to inform care providers on the importance of assessing physical activity levels and how cognitive behavioral principles (e.g., goal setting, problem-solving etc.) can be employed to assist patients to increase physical activity levels. We propose a dual strategy of developing both a smaller group of master trainers/supervisors (e.g., exercise physiologists and physiotherapists) and researchers and a larger group of practitioners (e.g., nurses) trained in the basics of cognitive behavioral physical activity strategies. This method has been successfully employed for cognitive behavioral therapy in trials in LMICs [59, 60]. A stepped-care approach, where patients start with self-management, may be a feasible strategy in LMIC settings. Then, if patients do not achieve guideline- specific levels of physical activity, they could continue with a manualized approach under the supervision of a non-specialist worker (e.g., nurses, occupational therapists). Patients would only be referred to a specialist supervisor (e.g., exercise physiologists and physiotherapists) if no significant increase in physical activity levels occurs, for example due to pain, sleep problems or mobility problems. It is known that inclusion of exercise physiologists or physiotherapists reduces drop out rates from physical activity interventions and consequently improves outcomes [61]. Careful consideration of what physical activity implementation strategies would be most efficacious, and evaluation of this stepped-care approach, is essential for chronic conditions. The current available evidence is, however, solely based on evidence from high-income countries. Efficacy trials of physical activity interventions among people with chronic conditions in different cultural settings across LMICs are urgently needed. In addition, effectiveness trials in diverse cultural settings could explore whether assisting people in fulfilling the following three universal and psychological needs will increase the likelihood that they adopt or maintain the prescribed recommendations/interventions of physical activity: (a) the need for autonomy (i.e., experiencing a sense of psychological freedom when engaging in physical activity), (b) the need for competence (i.e., ability to attain desired outcomes following the physical activity program), and (c) the need for relatedness (i.e., being socially connected when being physically active). If the efficacy and effectiveness of physical activity interventions are well established in better equipped scientific settings with research staff trained in physical activity prescription, the final step will be to fund interventions and initiatives to translate research findings into “real-world” settings while exploring its cost-effectiveness. In order to justify the inclusion of physical activity programs as a routine component in the treatment of chronic diseases and multimorbidity in LMICs, cost-benefit analyses should be conducted in order to quantify the financial implications of diverting resources or investing funds into such initiatives. Therefore, next to intervention studies exploring the efficacy of physical activity programs, effectiveness research capable of driving practice change, along with policy-level research, is urgently required. Ministries of health and education will play a critical role in this governance and policy development step.
If research shows that physical activity is efficacious and effective in the prevention and management of chronic diseases in LMICs, physical activity should be mainstreamed in existing health systems at all levels of care. Governments of LMICs will need to provide appropriate environments for physical activity including, space, infrastructure and tools. Such affirmative action is already required. Governmental and non-governmental agencies need to increase public health awareness of the importance of physical activity in people with chronic conditions. For example, physical activity should be integrated into the existing Information, Education and Communication public health awareness programs of the World Health Organization. Targeted messages should be developed in order to make these campaigns affordable. The benefits of engaging in physical activity should be properly outlined; any fears and wrongly held beliefs within various cultural contexts dispelled and, appropriate initiations steps (e.g., starting at a low intensity) and methods to maintain an active lifestyle should be included in community awareness programs.
Limitations and strengths
These findings should be interpreted in light of some limitations. First, the study is cross-sectional and cause and effect cannot be deduced. Therefore, it remains unclear whether lack of physical activity was caused by chronic conditions or vice versa. For example, physical inactivity is known to be a risk factor for cardiovascular diseases [62], while pain caused by arthritis or angina may limit the ability of an individual to engage in physical activity. Second, whilst we included all physical health conditions which were assessed within the WHS, other physical conditions such as stroke and hypertension which are frequently reported in multimorbidity indices [63] may have been present and not identified in the study. Third, since the information on chronic conditions and physical activity was based on self-report, reporting biases may exist. For example, across the entire sample only 29.2% were classified as being insufficiently active, which is lower than expected based on previous research. Therefore, the relationship between multimorbidity and low physical activity in our study may have been underestimated. Fourth, by separating the sample into dichotomous categories of sufficient and insufficient physical activity, we were not able to examine how different quantities of physical activity may affect morbidity. For instance, it is possible that achieving significantly more than 150 min confers additional benefits beyond simply meeting the minimum guidelines. Similarly, those who are almost completely inactive may have significantly more risk of chronic conditions than those who just fall short of the recommended 150 min. Future research to establish the optimal amounts of physical activity to reduce the risk of chronic illness would be useful to inform physical activity policy and practice guidelines. Fifth, the present study did not include institutionalized people, which may limit generalizability at a national level. Finally, we allowed for one missing variable when calculating the total number of chronic conditions. This was done to retain a larger sample size but some level of misclassification may exist. Nonetheless, the strengths of the study include the multi-national scope focused on LMICs, countries which are under-represented in the prior research literature. Additionally, we clarified numerous important mediators that can be targeted for future physical activity interventions.
Conclusions
The current study demonstrates that individuals with chronic conditions and multimorbidity are significantly more likely to engage in low levels of physical activity. Research on the efficacy and effectiveness of physical activity in management of chronic diseases and multimorbidity in LMICs should be a priority for funding bodies. When an evidence base is built, physical activity needs to be mainstreamed in existing health policies and strategies at all levels of care. To this end, policy makers and budget holders will need to invest in physical activity as part of a multidisciplinary treatment package for a wide range of chronic conditions, while health professionals will need to improve their physical activity assessment and prescriptions skills. Physiotherapists and exercise physiologists might assist in complex cases where pain, mobility difficulties, sleep problems and depression are important barriers for initiating or adopting an active lifestyle. Finally, (inter) national agencies will do well to increase public health awareness of the importance of physical activity in people with chronic diseases and multimorbidity in LMICs.
Abbreviations
- CI:
-
Confidence interval
- LMICs:
-
Low- and middle-income countries
- OR:
-
Odds ratio
- WHS:
-
World Health Survey
References
Murray CJ, Barber RM, Foreman KJ, Abbasoglu Ozgoren A, Abd-Allah F, Abera SF, Aboyans V, Abraham JP, Abubakar I, Abu-Raddad LJ, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015;386:2145–91.
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96.
Moran AE, Forouzanfar MH, Roth G, Mensah GA, Ezzati M, Flaxman A, Murray CJ, Naghavi M. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129(14):1493–501.
Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, Williams B, Gabriel S, Lassere M, Johns N. The global burden of rheumatoid arthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73:1316–22.
Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, Charlson F, Davis A, Degenhardt L, Dicker D. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800.
Hoy D, March L, Brooks P, Blyth F, Woolf A, Bain C, Williams G, Smith E, Vos T, Barendregt J. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(6):968–74.
Beulens JW, Grobbee DE, Nealb B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17:s3–8.
Jin L, Lamster I, Greenspan J, Pitts N, Scully C, Warnakulasuriya S. Global burden of oral diseases: emerging concepts, management and interplay with systemic health. Oral Dis. 2016;22(7):609–19.
Stevens G, Flaxman S, Brunskill E, Mascarenhas M, Mathers CD, Finucane M. Global and regional hearing impairment prevalence: an analysis of 42 studies in 29 countries. Eur J Publ Health. 2013;23:146–52.
Raviglione M, Sulis G. Tuberculosis 2015: burden, challenges and strategy for control and elimination. Infect Dis Rep. 2016;8(2):6570.
Stevens GA, White RA, Flaxman SR, Price H, Jonas JB, Keeffe J, Leasher J, Naidoo K, Pesudovs K, Resnikoff S. Global prevalence of vision impairment and blindness: magnitude and temporal trends, 1990–2010. Ophthalmology. 2013;120:2377–84.
Abegunde DO, Mathers CD, Adam T, Ortegon M, Strong K. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet. 2007;370:1929–38.
Fortin M, Stewart M, Poitras M-E, Almirall J, Maddocks H. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med. 2012;10:142–51.
Violan C, Foguet-Boreu Q, Flores-Mateo G, Salisbury C, Blom J, Freitag M, Glynn L, Muth C, Valderas JM. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS One. 2014;9:e102149.
Uijen AA, van de Lisdonk EH. Multimorbidity in primary care: prevalence and trend over the last 20 years. Eur J Gen Pract. 2008;14:28–32.
Salisbury C. Multimorbidity: redesigning health care for people who use it. Lancet. 2012;380:7–9.
Fortin M, Lapointe L, Hudon C, Vanasse A, Ntetu AL, Maltais D. Multimorbidity and quality of life in primary care: a systematic review. Health Qual Life Outcomes. 2004;2:1.
Lehnert T, Heider D, Leicht H, Heinrich S, Corrieri S, Luppa M, Riedel-Heller S, König H-H. Health care utilization and costs of elderly persons with multiple chronic conditions. Med Care Res Rev. 2011;68:387–420.
Gallo JJ, Hwang S, Joo JH, Bogner HR, Morales KH, Bruce ML, Reynolds III CF. Multimorbidity, depression, and mortality in primary care: randomized clinical trial of an evidence-based depression care management program on mortality risk. J Gen Int Med. 2016;31:380–6.
Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43.
Pedersen B, Saltin B. Exercise as medicine–evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25:1–72.
Bertheussen GF, Romundstad PR, Landmark T, Kaasa S, Dale O, Helbostad JL. Associations between physical activity and physical and mental health–a HUNT 3 study. Med Sci Sports Exerc. 2011;43:1220–8.
Hupin D, Roche F, Gremeaux V, Chatard J-C, Oriol M, Gaspoz J-M, Barthélémy J-C, Edouard P. Even a low-dose of moderate-to-vigorous physical activity reduces mortality by 22% in adults aged ≥ 60 years: a systematic review and meta-analysis. Br J Sports Med. 2015;49:1262–7.
Cimarras-Otal C, Calderón-Larrañaga A, Poblador-Plou B, González-Rubio F, Gimeno-Feliu LA, Arjol-Serrano JL, Prados-Torres A. Association between physical activity, multimorbidity, self-rated health and functional limitation in the Spanish population. BMC Publ Health. 2014;14:1.
Dhalwani NN, O’Donovan G, Zaccardi F, Hamer M, Yates T, Davies M, Khunti K. Long terms trends of multimorbidity and association with physical activity in older English population. Int J Behav Nutr Phys Act. 2016;13:8.
Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, Burnett R, Casey D, Coates MM, Cohen A. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2287–323.
Chow CK, Teo KK, Rangarajan S, Islam S, Gupta R, Avezum A, Bahonar A, Chifamba J, Dagenais G, Diaz R. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310:959–68.
Patel V, Araya R, Chatterjee S, Chisholm D, Cohen A, De Silva M, Hosman C, McGuire H, Rojas G, van Ommeren M. Treatment and prevention of mental disorders in low-income and middle-income countries. Lancet. 2007;370:991–1005.
Pengpid S, Peltzer K, Kassean HK, Tsala JPT, Sychareun V, Müller-Riemenschneider F. Physical inactivity and associated factors among university students in 23 low-, middle-and high-income countries. Int J Publ Health. 2015;60:539–49.
Atkinson K, Lowe S, Moore S. Human development, occupational structure and physical inactivity among 47 low and middle income countries. Prev Med Rep. 2016;3:40–5.
Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–8.
Vancampfort D, De Hert M, Skjerven LH, Gyllensten AL, Parker A, Mulders N, Nyboe L, Spencer F, Probst M. International Organization of Physical Therapy in Mental Health consensus on physical activity within multidisciplinary rehabilitation programmes for minimising cardio-metabolic risk in patients with schizophrenia. Disabil Rehabil. 2012;34:1–12.
Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–58.
Freeman EE, Roy-Gagnon MH, Samson E, Haddad S, Aubin MJ, Vela C, Zunzunegui MV. The global burden of visual difficulty in low, middle, and high income countries. PLoS One. 2013;8:e63315.
Goldhaber-Fiebert JD, Jeon CY, Cohen T, Murray MB. Diabetes mellitus and tuberculosis in countries with high tuberculosis burdens: individual risks and social determinants. Int J Epidemiol. 2011;40:417–28.
Stubbs B, Koyanagi A, Veronese N, Vancampfort D, Solmi M, Gaughran F, Carvalho AF, Lally J, Mitchell AJ, Mugisha J. Physical multimorbidity and psychosis: comprehensive cross sectional analysis including 242,952 people across 48 low-and middle-income countries. BMC Med. 2016;14:189.
Nuevo R, Chatterji S, Verdes E, Naidoo N, Arango C, Ayuso-Mateos JL. The continuum of psychotic symptoms in the general population: a cross-national study. Schizophr Bull. 2012;38:475–85.
Nuevo R, Van Os J, Arango C, Chatterji S, Ayuso-Mateos JL. Evidence for the early clinical relevance of hallucinatory-delusional states in the general population. Acta Psychiatr Scand. 2013;127:482–93.
Koyanagi A, Oh H, Stickley A, Haro JM, DeVylder J. Risk and functional significance of psychotic experiences among individuals with depression in 44 low- and middle-income countries. Psychol Med. 2016;46(12):2655–65.
Cifuentes M, Sembajwe G, Tak S, Gore R, Kriebel D, Punnett L. The association of major depressive episodes with income inequality and the human development index. Soc Sci Med. 2008;67:529–39.
Worldbank. Classification of economies. Washington DC; 2004. http://siteresources.worldbank.org/INTRGEP2004/Resources/classification.pdf. Accessed 15 May 2016.
Koyanagi A, Garin N, Olaya B, Ayuso-Mateos JL, Chatterji S, Leonardi M, Koskinen S, Tobiasz-Adamczyk B, Haro JM. Chronic conditions and sleep problems among adults aged 50 years or over in nine countries: a multi-country study. PLoS One. 2014;9:e114742.
Breen R, Karlson KB, Holm A. Total, direct, and indirect effects in logit and probit models. Soc Method Res. 2013;42:164–91.
Koyanagi A, Stickley A. The association between sleep Problems and psychotic symptoms in the general population: a global perspective. Sleep. 2015;38:1875–85.
Stubbs B, Hurley M, Smith T. What are the factors that influence physical activity participation in adults with knee and hip osteoarthritis? A systematic review of physical activity correlates. Clin Rehabil. 2015;29:80–94.
Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;49(24):1554–7.
Kelley GA, Kelley KS, Hootman JM. Effects of exercise on depression in adults with arthritis: a systematic review with meta-analysis of randomized controlled trials. Arthritis Res Ther. 2015;17:21.
Verhoeven F, Tordi N, Prati C, Demougeot C, Mougin F, Wendling D. Physical activity in patients with rheumatoid arthritis. Joint Bone Spine. 2016;83:265–20.
Hernández-Hernández MV, Díaz-González F. Role of physical activity in the management and assessment of rheumatoid arthritis patients. Reumatol Clín. 2016. doi: 10.1016/j.reuma.2016.04.003
Bartels EM, Juhl CB, Christensen R, Hagen KB, Danneskiold‐Samsøe B, Dagfinrud H, Lund H. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016. doi: 10.1002/14651858.CD005523.pub3
Faurholt-Jepsen M, Faurholt-Jepsen D, Range N, Praygod G, Jeremiah K, Aabye M, Changalucha J, Krarup H, Christensen DL, Andersen Å. The use of combined heart rate response and accelerometry to assess the level and predictors of physical activity in tuberculosis patients in Tanzania. Epidemiol Infect. 2014;142:1334–42.
Wallis RS, Maeurer M, Mwaba P, Chakaya J, Rustomjee R, Migliori GB, Marais B, Schito M, Churchyard G, Swaminathan S. Tuberculosis—advances in development of new drugs, treatment regimens, host-directed therapies, and biomarkers. Lancet Infect Dis. 2016;16:e34–46.
Giannuzzi P, Mezzani A, Saner H, Bj√∂rnstad H, Fioretti P, Mendes M, Cohen-Solal A, Dugmore L, Hambrecht R, Hellemans I. Physical activity for primary and secondary prevention. Position paper of the Working Group on Cardiac Rehabilitation and Exercise Physiology of the European Society of Cardiology. Eur J Cardiovasc Prev Rehabil. 2003;10:319–27.
Gispen FE, Chen DS, Genther DJ, Lin FR. Association between hearing impairment and lower levels of physical activity in older adults. J Am Geriatr Soc. 2014;62:1427–33.
Marmeleira J, Laranjo L, Marques O, Pereira C. Physical activity patterns in adults who are blind as assessed by accelerometry. Adapt Phys Act Quart. 2014;31:283–96.
Mesas AE, De Andrade SM, Cabrera MAS. Factors associated with negative self‐perception of oral health among elderly people in a Brazilian community. Gerodontology. 2008;25:49–56.
Mugisha J, Ssebunnya J, Kigozi FN. Towards understanding governance issues in integration of mental health into primary health care in Uganda. Int J Ment Health Syst. 2016;10:1.
Kagee A, Le Roux M, Dick J. Treatment adherence among primary care patients in a historically disadvantaged community in South Africa a qualitative study. J Health Psychol. 2007;12:444–60.
Naeem F, Saeed S, Irfan M, Kiran T, Mehmood N, Gul M, Munshi T, Ahmad S, Kazmi A, Husain N. Brief culturally adapted CBT for psychosis (CaCBTp): a randomized controlled trial from a low income country. Schizophr Res. 2015;164:143–8.
Naeem F, Sarhandi I, Gul M, Khalid M, Aslam M, Anbrin A, Saeed S, Noor M, Fatima G, Minhas F. A multicentre randomised controlled trial of a carer supervised culturally adapted CBT (CaCBT) based self-help for depression in Pakistan. J Affect Disord. 2014;156:224–7.
Vancampfort DRS, Schuch FB, Ward PB, Probst M, Stubbs B. Prevalence and predictors of treatment dropout from physical activity interventions in schizophrenia: a meta-analysis. Gen Hosp Psychiatr. 2015;39:15–23.
Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, Alter DA. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Int Med. 2015;162:123–32.
Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases—a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci. 2011;66:301–11.
Acknowledgements
None.
Funding
Brendon Stubbs receives funding from the National Institute for Health Research Collaboration for Leadership in Applied Health Research & Care Funding scheme. Ai Koyanagi’s work is supported by the Miguel Servet contract financed by the CP13/00150 and PI15/00862 projects, integrated into the National R + D + I and funded by the ISCIII - General Branch Evaluation and Promotion of Health Research - and the European Regional Development Fund (ERDF-FEDER). Davy Vancampfort is funded by the Research Foundation – Flanders (FWO – Vlaanderen). The views expressed in this publication are those of the authors and not necessarily those of the any funding agencies.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available in the World Health Survey repository, available at http://www.who.int/healthinfo/survey/en/.
Authors’ contributions
Access to the World Health Survey data collection was obtained by Dr. Brendon Stubbs. Analyses were performed by Dr. Ai Koyanagi and Dr. Brendon Stubbs. Dr. Davy Vancampfort wrote a first draft which was reviewed and revised in several rounds by the other co-authors. All authors approved the final version and all authors certify that they have participated sufficiently in the work to believe in its overall validity and to take public responsibility for appropriate portions of its content.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethical approval was obtained from ethical boards at each study site. Participants give written informed consent. Details are available at http://www.who.int/healthinfo/survey/en/.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1: Table S1.
Questions used to assess health status. (DOCX 11 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Vancampfort, D., Koyanagi, A., Ward, P.B. et al. Chronic physical conditions, multimorbidity and physical activity across 46 low- and middle-income countries. Int J Behav Nutr Phys Act 14, 6 (2017). https://doi.org/10.1186/s12966-017-0463-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12966-017-0463-5