Abstract
Background
To explore if exogenous progestin required for progestin primed ovarian stimulation (PPOS) protocol compromises the euploidy rate of patients who underwent preimplantation genetic testing cycles when compared to those who received the conventional gonadotropin-releasing hormone (GnRH) antagonist protocol.
Methods
This retrospective cohort study analyzed 128 preimplantation genetic testing for aneuploidy (PGT-A) cycles performed from January 2018 to December 2021 in a single university hospital-affiliated fertility center. Infertile women aged 27 to 45 years old requiring PGT-A underwent either PPOS protocol or GnRH-antagonist protocol with in-vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) for fertilization. Frozen embryo transfers were performed following each PGT-A cycle. Data regarding the two groups were analyzed using the Statistical Package for Social Sciences (SPSS) version 22.0 (SPSS Inc., Chicago, IL).
Results
Patients who underwent PPOS treatment had significantly reduced blastocyst formation rate and euploidy rate compared to those who received the GnRH antagonist protocol. Subgroup-analysis was performed by stratifying patients’ age into elder and young subgroups (elder: ≥ 38-year-old, young: < 38-year-old). In the elder sub-population, the blastocyst formation rate of the PPOS group was significantly lower than that of the GnRH-antagonist group (45.8 ± 6.1% vs. 59.9 ± 3.8%, p = 0.036). Moreover, the euploidy rate of the PPOS group was only about 20% of that of the GnRH-antagonist group (5.4% and 26.7%, p = 0.006). In contrast, no significant differences in blastocyst formation rate (63.5 ± 5.7% vs. 67.1 ± 3.2%, p = 0.45) or euploidy rate (30.1% vs. 38.5%, p = 0.221) were observed in the young sub-population. Secondary outcomes, which included implantation rate, biochemical pregnancy rate, clinical pregnancy rate, live birth rate, and miscarriage rate, were comparable between the two treatment groups, regardless of age.
Conclusion
When compared to the conventional GnRH-antagonist approach, PPOS protocol could potentially reduce the euploidy rate in aging IVF patients. However, due to the retrospective nature of this study, the results are to be interpreted with caution. Before the PPOS protocol is widely implemented, further studies exploring its efficacy in larger populations are needed to define the optimal patient selection suitable for this method.
Trial registration
Human Investigation and Ethical Committee of Chang Gung Medical Foundation (202200194B0).
Similar content being viewed by others
Background
Controlled ovarian hyperstimulation (COH), which involves the administration of exogenous gonadotropins to stimulate ovarian follicle growth, is a crucial checkpoint of the in vitro fertilization (IVF)/artificial reproductive technology (ART) procedure. During COH, the rise in estradiol level can sometime induce a premature luteinizing hormone (LH) surge and early release of oocytes that compromise the retrieval of a cohort of mature oocytes. To overcome this obstacle, various COH protocols have been established to suppress premature LH surge. Notably, the gonadotropin-releasing hormone antagonist (GnRH-antagonist) protocol is among the most widely used methods, because it effectively prevents premature LH surge [1, 2] and reduces the risk of ovarian hyperstimulation syndrome (OHSS) [3]. However, such protocol is associated with disadvantages like multiple injections, frequent monitoring and dose adjustment, and high therapeutic cost [4]. Recently, a newer stimulation regimen, progestin primed ovarian stimulation (PPOS), has emerged with the goal of preventing premature LH surge in a more patient-friendly manner [5].
The PPOS protocol uses exogenous progesterone to suppress endogenous LH secretion in the early follicular phase and maintain a stable hormonal environment for the induction of final oocyte maturation. Earlier studies have shown that the clinical outcomes of PPOS procedures are comparable to that of conventional methods [6,7,8]. Owing to its ease in drug administration, PPOS protocol has gained popularity as the more flexible and patient-friendly COH approach [9,10,11]. Although PPOS is effective, there are concerns about the potential side effects of progestin stimulation on oocyte competence, embryo availability, embryo implantation, and obstetric outcome, especially for patients who have difficulties achieving a successful IVF outcome. In addition, the PPOS protocol has been reported to yield a lower number of retrieved oocytes [12] and exert negative influences on granular cell functions [13,14,15,16] and follicle growths [17]. In some patient populations, the PPOS protocol could even result in a lower cumulative live birth rate and higher cycle cancellation rate when compared to the GnRH-antagonist protocol [18].
In this retrospective study, we seek to clarify whether the PPOS protocol has an adverse impact on oocyte competence and clinical outcomes when compared to the conventional GnRH-antagonist protocol in a cohort of patients who received PGT-A cycles. The results of this study indicate that the PPOS protocol may negatively impact the embryo quality in elder patients, and age may be a critical factor in determining its utility.
Materials and methods
Study subjects
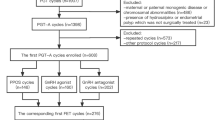
This retrospective study included infertile patients who received PGT-A cycles for advanced maternal age (46.9%, 60/128), recurrent miscarriage (12.5%, 16/128), recurrent IVF failure (22.6%, 29/128), severe male infertility (0.8%, 1/128), or personal request regarding the chromosomal status of the embryos (17.2%, 22/128) from January 2018 to December 2021 in the Chang Gung Memorial Hospital Fertility Center.
Exclusion criteria included those with body mass index (BMI) greater than 30 kg/m2, known chromosomal translocation, endocrine disorders, systemic diseases, and Mullerian malformations. Baseline characteristics, such as the main cause of infertility and ovarian reserve status, were not subjected to exclusion. The study included 128 patients, with an average age of 37.5 years old, categorized into the PPOS arm (34 patients) and the conventional GnRH-antagonist arm (94 patients) (Table 1). The number of previous IVF treatment failure (averaged 1.8 for PPOS and 1.9 for GnRH-antagonist, p = 0.58) and previous miscarriage (averaged 1.1 for PPOS and 0.8 for GnRH-antagonist, p = 0.09) were comparable between the two arms. Choices of ovarian stimulation protocol and gonadotropin dosage were adjusted based on the patient's age, BMI, hormone levels, antral follicle count (AFC), and prior responses to ovarian stimulation. The study was reviewed and approved by the institutional review board of the Human Investigation and Ethical Committee of Chang Gung Medical Foundation (202200194B0).
Treatment protocols
Starting on the second or third day of the menstrual cycle, individualized doses of gonadotropins, between 150–300 IU per day, were typically used, which included the following types of medications: recombinant-follicle-stimulating hormone (r-FSH) combined with recombinant-luteinizing hormone (r-LH, Pergoveris®, 150 IU/75 IU/vial, Merck Serono, Switzerland), recombinant-follicle-stimulating hormone (r-FSH, Gonal-F®, 5.5 mcg/vial, Merck Serono, Switzerland), human menopausal gonadotrophin (HMG, Menopur®, 75 IU/75 IU/vial, Ferring, Germany), or long-acting r-FSH (Elonva® 100 μg/0.5 ml or 150 µg/0.5 ml, Vetter Pharma-Fertigung, Germany).
On the fifth or sixth day of stimulation, follicular size and hormone levels were measured with laboratory and sonographic assessment, and the doses of gonadotropins would be adjusted accordingly. Either oral synthetic progestins or subcutaneous injections of a GnRH antagonist was used for the inhibition of premature LH surge.
In the PPOS arm, medical suppression would begin around day 3 of the menstrual cycle until the trigger day, with a daily dose of medroxyprogesterone acetate (MPA) tablet (Medrone, 10 mg, U-liang, Taiwan) or twice daily dose of dydrogesterone film-coated tablet (Duphaston, 10 mg, Abbott, Netherlands). Patients in the conventional GnRH-antagonist arm received an injection of cetrorelix acetate (Cetrotide®, 0.25 mg/vial, Merck Serono, Switzerland) daily, starting from the fifth day of ovarian stimulation until the trigger day. Final oocyte maturation was induced with 0.2 mg of triptorelin acetate (Decapeptyl, 0.1 mg/ml, Ferring, Germany) or 250 mcg of choriogonadotropin alfa (Ovidrel, 250 mcg, Merck Serono Italy), once the leading follicle reached 18 mm in diameter or greater. Transvaginal oocyte pick-up was performed 36–38 h later.
Oocyte insemination, embryo culture and biopsy preimplantation genetic analysis
Retrieved oocytes were washed with fertilization medium (Sydney fertilization medium, COOK) and incubated in a 6% CO2, 5% O2, and 89% N2 37 °C dry benchtop incubator (G210, K-Systems) for approximately two hours prior to the removal of cumulus cells. Oocytes were transferred to a medium with 40 ~ 120 IU/ml of recombinant human hyaluronidase (ICSI Cumulase, Origio) with a micropipette (Stripper; 275-μm inner diameter, Origio). Subsequent aspiration of the oocytes in and out of a micropipette (Flexipet; 140-μm inner diameters, Cook) multiple times completed the denuding process.
Around four hours after retrieval, intracytoplasmic sperm injection (ICSI) was performed on denuded, metaphase II oocytes. Bathed in culture medium (Continuous Single Culture-NX Complete, Irvine Scientific) under paraffin oil (OVOIL, Vitrolife), the inseminated oocytes were incubated in a 6% CO2, 5% O2, and 89% N2 dry benchtop incubator. Medium change-over (Continuous Single Culture-NX Complete, Irvine Scientific) took place on day 1 and day 3 of sequential culture. Fertilized embryos were observed and evaluated daily. Laserassisted hatching was performed on morula stage embryos, and blastocysts were graded with Gardner Classification prior to biopsy of the trophectoderm. Only blastocysts with a score of AA, AB, BA, BB, or BC were biopsied, which involved mechanical excision of 8–10 trophectoderm cells. The biopsied cells were aspirated with a biopsy pipette (Biopsy Pipette 15,115, 25-μm inner diameter, Vitrolife), washed twice with sterile 1 × phosphate-buffered saline solution (PBS-20X #9808, Cell Signaling), and centrifuged immediately. Cell pellets were stored at -20 °C prior to transporting to a reference laboratory for genetic analysis using next generation sequencing methods.
Clinical outcomes and statistical analysis
The primary outcome evaluated euploidy rate while secondary outcomes included oocyte fertilization rate, blastocyst formation rate, number of oocytes retrieved, biochemical pregnancy rate, clinical pregnancy rate, live birth rate (per cycle), and miscarriage rate. To investigate the potential influence of maternal age, age-stratified subgroup analysis was performed by categorizing patients into those older than or equal to 38 years old or less than 38 years old.
Fertilization rate was calculated by taking the total number of 2 pronuclei stage zygotes divided by the total number of MII oocytes. Euploidy rate was defined as the total number of embryos proven to be euploid divided by the total number of embryos that were biopsied.
Data were analyzed using the Statistical Package for Social Sciences (SPSS) version 22.0 (SPSS Inc., Chicago, IL). The Kruskal–Wallis test was used to compare parameters between groups with non-normal distributions. The Mann–Whitney U test was applied to identify the group causing the difference. For qualitative data, X2 test was implemented while Student's t-test was used for small sample size sets. Results were presented as mean ± standard deviation or mean ± SEM, and a p-value of < 0.05 was considered statistically significant.
Results
A total of 128 infertile women were included in this study, with 94 patients in the conventional GnRH-antagonist protocol and 34 patients in the PPOS protocol treatment groups (Table 1). The average age of the PPOS and GnRH-antagonist groups were 37.9 ± 4.5 and 37.3 ± 4.4 years, respectively. Baseline characteristics, such as age, body mass index (BMI), years of infertility, AMH level, were comparable between the two treatment groups. A total of 407 blastocysts derived from 1527 oocytes were obtained and eligible for analysis (Table 2). In both groups, there were no reported cases of severe OHSS or incidences of premature LH surge.
Analysis of the entire study population showed that the total gonadotropin dose, FSH and LH levels on Day 3, and the LH level on trigger day were comparable between the treatment groups (Table 1). On the other hand, the day of trigger (10.9 ± 1.7 vs. 9.3 ± 1.2, p < 0.001) and the E2 level on trigger day (2314.9 ± 1956.5 vs. 1505.8 ± 1166.2, p = 0.008) were significantly greater in the PPOS group when compared to that of the GnRH-antagonist group.
While the number of oocytes retrieved and the fertilization rate of the two groups were similar, the percentage of MII oocytes in the PPOS group was significantly higher than that of the GnRH-antagonist group (86.6% vs. 80.0%, p = 0.004). However, patients in the PPOS group had significantly lower blastocyst formation rate (53.6 ± 4.5% vs. 63.5 ± 2.5%, p = 0.042) and euploidy rate (17.0 ± 4.6% vs. 31.8 ± 3.5%, p = 0.027) when compared to the GnRH-antagonist group. Additionally, analysis focused on a per embryo basis showed that the euploidy rate of PPOS group remains significantly lower than that of the GnRH-antagonist group (26.8% vs. 33.0%, p = 0.029) (Table 2), suggesting that the progestin protocol could affect the developmental processes of retrieved oocytes after fertilization.
Evaluation of clinical outcomes showed that after transfer of an euploid embryo or euploid embryos in either group results in similar clinical outcomes (Table 3). There were no significant differences in implantation rate (36.7% vs. 39.8%, p = 0.766), biochemical pregnancy rate (55% vs. 53%, p = 0.897), clinical pregnancy rate (40% vs. 41.7% p = 0.896), live birth rate (35% vs. 33.3%, p = 0.891), or miscarriage rate (5% vs. 8.3% p = 0.624) between the treatment groups (Table 3).
Sub-analysis by stratifying patients into young (< 38 years old) and elder (≥ 38 years old or older) revealed that the overall pattern of clinical differences between the treatment groups was only apparent in the elder subgroup (Tables 1 and 2). For patients ≥ 38 years old, those in the PPOS group (mean age = 41.1 ± 1.9) had significantly lower blastocyst formation rate (45.8 ± 6.1% vs. 59.9 ± 3.8%, p = 0.036) and euploidy rate (8.3 ± 6.1% vs. 24 ± 4.2%, p = 0.003) when compared to those in the GnRH-antagonist group (mean age = 40.9 ± 2.0) (Table 1). Likewise, when the data was analyzed on a per embryo basis, the euploidy rate of the PPOS group remained significantly lower than that of the GnRH-antagonist group (5.4% vs. 26.7%, p = 0.006) (Table 2). The rate of euploid blastocyst per injected MII oocyte of the elder PPOS and elder GnRH antagonist subgroup was 1.3% and 8.3%, respectively. The elder PPOS subgroup was triggered at a later day (10.8 ± 1.5 vs. 9.3 ± 1.2, p < 0.001) and had a significantly higher rate of MII formation (89.5% vs. 81.9%, p = 0.024) compared to the elder GnRH-antagonist subgroup (Table 1).
In contrast, there were no significant differences in blastocyst formation rate or euploidy rate between the young PPOS and young GnRH-antagonist subgroups (Table 1). Similarly, no significant difference in the euploidy rate was observed between the young subgroups when the data was analyzed on a per embryo basis (30.1% vs. 38.5%, P = 0.221) (Table 2). The rate of euploid blastocyst per injected MII oocyte of the young PPOS and young GnRH-antagonist subgroup was 10.5% and 13.1%, respectively. While patients in the young PPOS subgroup were also triggered later (day 11.1 ± 1.9 vs. day 9.4 ± 1.2, p < 0.001) when compared to those of the young GnRH-antagonist subgroup, they had a significantly higher level of E2 on the day of trigger (2630.4 ± 1692.0 vs. 1526.0 ± 1067.6, p = 0.006) compared to patients in the young GnRH-antagonist subgroup (Table 1).
Similar to the results from the overall study population, there were no significant differences in implantation rate (36.7% vs 39.8% p = 0.766), biochemical pregnancy rate (55% vs 53% p = 0.897), clinical pregnancy rate (40% vs 41.7% p = 0.896), live birth rate (35% vs 33.3% p = 0.891), and miscarriage rate (5% vs. 8.3% p = 0.624) between the protocols in both the elder and young subgroups (Table 3).
Discussion
The current retrospective analysis showed that while clinical outcomes were comparable, regardless of whether the patients received the PPOS or GnRH-antagonist protocol, there were significant differences in the blastocyst formation rate and euploidy rate between the two treatment groups in the older subset of patients. It is possible that: (1) the PPOS protocol is not be as effective as the GnRH-antagonist protocol for patients who are prone to aneuploidy formation, and (2) progestins mainly impact the quality of oocytes and embryos during early embryo formation but not oocyte formation or post-implantation development. Our results, furthermore, raised the question of whether, in elder patients, PPOS protocol is truly noninferior to the standard GnRH-antagonist protocol, in terms of embryo quality.
To prevent premature LH surge during COH, GnRH agonists like leuprolide were initially used to suppress the hypothalamic-pituitary-ovarian (HPO) axis prior to ovarian hyperstimulation. However, such practice occasionally led to prolonged suppression and reduced number of oocytes retrieved [19]. Meanwhile, the use of GnRH antagonists, such as ganirelix or cetrorelix, appeared to allow better control of ovulation and oocyte yield. While the application of GnRH-antagonist protocol had become increasingly prevalent [20], concerns about its cumbersome administration and risk of premature LH surge and OHSS remained [21].
In recent years, an alternative protocol that utilizes synthetic progestin to prevent premature ovulation has emerged and evolved into the PPOS protocol. Similar to the corpus luteum-derived progesterone, high doses of synthetic progestin, such as medroxyprogesterone 17-acetate (MPA), during COH can suppress LH level and prevent premature ovulation [1, 6, 22]. Studies in the past few years had shown that the use of PPOS and GnRH-antagonist protocols yielded comparable number of mature oocytes, blastocyst formation rate, number of good-quality embryos, implantation rate, clinical pregnancy rate, and live birth rate [23]. A meta-analysis further demonstrated that the PPOS protocol resulted in higher number of retrieved oocytes, MII oocytes, viable embryos, as well as a lower rate of premature LH surge than the control protocol in patients with diminished ovarian reserve or normal ovarian reserve [24]. Furthermore, the PPOS protocol seemed to lower the risk of OHSS when compared to the conventional methods, especially in patients with polycystic ovarian syndrome (PCOS) [25].
Because progestins can be administered orally, the PPOS protocol is considered more patient-friendly and has gained popularity and clinical advocates since 2015 [24]. A major short-coming, however, comes from progestin’s negative impact on endometrial receptivity [1], so such application requires freeze-all and FET cycles. As such, it has been proposed that PPOS should be the first choice for cases that require fertility preservation, not suitable for fresh embryo transfers (e.g., oocyte donors, PGT-A, and PGT-M cycles), or at high risk for OHSS.
However, since high levels of progestins had been shown to affect some aspects of ovarian physiology, we hypothesized that the PPOS protocol could impact specific steps of embryogenesis. Consistent with this theory, we noticed that while the PPOS protocol produced clinical outcomes comparable to that of the conventional protocol, embryos from the PPOS group had reduced euploidy and blastocyst rates. As euploidy rate is a crucial determinant of a successful IVF and is strongly correlated with maternal age, we further analyzed the data in age-stratified subgroups to factor in the influences of age. The age of 38 was used as the cutoff to divide the study population, because euploidy rate drastically declined after that age [26]. Importantly, we found that the difference in euploidy rate between the two treatment groups could be largely attributed to the results of the elder sub-population. These data suggested that, in selected patients, the use of PPOS protocol could significantly reduce the success of early embryo development but have negligible effect on oocyte yield under COH condition or post-implantation development [11, 25, 27, 28].
While the PPOS protocol's effects on diminished euploidy rate in the elder patients remain to be elucidated, we speculate that multiple factors were at play. First, it could be the result of local effects from supra-physiological progesterone. Not to mention, synthetic progestins exerted effects different from that of natural progesterone, owing to their distinct pharmacodynamic characteristics [29]. With unique affinities not just to progesterone receptors, synthetic progestins also act on other steroid receptors, such as androgen and mineralocorticoid receptors. Exposure of pharmacological progesterone had been shown to significantly reduce blastocyst formation rate in bovine cumulus–oocyte complexes [30] and inhibit the resumption of meiosis in mouse oocytes [31, 32]. Additionally, periovulatory exposure to high doses of progesterone could reduce the number of antral follicles and ovulation rate in hamsters, rabbits, and monkeys [33,34,35] and negatively impact oocyte health, meiosis, cytoplasmic maturation, and fertilization in nonhuman primates [36].
Likewise, earlier clinical studies of IVF patients had insinuated possible negative influences of elevated progesterone level on embryo quality [37,38,39,40]. A reduced cumulative live birth rate (CLBR) [39] and top-quality embryo formation rate [38, 40] were observed in cases with elevated serum progesterone on triggering day [39]. In addition, in a study with oocyte donation cycles, the PPOS protocol significantly reduced the biochemical pregnancy rate, clinical pregnancy rate, and live birth rate when compared to the use of conventional GnRH antagonist despite a comparable number of mature oocytes were retrieved [41]. Furthermore, the use of PPOS protocol had been consistently shown to associate with longer time to live birth (TTLB) in both unselected women [17] and patients of Poseidon Group 1 [42]. As such, pharmacological level of progestin required for the PPOS protocol could contribute to the observed reduction of euploidy rate.
Second, our results indicated that age could be the main contributing factor for the negative impact of PPOS on euploidy rate. While a recent study reported that PPOS had no impact on embryo quality [43], the discordant finding could be associated with the characteristics of patient populations. In the previous study, the average age of the patients was 37 years old, with 39 being the highest age . In contrast, 44.5% (57/128) of the patients in our study was ≥ 39 years old. The average age of elder PPOS and GnRH-antagonist subgroups was 41.1 ± 1.9 years and 40.9 ± 2.0 years, respectively. The lack of impact on euploidy rate in the prior study was more consistent with our observation of the young IVF patients. In addition, the difference in years of infertility between the populations of these studies could partly contribute to the dissimilarities in the results. While the prior study reported an average of 28–30 months of infertility, the elder patients in the present study experienced infertility for around 3.7–4.1 years. It is plausible that the damaging effects of progestins on embryo quality was exacerbated by the longer duration of infertility and the more advanced age. Taken together, these results imply that the PPOS protocol may reduce euploidy rate in aging patients due to (1) an altered hormonal environment that affects the maturation process and chromosomal integrity of early embryos; and (2) age- and infertility duration (i.e., years of infertility)-related decline in follicular environment.
Finally, it is important to note that our finding is unique because prior studies focused on other components of COH protocols and demonstrated that the dose of gonadotropins [44, 45], follicular phase progesterone level prior to oocyte retrieval [46], and the methods of LH suppression have negligible impacts on euploidy rate in IVF patients. Our results revealed that althoughy the PPOS protocol is a clinically acceptable method, given its comparable clinical outcomes in the general population, it may have an impact on embryogenesis of oocytes for aging women. Therefore, cautions should be taken for whom or how a COH protocol is selected.
Major limitations of our study included the retrospective, non-randomized design and small sample size, which caused differences in baseline characteristics of the two treatment groups. Additionally, the lack of standardization in the initial dose and type of medications prescribed could have affected the final outcomes. Moreover, due to the chemical structure of dydrogesterone, we could not accurately monitor the progestin levels with simple blood tests. Overall, a study with a larger sample size could provide more robust results.
Conclusion
In summary, our study indicates that the PPOS protocol yields a euploidy rate four times lower than that of the conventional GnRH-antagonist protocol for infertile patients aged 38 and above. While earlier studies that compared PPOS with other protocols have produced conflicting results, our study suggests that age may be an important factor to consider. Despite the advantages of a reduced cost and convenient drug administration, the application of PPOS protocol in aging IVF patients should be carefully reviewed to ensure favorable obstetrical and neonatal outcomes. Further studies regarding the efficacy of the PPOS protocol in a larger population are needed to help elucidate the optimal patient population suitable for this method before it is widely implemented.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AMH:
-
Anti-mullerian hormone
- ART:
-
Artificial reproductive technology
- BMI:
-
Body mass index
- COH:
-
Controlled ovarian hyperstimulation
- E2:
-
Estradiol
- FSH:
-
Follicle-stimulating hormone
- GnRH:
-
Gonadotropin-releasing hormone
- HMG:
-
Human menopausal gonadotrophin
- HPO:
-
Hypothalamic-pituitary-ovarian
- ICSI:
-
Intracytoplasmic sperm injection
- IVF:
-
In-vitro fertilization
- LH:
-
Luteinizing hormone
- MII:
-
Metaphase II
- OHSS:
-
Ovarian hyperstimulation syndrome
- PCOS:
-
Polycystic ovarian syndrome
- PGT-A:
-
Preimplantation genetic testing for aneuploidy
- PPOS:
-
Progestin primed ovarian stimulation
- r-FSH:
-
Recombinant-follicle-stimulating hormone
- r-LH:
-
Recombinant-luteinizing hormone
References
Bosch EVI, Escudero E, Crespo J, Simón C, Remohí J, Pellicer A. Premature luteinization during gonadotropin-releasing hormone antagonist cycles and its relationship with in vitro fertilization outcome. Fertil Steril. 2003;80:1444–9.
Segal S, Glatstein I, McShane P, Hotamisligil S, Ezcurra D, Carson R. Premature luteinization and in vitro fertilization outcome in gonadotropin/gonadotropin-releasing hormone antagonist cycles in women with polycystic ovary syndrome. Fertil Steril. 2009;91:1755–9.
Lambalk CB, Banga FR, Huirne JA, Toftager M, Pinborg A, Homburg R, et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update. 2017;23:560–79.
Ata B, Seli E. Strategies for Controlled Ovarian Stimulation in the Setting of Ovarian Aging. Semin Reprod Med. 2015;33:436–48.
Massin N. New stimulation regimens: endogenous and exogenous progesterone use to block the LH surge during ovarian stimulation for IVF. Hum Reprod Update. 2017;23:221–220.
Kuang Y, Chen Q, Fu Y, Wang Y, Hong Q, Lyu Q, et al. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril. 2015;104(62–70): e3.
Kuang Y, Hong Q, Chen Q, Lyu Q, Ai A, Fu Y, et al. Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen-thawed embryo transfer cycles. Fertil Steril. 2014;101:105–11.
Huang P, Tang M, Qin A. Progestin-primed ovarian stimulation is a feasible method for poor ovarian responders undergoing in IVF/ICSI compared to a GnRH antagonist protocol: A retrospective study. J Gynecol Obstet Hum Reprod. 2019;48:99–102.
Chen H, Wang Y, Lyu Q, Ai A, Fu Y, Tian H, et al. Comparison of live-birth defects after luteal-phase ovarian stimulation vs. conventional ovarian stimulation for in vitro fertilization and vitrified embryo transfer cycles. Fertil Steril. 2015;103:1194–201 e2.
Qin N, Chen Q, Hong Q, Cai R, Gao H, Wang Y, et al. Flexibility in starting ovarian stimulation at different phases of the menstrual cycle for treatment of infertile women with the use of in vitro fertilization or intracytoplasmic sperm injection. Fertil Steril. 2016;106:334-41e1.
Yildiz S, Turkgeldi E, Angun B, Eraslan A, Urman B, Ata B. Comparison of a novel flexible progestin primed ovarian stimulation protocol and the flexible gonadotropin-releasing hormone antagonist protocol for assisted reproductive technology. Fertil Steril. 2019;112:677–83.
Long H, Yu W, Yu S, Yin M, Wu L, Chen Q, et al. Progesterone affects clinic oocyte yields by coordinating with follicle stimulating hormone via PI3K/AKT and MAPK pathways. J Adv Res. 2021;33:189–99.
Peluso JJ, Pappalardo A, Losel R, Wehling M. Progesterone Membrane Receptor Component 1 Expression in the Immature Rat Ovary and Its Role in Mediating Progesterone’s Antiapoptotic Action. Endocrinology. 2006;147:3133–40.
Peluso JJ. Progesterone receptor membrane component 1 and its role in ovarian follicle growth. Front Neurosci. 2013;7:99,1–7.
LA Chaffkin LM, Peluso JJ. The role of progesterone in regulating human granulosa cell proliferation and differentiation in vitro. J Clin Endocrinol Metab. 1993;76:696–700.
Peluso JJ, Liu X, Gawkowska A, Lodde V, Wu CA. Progesterone inhibits apoptosis in part by PGRMC1-regulated gene expression. Mol Cell Endocrinol. 2010;32:153–61.
Chen H, Teng XM, Sun ZL, Yao D, Wang Z, Chen ZQ. Comparison of the cumulative live birth rates after 1 in vitro fertilization cycle in women using gonadotropin-releasing hormone antagonist protocol vs. progestin-primed ovarian stimulation: a propensity score–matched study. Fertil Steril. 2022;118:701–12.
Komatsu KMS. The concentration-dependent effect of progesterone on follicle growth in the mouse ovary. J Reprod Dev. 2017;63:271–7.
Ren J, Sha A, Han D, Li P, Geng J, Ma C. Does prolonged pituitary down-regulation with gonadotropin-releasing hormone agonist improve the live-birth rate in in vitro fertilization treatment? Fertil Steril. 2014;102:75–81.
Tarlatzis BC, Fauser BC, Kolibianakis EM, Diedrich K, Rombauts L, Devroey P. GnRH antagonists in ovarian stimulation for IVF. Hum Reprod Update. 2006;12:333–40.
Tarlatzis BC, Kolibianakis EM. GnRH agonists vs antagonists. Best Pract Res Clin Obstet Gynaecol. 2007;21:57–65.
Di Renzo GC, Tosto V, Tsibizova V. Progesterone: History, facts, and artifacts. Best Pract Res Clin Obstet Gynaecol. 2020;69:2–12.
Ata B, Capuzzo M, Turkgeldi E, Yildiz S, La Marca A. Progestins for pituitary suppression during ovarian stimulation for ART: a comprehensive and systematic review including meta-analyses. Hum Reprod Update. 2021;27:48–66.
Guan S, Feng Y, Huang Y, Huang J. Progestin-Primed Ovarian Stimulation Protocol for Patients in Assisted Reproductive Technology: A Meta-Analysis of Randomized Controlled Trials. Front Endocrinol (Lausanne). 2021;12: 702558.
Wang Y, Chen Q, Wang N, Chen H, Lyu Q, Kuang Y. Controlled Ovarian Stimulation Using Medroxyprogesterone Acetate and hMG in Patients With Polycystic Ovary Syndrome Treated for IVF: A Double-Blind Randomized Crossover Clinical Trial. Medicine (Baltimore). 2016;95: e2939.
Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(656–63): e1.
La Marca A, Capuzzo M. Use of progestins to inhibit spontaneous ovulation during ovarian stimulation: the beginning of a new era? Reprod Biomed Online. 2019;39:321–31.
Sighinolfi G, Sunkara SK, La Marca A. New strategies of ovarian stimulation based on the concept of ovarian follicular waves: From conventional to random and double stimulation. Reprod Biomed Online. 2018;37:489–97.
Piette PCM. The pharmacodynamics and safety of progesterone. Best Pract Res Clin Obstet Gynaecol. 2020;69:13–29.
Silva CCKP. Effects of androgens, progesterone and their antagonists on the developmental competence of in vitro matured bovine oocytes. J Reprod Fertil. 2000;119:261–9.
Salehnia M, Zavareh S. The Effects of Progesterone on Oocyte Maturation and Embryo Development. Int J Fertil Steril. 2012;7:74–81.
Zavareh S, Saberivand A, Salehnia M. The Effect of Progesterone on the In vitro Maturation and Developmental Competence of Mouse Germinal Vesicle Oocytes. Int J Fertil Steril. 2009;3:21–8.
diZerega GSHG. The interovarian progesterone gradient: a spatial and temporal regulator of folliculogenesis in the primate ovarian cycle. J Clin Endocrinol Metab. 1982;54:495–9.
Kim IGG. Stimulatory and inhibitory effects of progesterone on follicular development in the hypophysectomized follicle-stimulating hormone/luteinizing hormone-treated hamster. Biol Reprod. 1987;36:270–6.
Setty SLMT. The effects of progesterone on follicular growth in the rabbit ovary. Biol Reprod. 1987;36:1247–52.
Borman SM, Chaffin CL, Schwinof KM, Stouffer RL, Zelinski-Wooten MB. Progesterone promotes oocyte maturation, but not ovulation, in nonhuman primate follicles without a gonadotropin surge. Biol Reprod. 2004;71:366–73.
Healy MW, Yamasaki M, Patounakis G, Richter KS, Devine K, DeCherney AH, et al. The slow growing embryo and premature progesterone elevation: compounding factors for embryo-endometrial asynchrony. Hum Reprod. 2017;32:362–7.
Huang B, Ren X, Wu L, Zhu L, Xu B, Li Y, et al. Elevated Progesterone Levels on the Day of Oocyte Maturation May Affect Top Quality Embryo IVF Cycles. PLoS ONE. 2016;11: e0145895.
Racca A, Santos-Ribeiro S, De Munck N, Mackens S, Drakopoulos P, Camus M, et al. Impact of late-follicular phase elevated serum progesterone on cumulative live birth rates: is there a deleterious effect on embryo quality? Hum Reprod. 2018;33:860–8.
Vanni VS, Somigliana E, Reschini M, Pagliardini L, Marotta E, Faulisi S, et al. Top quality blastocyst formation rates in relation to progesterone levels on the day of oocyte maturation in GnRH antagonist IVF/ICSI cycles. PLoS ONE. 2017;12: e0176482.
Beguería R, García D, Vassena R, Rodríguez A. Medroxyprogesterone acetate versus ganirelix in oocyte donation: a randomized controlled trial. HumReprod. 2019;34:872–80.
Du M, Zhang J, Li Z, Liu X, Li J, Liu W, et al. Comparison of the Cumulative Live Birth Rates of Progestin-Primed Ovarian Stimulation and Flexible GnRH Antagonist Protocols in Patients With Low Prognosis. Front Endocrinol. 2021;12: 705264.
La Marca A, Capuzzo M, Sacchi S, Imbrogno MG, Spinella F, et al. Comparison of euploidy rates of blastocysts in women treated with progestins or GnRH antagonist to prevent the luteinizing hormone surge during ovarian stimulation. Hum Reprod. 2020;35:1325–31.
Barash O, Hinckley MD, Rosenbluth EM, Ivani KA, Weckstein LN. High Gonadotropin Dosage Does Not Affect Euploidy and PregnancyRates in IVF PGS Cycles With Single Embryo Transfer. Hum Reprod. 2017;32:2209–17.
Sekhon L, Shaia K, Santistevan A, Cohn KH, Lee JA, Beim PY, et al. The cumulative dose of gonadotropins used for controlled ovarian stimulation does not influence the odds of embryonic aneuploidy in patients with normal ovarian response. J Assist Reprod Genet. 2017;34:749–58.
Hernandez-Nieto C, Lee JA, Alkon-Meadows T, Luna-Rojas M, Mukherjee T, Copperman AB, et al. Late follicular phase progesterone elevation during ovarian stimulation is not associated with decreased implantation of chromosomally screened embryos in thaw cycles. Hum Reprod. 2020;35:1889–99.
Acknowledgements
We thank Professor YK Song at Chang Gung Memorial Hospital for the encouragement provided throughout the study.
Attestation statements
Data regarding any of the subjects in the study has not been previously published. Data will be made available for review or query upon request.
Funding
This study received no financial support.
Author information
Authors and Affiliations
Contributions
All authors contributed greatly to this work. In specifics, CC directed the study and supervised the data collection, analytical calculations, writing of the manuscript, and interpretation of the results; AP contributed to the study design, analysed the results, constructed the tables, and drafted the manuscript; YS developed the study idea, collected data, performed statistical analyses, and finalized data presentation; both CJL and CYL performed the laboratory procedures and ensured the integrity and accuracy of all laboratory methods and results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was reviewed and approved by the institutional review board of the Human Investigation and Ethical Committee of Chang Gung Medical Foundation (202200194B0).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pai, A.HY., Sung, Y.J., Li, CJ. et al. Progestin Primed Ovarian Stimulation (PPOS) protocol yields lower euploidy rate in older patients undergoing IVF. Reprod Biol Endocrinol 21, 72 (2023). https://doi.org/10.1186/s12958-023-01124-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12958-023-01124-3