Abstract
Background
Thrombus formation is an important factor affecting cardiovascular events and venous thromboembolism in type 2 diabetes. However, it is unclear whether glycemic control reduces thrombogenicity. We investigated the effect of short-term glycemic control (STUDY 1) and hypoglycemia (STUDY 2) on thrombus formation using an automated microchip flow chamber system.
Methods
For STUDY 1, we recruited 10 patients with type 2 diabetes. Before and after 2 weeks of treatment, blood glucose was analyzed with a continuous glucose monitoring system, and thrombogenicity was analyzed with an automated microchip flow chamber system. For STUDY 2, we recruited 10 subjects without diabetes who underwent an insulin tolerance test. We evaluated the change in thrombogenic potential with hypoglycemia.
Results
STUDY1: The mean blood glucose level reduced from 10.1 ± 2.6 to 6.9 ± 0.97 mM (P < 0.01). T10, an indicator of thrombogenicity, significantly attenuated after glycemic control (338 ± 65 vs. 425 ± 117 s, P < 0.05). The attenuation in T10 was significantly correlated with changes in mean blood glucose level after treatment (r = − 0.718, P < 0.05). STUDY 2: Platelet function was enhanced with decreasing blood glucose; increased platelet function was strongly correlated with an increase in epinephrine.
Conclusions
We demonstrated attenuation in thrombogenicity with short-term comprehensive diabetes care and enhancement in thrombogenicity with hypoglycemia, using a new flow chamber system.
Trial registration
UMIN-CTR UMIN 000019899, registered 26-Jan-2015 (STUDY 2).
Similar content being viewed by others
Background
Atherothrombotic disease is a major cause of death in patients with diabetes [1]. The mechanism of increased thrombosis in patients with diabetes involves multiple pathways. Thrombus formation, the last step in the atherothrombotic process, is an important factor influencing the risk and prognosis associated with cardiovascular events [2]. Moreover, diabetes is a risk factor for arterial and venous thrombosis. A recent meta-analysis found that patients with diabetes were at increased risk of developing venous thromboembolism (VTE), which can occur in a hypercoagulable state [3].
Several studies have shown that patients with diabetes have increased thrombogenicity owing to platelet hyperreactivity, activation of coagulation factors and hypo-fibrinolysis [4,5,6]. However, it is unclear whether glycemic control reduces thrombus formation. Especially, the influence of glycemic control over short periods of time, such as one or two weeks, has not been assessed, even though it is a clinically-important issue for preventing post-surgical complications [7].
On the other hand, evidence is accumulating that hyperglycemia and hypoglycemia, which can occur when trying to achieve strict glycemic control, is associated with increased risk of cardiovascular and cerebrovascular death. In the ACCORD study, the mortality rate was higher in the strict-treatment than in the standard-treatment group [8]. The involvement of hypoglycemia as a risk factor for thrombosis is a topic of active discussion.
A previous study evaluated thrombogenicity using plasma markers [9] and platelet function tests with an agonist, above physiological concentrations; however, the effects of blood flow were not considered and blood flow is a well-known influence on the thrombus formation process [10]. Therefore, it is necessary to evaluate thrombogenicity comprehensively, using close-to-physiological conditions and considering blood flow. Recently, a Total Thrombus formation Analysis System (T-TAS, Fujimori Kogyo Co., Yokohama, Kanagawa) [11] was found useful [12] for quantitative analysis of thrombus formation. The T-TAS continuously monitors the pressure on microchips with thrombogenic surfaces that simulate pathological blood vessels [11, 13]. Compared with conventional systems, the T-TAS can evaluate thrombogenicity under blood flow conditions; therefore, it is possible to perform analyses that more closely resemble in vivo conditions.
This pilot study aimed at investigating the influence of short-term glycemic control (STUDY 1) and change in hypoglycemia (STUDY 2) on thrombus formation using T-TAS.
Materials and methods
Study 1
Glycemic control efforts were started immediately after hospitalisation. Blood glucose levels were controlled with insulin, GLP-1 analogues and oral hypoglycemic agents. The goal of treatment was to achieve a fasting plasma glucose (FPG) level < 7.2 mM and a postprandial PG level < 11.1 mM. A continuous glucose monitoring (CGM) device (iPro2: Medtronic Minimed, Northridge, CA, USA) was attached to each patient from the day of hospitalization, and monitoring was performed for 3 days. Blood collection and evaluation for T-TAS were carried out on the day after hospitalization. These evaluations were performed again after glycemic control was achieved. The exclusion criteria were antiplatelet drug or anticoagulant use, infectious diseases, malignant tumors, drug-induced diabetes, ketoacidosis and possible pregnancy.
Blood samples
Following overnight fasting, blood was drawn for the measurement of blood glucose, hematocrit (Ht), platelet, plasminogen activator inhibitor 1 (PAI-1), fibrinogen, factor VII (FVII), factor VIII (FVIII) and prothrombin fragment 1 + 2 (F1 + 2), and for assessment using T-TAS.
Glucose levels were measured using a GA echo buffer solution, Glucose BP standard solution and automated glucose analyzer GA08III (A&T corporation, Kanagawa, Japan). The LPIA·tPAI test with the automated immunoanalyzer STACIA (LSI Medience Corporation, Tokyo, Japan) for PAI-1, Thrombo check Fib (L) with CS-5100 Automated Coagulation Analyzer (Sysmex Corporation, Hyogo, Japan) for fibrinogen, HemosIL RecombiPlasTin or HemosIL Synth Asil with ACL TOP automated analyzer (Instrumentation Laboratory, Bedford, MA) for FVII or FVII and Enzygnost with Microplate Reader Emax (Molecular Devices Japan, Tokyo, Japan) for F1 + 2 were applied respectively.
CGM
We used a CGM device and considered the result as an indicator of short-term glycemic control. The mean blood glucose level, peak blood glucose levels and standard deviation (SD) and mean amplitude of glycemic excursion (MAGE) were calculated from the results.
T-TAS
T-TAS evaluations were carried out using two kinds of microchips (PL chip and AR chip). The PL chip, which is coated with collagen, represents platelet thrombus formation. The AR chip, which is coated with collagen plus tissue thromboplastin, represents thrombus formation mediated by the coagulo-fibrinolysis system and platelets. Whole blood anticoagulated with hirudin was perfused through the PL chip at a shear rate of 1500 S− 1; then, PL-T10 (time to 10 kPa), PL-OT (occlusion time: time to 60 kPa) and PL-AUC10 (area under the flow curve in 10 min) were used to evaluate thrombus formation. Recalcified whole blood containing corn trypsin inhibitor was perfused through the AR chip at a sheer rate of 600 S− 1; then, AR-T10 (time to 10 kPa), AR-OT (occlusion time: time to 80 kPa) and AR-AUC30 (area under the flow curve in 30 min) were evaluated. The intra-assay coefficient of variability of PL-AUC and AR-AUC are 5.85 and 1.24% respectively (Additional file 1: Figure S1). The reference ranges (mean ± SD) of PL-T10, PL-OT and PL-AUC in healthy volunteers were reported to be 163 ± 51 s, 347 ± 96 s, and 369.1 ± 71.8 kPa × min, respectively [14].
Study 2
Human regular insulin (Eli Lilly, Indianapolis, IN) was injected intravenously at a dose of 0.05–0.10 U/kg body weight. Blood samples for glucose and epinephrine were collected before the injection and at 15, 30, 45, 60, 90 and 120 min after the injection; for T-TAS blood samples were collected before the injection and at 15, 30, 60, 90 and 120 min; and for WBC blood samples were collected before the injection and at 30 and 60 min after the injection. For measurement of epinephrine, the CA Test 「TOSOH」 (Tosoh Corporation, Tokyo, Japan) and HPLC system (SHIMADZU CORPORATION, Kyoto, Japan) were used.
Statistical analysis
Normally distributed data are presented as means ± SDs, and the paired t-test was used to assess the presence of significant differences. Non-normally distributed data are presented as medians (quartile range), and the Wilcoxon signed rank test was used for assessment. Spearman’s rank correlation was used for assessing correlation. IBM SPSS 20 statistical package (IBM Corp., Armonk, NY) was used to perform all the statistical analyses. A P-value < 0.05 was considered statistically significant.
Results
Study 1
We included 10 patients with type 2 diabetes who were admitted to Kagoshima University Hospital for glycemic control (Table 1) (Additional file 1: Table S1). The mean duration of the period between pre- and post-glycemic control was 10.4 days. The levels of fasting plasma glucose (FPG) reduced from 9.6 ± 2.3 mM to 6.3 ± 1.4 mM (P < 0.01), mean blood glucose reduced from 10.1 ± 2.5 mM to 6.8 ± 0.9 mM (P < 0.01), and peak blood glucose reduced from 15.6 ± 4.3 mM to 11.5 ± 4.1 mM (P < 0.05). The SD of blood glucose and MAGE were unchanged (2.1 ± 1.3 mM vs. 1.6 ± 1.2 mM, P = 0.24 and 87.5 ± 42.4 vs. 68.5 ± 51.2, P = 0.31, respectively) (Table 2). In one case, mild hypoglycemia (3.1 mM) was recognized in the night during CGM; however, this was not observed in other patients.
Hematologically, no significant changes were observed in WBC count, Ht level, platelet count, fibrinolysis marker (PAI-1) level and coagulation marker (fibrinogen, FVII, FVIII, F1 + 2) levels (Table 2).
In STUDY 1, the T-TAS and two microchips were used to evaluate thrombus formation. Inside the PL chip, activation of the platelet is triggered on the surface of collagen [13]. Inside the other AR chip, activation of both platelets and coagulation is triggered by collagen and tissue thromboplastin [11]. We evaluated thrombogenicity by assessing the changes in flow pressure during thrombus formation.
AR-T10 and AR-OT increased from 337.8 ± 64.7 s to 425.2 ± 116.9 s (P = 0.005) and from 441.2 ± 73.0 s to 527.7 ± 115.4 s (P = 0.006), respectively, while AR-AUC30 reduced from 1890.3 ± 91.3 to 1775.9 ± 154.5 kPa × min (P = 0.006) (Fig. 1). No PL chip parameters changed (Table 2).
We compared the change in the mean PG (Δmean PG) and SD (ΔSD) with the change in T-TAS parameters (Fig. 2). The change in AR-T10 (ΔAR-T10) was significantly correlated with Δmean PG after treatment (r = − 0.718, P < 0.05), but not with FPG, SD and MAGE.
Although the correlation between Δmean PG and ΔAR-OT or ΔAR-AUC30 was not statistically significant, non-significant trend was observed. Additional file 1: Table S2 presents individual data on mean PG, AR-T10, AR-OT and AR-AUC30 in STUDY 1.
Study 2
We included 10 patients who underwent an insulin tolerance test for hypothalamic pituitary evaluation (Table 3). After insulin injection, PG decreased from 5.2 ± 0.7 mM (baseline) to 1.7 ± 0.5 mM (45 min), and Ht increased from 37.5 ± 3.5% to 39.1 ± 3.7% (P < 0.01) and epinephrine increased from 19.5 ± 11.2 pg/mL to 449.5 ± 289.6 pg/mL (P < 0.01). However, the platelet count did not change (24.5 × 104/μL to 25.8 × 104/μL, P = 0.053).
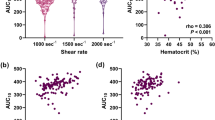
PL-T10 decreased from 156.4 ± 55.5 s to 109.7 ± 15.2 s (P < 0.05), while PL-AUC10 increased from 380.3 ± 72.4 s to 428.3 ± 31.9 s (P < 0.05). PL-T10 was significantly lower, and PL-AUC10 was significantly higher, at 60 min compared to the baseline values (Fig. 3c, d).
Time course of PG (a) and epinephrine (b) and changes in PL-T10 (c), PL-AUC 10 (d), WBC (e) before and 60 min after insulin injection in insulin tolerance test (STUDY 2). Data are presented as mean ± SD. *P < 0.05 vs basal, **P < 0.01 vs basal, ***P < 0.005 vs basal, **** P < 0.001 vs basal. The change in WBC was evaluated by two-tailed t-test, the others were evaluated by Wilcoxon signed rank test (n = 10)
Along with hypoglycemia, epinephrine and WBC count significantly changed (Fig. 3e). In addition, there was a correlation between the decrease in PG (ΔPG) and the change in epinephrine (Δepinephrine). Furthermore, Δepinephrine was correlated with ΔPL-T10 and ΔPL-AUC10 (Fig. 4). We found the significant correlations between ΔPL-T10 and change in Ht (r = − 0.772, P = 0.009), change in WBC count (r = − 0.915, P < 0.001) or change in platelet count (r = − 0.648, P = 0.043) (Additional file 1: Figure S2). Additional file 1: Table S3 shows individual data on the PG, epinephrine, PL-T10 and PL-AUC10 in STUDY2.
Correlation between the change in PG and the change in epinephrine (a), the change in epinephrine and the change in PL-T10 (b) and the change in epinephrine and the change in PL-AUC10 (c) in STUDY 2. Each data is the difference between the value before administration of insulin and the bottom value (PG and PL-T10) or the peak value (epinephrine and PL-AUC10) at any time point after administration of insulin. Correlation between the change in PG and the change in epinephrine was analzsed by Pearson’s rank correlation, and others were analyzed by Spearman’s rank correlation (n = 10)
Discussion
We aimed to elucidate the effects of short-term glycemic control on blood thrombogenicity in patients with diabetes (STUDY1) and change of platelet function during hypoglycemia (STUDY2) using a newly-developed, microchip-based flow chamber system. We used two types of microchips [13]: the PL chip to evaluate thrombogenicity, mainly involving platelets, and the AR chip to evaluate platelet activation and the coagulo-fibrinolytic reaction [11].
Although it has been reported that thrombogenic potential improves as glycemic control improves, for at least several months [15], it was not clear whether short-term control reduces thrombus formation. Our study (STUDY1) demonstrated that short-term glycemic control attenuates thrombogenicity. Of note, improvements in mean blood glucose level, but not glucose excursion, were strongly correlated with reduced thrombogenicity. In impaired glucose tolerance patients, it has been reported that suppression of postprandial hyperglycemia can prevent the onset of cardiovascular events [16]. But in type 2 diabetic patients, the protective effect of the attenuation of glucose spikes remains controversial [17]. Furthermore, there is a large-scale clinical research showing that the cardiovascular risk is lower in patients with low HbA1c values, which represent low mean blood glucose levels [18]. These results underscore the clinical significance of short-term pre-operative glycemic control to improve patients’ prognosis.
The mean value of AR-OT before glycemic control was 425.2 s, shorter than the mean value in healthy adults (489 +/− 96 s, unpublished data). After glycemic control, AR-OT improved to 527.7 s. The other AR chip parameters (AR-T10 and AR-AUC30) also improved after glycemic control. The PL chip parameters, which represent platelet-mediated thrombogenicity, showed no attenuation.
One possible explanation for the reduced thrombogenicity of the AR chip, without attenuation of PL chip thrombogenicity and coagulo-fibrinolysis markers, is glycation of fibrinogen and plasminogen because of high blood glucose. In the studies that used the purified-fibrinogen model, chronic hyperglycemia accelerated glycation of fibrinogen non-enzymatically. Glycated fibrinogen rapidly constructed a fibrin network, increasing the resistance to the fibrinolytic system, compared to non-diabetic patients [19, 20], due to changes in the kinetics of fibrin polymerization [20]. Past reports found that patients with diabetes and rapidly-improving glycemic control had significantly decreased glycated fibrinogen over 3 days [21]. In patients with diabetes, glycation of plasminogen reduced plasmin generation and impaired plasminogen function, both of which were ameliorated by glycemic control [22]. Based on these results, the hypo-fibrinolytic state may have improved secondary to decreases in glycated fibrinogen and plasminogen, even during short-term glycemic control.
It is not clear what factor, among all those altered by comprehensive diabetes care, contributed to the attenuation of thrombotic occlusion. Since the decrease in the mean PG change showed a relatively strong negative correlation with the change in AR-T10, mean PG might be an important factor. However, it is also possible that some specific treatment options, such as metformin, glucagon-like peptide-1 receptor agonists or statins, contribute to reduction of thrombogenic potentials. A large-scale study with a stratified analysis is necessary to examine this issue further.
The findings of this study differ from the findings of previous studies [23, 24] of the relationship between glycemic control and thrombogenicity. Several reported that levels of fibrinogen, PAI-1, FVII, FVIII and F1 + 2 are increased in patients with diabetes [25]; however, in the present study, the levels were within normal limits and did not change after glycemic control. These markers are influenced by various factors, such as low-grade inflammation [26], insulin resistance [27] and hyperglycemia itself [28]; therefore, our results may reflect differences in patients’ backgrounds.
The relationship between glycemic control and platelet function is controversial [29, 30]. Factors other than the glucose level may be involved in platelet function among patients with type 2 diabetes [31]. In a previous clinical study, aspirin administered for primary prevention in patients with type 2 diabetes failed to reduce the risk of macrovascular events [32]. The role of platelets in thrombus formation, among patients with type 2 diabetes, may be limited.
Conventionally, there is a recognized relationship between hypoglycaemia and increased platelet function [33]. However, prior results were verified in the absence of blood flow or with supra-physiological concentrations. Moreover, the samples consisted of platelet-rich plasma (PRP) and not whole blood. To the best of our knowledge, this is the first study to evaluate thrombus formation associated with hypoglycaemia using a flow chamber system with microchips simulated pathological blood vessels.
Platelet function was enhanced with decreasing blood glucose, and increased platelet function was strongly correlated with an increase in epinephrine (STUDY2). Several studies have shown that plasma epinephrine may have an important role in platelet activation during hypoglycemia in patients with type 2 diabetes [34, 35]. Epinephrine is secreted from the adrenal medulla through the activation of the sympathetic nervous system as a physiological defence when PG levels fall [36]. Increased epinephrine levels cause platelet aggregation through the activation of α2-adrenergic receptors [37]. Additionally, the changes in WBC count and Ht levels correlated with changes in PL chip parameters. Increased WBC count, partially that induced by epinephrine, may contribute to thrombus formation and progress. WBCs, especially monocytes and neutrophils, induce thrombus formation in collaboration with platelets [38]. Furthermore, increased Ht level after insulin injection could have affected blood viscosity inside the PL chip.
Our patients included one with acromegaly and one with adrenal insufficiency; however, there was no association of cortisol and growth hormone levels with platelet function [39].
It has been reported that, in patients with acute coronary syndrome, hyperglycemia is associated with poor prognosis and high rates of subsequent ischaemic events [40]. However, there is no firm evidence that glycemic control improves prognosis in patients who have experienced cardiovascular events. Our results suggest that glycemic control improves prognosis in critical situations and underscore the importance of avoiding hypoglycemia.
Although there is no study on the relationship between glycemic control and development of VTE, glycemic control-related improvements in the hypo-fibrinolysis state may lower the risk of VTE while improving prognosis.
Earlier studies investigated the effects of anti-platelet or anti-coagulant drugs on T-TAS measurements [41, 42]. One study that evaluated the effects of warfarin or direct-acting oral coagulants (DOACs) with T-TAS [43] showed a decrease in AR-AUC30, similar to the results of STUDY 1. Changes in T-TAS parameters, obtained by comprehensive diabetes care involving short-term glycemic control, appear physiologically-relevant.
Our study has several limitations. First, the number of participants was small. Nevertheless, we think that our preliminary findings are worth reporting and may inform future, more scientifically-rigorous studies. Second, we enrolled patients without diabetes in STUDY 2, because patients with diabetes have increased risk of ischaemic events associated with hypoglycaemia. Third, we did not evaluate thrombus formation with the AR chip in STUDY2. Interpretation of AR chip results might be difficult if significant changes in platelet function are observed because the AR chip results reflected either platelet aggregation or coagulation factor. Finally, we could not obtain direct evidence of glycated state alteration.
Conclusions
In summary, we demonstrated attenuation in thrombogenicity with comprehensive diabetes care and enhancement in thrombogenicity with hypoglycemia, using a new flow chamber system. To confirm these findings, further large-scale clinical studies are needed.
Availability of data and materials
All data in this study are included in this article or are available from the corresponding author upon reasonable request.
Abbreviations
- AR-AUC30:
-
area under the flow curve in 30 min
- AR-OT:
-
occlusion time: time to 80 kPa
- AR-T10:
-
time to 10 kPa
- CGM:
-
Continuous glucose monitoring
- DOAC:
-
Direct-acting oral coagulants
- F1 + 2:
-
Prothrombin fragment 1 + 2
- FPG:
-
Fasting plasma glucose
- FVII :
-
Factor VII
- FVIII:
-
Factor VIII
- Ht:
-
Hematocrit
- MAGE:
-
Mean amplitude of glycemic excursion
- PAI-1:
-
Plasminogen activator 1
- PL-AUC10:
-
area under the flow curve in 10 min
- PL-OT:
-
occlusion time: time to 60 kPa
- PL-T10:
-
time to 10 kPa
- PRP:
-
Platelet-rich plasma
- SD:
-
Standard deviation
- T-TAS:
-
Total Thrombus formation Analysis System
- WBC:
-
White blood cell
References
Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22.
Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–49.
Gariani K, Mavrakanas T, Combescure C, Perrier A, Marti C. Is diabetes mellitus a risk factor for venous thromboembolism? A systematic review and meta-analysis of case-control and cohort studies. Eur J Intern Med. 2016;28:52–8.
Colwell JA, Nesto RW. The platelet in diabetes: focus on prevention of ischemic events. Diabetes Care. 2003;26:2181–8.
Boden G, Vaidyula VR, Homko C, Cheung P, Rao AK. Circulating tissue factor procoagulant activity and thrombin generation in patients with type 2 diabetes: effects of insulin and glucose. J Clin Endocrinol Metab. 2007;92:4352–8.
Seljeflot I, Larsen JR, Dahl-Jorgensen K, Hanssen KF, Arnesen H. Fibrinolytic activity is highly influenced by long-term glycemic control in type 1 diabetic patients. J Thromb Haemost. 2006;4:686–8.
Newman JD, Wilcox T, Smilowitz NR, Berger JS. Influence of diabetes on trends in perioperative cardiovascular events. Diabetes Care. 2018.
Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59.
Fonseca VA, Reynolds T, Hemphill D, Randolph C, Wall J, Valiquet TR, Graveline J, Fink LM. Effect of troglitazone on fibrinolysis and activated coagulation in patients with non-insulin-dependent diabetes mellitus. J Diabetes Complicat. 1998;12:181–6.
Brass LF, Diamond SL. Transport physics and biorheology in the setting of hemostasis and thrombosis. J Thromb Haemost. 2016;14:906–17.
Hosokawa K, Ohnishi T, Kondo T, Fukasawa M, Koide T, Maruyama I, Tanaka KA. A novel automated microchip flow-chamber system to quantitatively evaluate thrombus formation and antithrombotic agents under blood flow conditions. J Thromb Haemost. 2011;9:2029–37.
Hosokawa K, Ohnishi T, Sameshima H, Miura N, Ito T, Koide T, Maruyama I. Analysing responses to aspirin and clopidogrel by measuring platelet thrombus formation under arterial flow conditions. Thromb Haemost. 2013;109:102–11.
Hosokawa K, Ohnishi T, Fukasawa M, Kondo T, Sameshima H, Koide T, Tanaka KA, Maruyama I. A microchip flow-chamber system for quantitative assessment of the platelet thrombus formation process. Microvasc Res. 2012;83:154–61.
Yamaguchi Y, Moriki T, Igari A, Matsubara Y, Ohnishi T, Hosokawa K, Murata M. Studies of a microchip flow-chamber system to characterize whole blood thrombogenicity in healthy individuals. Thromb Res. 2013;132:263–70.
Osende JI, Badimon JJ, Fuster V, Herson P, Rabito P, Vidhun R, Zaman A, Rodriguez OJ, Lev EI, Rauch U, et al. Blood thrombogenicity in type 2 diabetes mellitus patients is associated with glycemic control. J Am Coll Cardiol. 2001;38:1307–12.
Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072–7.
Raz I, Wilson PW, Strojek K, Kowalska I, Bozikov V, Gitt AK, Jermendy G, Campaigne BN, Kerr L, Milicevic Z, Jacober SJ. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care. 2009;32:381–6.
Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Bmj. 2000;321:405–12.
Dunn EJ, Ariens RA, Grant PJ. The influence of type 2 diabetes on fibrin structure and function. Diabetologia. 2005;48:1198–206.
Pieters M, Covic N, van der Westhuizen FH, Nagaswami C, Baras Y, Toit Loots D, Jerling JC, Elgar D, Edmondson KS, van Zyl DG, et al. Glycaemic control improves fibrin network characteristics in type 2 diabetes - a purified fibrinogen model. Thromb Haemost. 2008;99:691–700.
Hammer MR, John PN, Flynn MD, Bellingham AJ, Leslie RD: Glycated fibrinogen: a new index of short-term diabetic control. Ann Clin Biochem 1989, 26 ( Pt 1):58–62.
Ajjan RA, Gamlen T, Standeven KF, Mughal S, Hess K, Smith KA, Dunn EJ, Anwar MM, Rabbani N, Thornalley PJ, et al. Diabetes is associated with posttranslational modifications in plasminogen resulting in reduced plasmin generation and enzyme-specific activity. Blood. 2013;122:134–42.
Derosa G, Dangelo A, Ragonesi PD, Ciccarelli L, Piccinni MN, Pricolo F, Salvadeo S, Montagna L, Gravina A, Ferrari I, et al. Effects of rosiglitazone and pioglitazone combined with metformin on the prothrombotic state of patients with type 2 diabetes mellitus and metabolic syndrome. J Int Med Res. 2006;34:545–55.
Bruno G, Cavallo-Perin P, Bargero G, Borra M, D'Errico N, Pagano G. Association of fibrinogen with glycemic control and albumin excretion rate in patients with non-insulin-dependent diabetes mellitus. Ann Intern Med. 1996;125:653–7.
Barazzoni R, Kiwanuka E, Zanetti M, Cristini M, Vettore M, Tessari P. Insulin acutely increases fibrinogen production in individuals with type 2 diabetes but not in individuals without diabetes. Diabetes. 2003;52:1851–6.
Sam S, Haffner S, Davidson MH, D'Agostino RB Sr, Feinstein S, Kondos G, Perez A, Mazzone T. Relation of abdominal fat depots to systemic markers of inflammation in type 2 diabetes. Diabetes Care. 2009;32:932–7.
Juhan-Vague I, Thompson SG, Jespersen J. Involvement of the hemostatic system in the insulin resistance syndrome. A study of 1500 patients with angina pectoris. The ECAT angina pectoris study group. Arterioscler Thromb. 1993;13:1865–73.
Ceriello A, Giugliano D, Quatraro A, Dello Russo P, Torella R. Blood glucose may condition factor VII levels in diabetic and normal subjects. Diabetologia. 1988;31:889–91.
Konya H, Hasegawa Y, Hamaguchi T, Satani K, Umehara A, Katsuno T, Ishikawa T, Miuchi M, Kohri K, Suehiro A, et al. Effects of gliclazide on platelet aggregation and the plasminogen activator inhibitor type 1 level in patients with type 2 diabetes mellitus. Metabolism. 2010;59:1294–9.
Singer J, Weissler Snir A, Leshem-Lev D, Rigler M, Kornowski R, Lev EI. Effect of intensive glycemic control on platelet reactivity in patients with long-standing uncontrolled diabetes. Thromb Res. 2014;134:121–4.
Schneider DJ. Factors contributing to increased platelet reactivity in people with diabetes. Diabetes Care. 2009;32:525–7.
Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N, Jinnouchi H, Sugiyama S, Saito Y. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. Jama. 2008;300:2134–41.
Trovati M, Anfossi G, Cavalot F, Vitali S, Massucco P, Mularoni E, Schinco P, Tamponi G, Emanuelli G. Studies on mechanisms involved in hypoglycemia-induced platelet activation. Diabetes. 1986;35:818–25.
Mikhailidis DP, Barradas MA, Jeremy JY, Dandona P. Effect of alpha a-adrenoceptor antagonist on platelet activation during insulin-induced hypoglycaemia in type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1989;32:147–9.
Takeda H, Kishikawa H, Shinohara M, Miyata T, Suzaki K, Fukushima H, Ichinose K, Shichiri M. Effect of alpha 2-adrenoceptor antagonist on platelet activation during insulin-induced hypoglycaemia in type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1988;31:657–63.
Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2013;369:362–72.
Kishikawa H, Takeda H, Kiyota S, Sakakida M, Fukushima H, Ichinose K, Matsuda H, Nakamura N, Uzawa H. Role of alpha 2-adrenergic receptor in platelet activation during insulin-induced hypoglycemia in normal subjects. Diabetes. 1987;36:407–12.
Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–84.
Hutton RA, Mikhailidis D, Dormandy KM, Ginsburg J. Platelet aggregation studies during transient hypoglycaemia: a potential method for evaluating platelet function. J Clin Pathol. 1979;32:434–8.
De Caterina R, Madonna R, Sourij H, Wascher T. Glycaemic control in acute coronary syndromes: prognostic value and therapeutic options. Eur Heart J. 2010;31:1557–64.
Arima Y, Kaikita K, Ishii M, Ito M, Sueta D, Oimatsu Y, Sakamoto K, Tsujita K, Kojima S, Nakagawa K, et al. Assessment of platelet-derived thrombogenicity with the total thrombus-formation analysis system in coronary artery disease patients receiving antiplatelet therapy. J Thromb Haemost. 2016;14:850–9.
Idemoto Y, Miura SI, Norimatsu K, Suematsu Y, Hitaka Y, Shiga Y, Morii J, Imaizumi S, Kuwano T, Iwata A, et al. Evaluation of the antithrombotic abilities of non-vitamin K antagonist oral anticoagulants using the Total Thrombus-formation analysis system((R)). Heart Vessel. 2017;32:309–16.
Ishii M, Kaikita K, Ito M, Sueta D, Arima Y, Takashio S, Izumiya Y, Yamamoto E, Yamamuro M, Kojima S, et al. Direct Oral anticoagulants form Thrombus different from warfarin in a microchip flow chamber system. Sci Rep. 2017;7:7399.
Acknowledgements
None.
Funding
No applicable funding.
Author information
Authors and Affiliations
Contributions
KY, TI, AA, HA, HH, TD, IM and NY participated in the design and conception of the design. KY, TI and TN collected the data. KY and TI analyzed the data. SGD, AS, MK, AA and HA participated in recruitment and study conduction. TI and YN interpreted the data and edited the manuscript. YN was the principal investigator of the study who was primarily responsible for the conception and design of the study and oversaw the entire conduction, data collection and analysis the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Kagoshima University Graduate School of Medicine and Dentistry and registered under UMIN000019899 (STUDY 2). All the patients provided written informed consent to participate.
Consent for publication
Not applicable.
Competing interests
T-TAS is a product of Fujimori Kogyo Co., Ltd. where T.N. are employed. I.M. received research support from Fujimori Kogyo Co., Ltd., however, it is for academic promotion and is not directly related to this study. The remaining authors have disclosed they do not have any conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Glucose-lowering medication and lipid-lowering drugs before and after glycemic control in STUDY1. Table S2. Changes in the mean PG, AR-T10, AR-OT and AR-AUC30 in STUDY1. Table S3. Changes in the PG, epinephrine, PL-T10 and PL-AUC10 in STUDY2. Figure S1. Intra-assay precision of the T-TAS AR assay (A) and the PL assay (B) was analyzed using a whole blood sample. Figure S2. Correlations between the change in PL-T10 and the change in Ht (A), WBC count (B), or platelet count (C) in STUDY2. Spearman's rank correlation was used for assessing correlation (n=10) (DOCX 254 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Yamamoto, K., Ito, T., Nagasato, T. et al. Effects of glycemic control and hypoglycemia on Thrombus formation assessed using automated microchip flow chamber system: an exploratory observational study. Thrombosis J 17, 17 (2019). https://doi.org/10.1186/s12959-019-0206-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12959-019-0206-8