Abstract
Purpose
We aimed to evaluate perioperative complications of radical cystectomy (RC) by using standardized methodology. Additionally, we identified independent risk factors associated with perioperative complications.
Materials and methods
We retrospectively analyzed 30-day and 90-day perioperative complications of 211 consecutive RC patients. The intraoperative and postoperative complications were defined according to Clavien-Dindo classification (CDC) and reported based on the ICARUS criteria, Martin, and EAU quality criteria. Age-adjusted Charlson comorbidity index (ACCI), systemic inflammatory response index (SIRI), body mass index (BMI) ≥ 25 kg/m2, and neoadjuvant chemotherapy (NAC) were also evaluated. Multivariable regression models according to severe (CDC ≥ IIIb grade) complications were tested.
Results
Overall, 88.6% (187/211) patients experienced at least one intraoperative complication. Bleeding during cystectomy was the most common complication observed (81.5% [172/211]). Severe intraoperative complications (EAUiaiC grade > 2) were recorded in 8 patients. Overall, 521 postoperative complications were recorded. Overall, 69.6% of the patients experienced complications. Thirty-nine patients suffered from most severe (CDC ≥ IIIb grade) complications. ACCI (OR: 1.492 [1.144–1.947], p = 0.003), SIRI (OR: 1.279 [1.029–1.575], p = 0.031), BMI (OR: 3.62 [1.58–8.29], p = 0.002), and NAC (OR: 0.342 [0.133–0.880], p = 0.025) were significant independent predictive factors for 90-day most severe complications (CDC ≥ IIIb grade).
Conclusions
RC complications were reported within a standardized manner, concordant with the ICARUS and Martin criteria and EAU guideline recommendations. Complication reporting seems to be improved with the use of standard methodology. Our results showed that ACCI, SIRI, and BMI ≥ 25 kg/m2 and the absence of NAC were significant predictive factors for most severe complications.
Similar content being viewed by others
Introduction
Radical cystectomy (RC) is one of the most difficult and invasive surgical procedures in urology. The surgery involves both gastrointestinal and urinary systems after radical resection of the bladder. Hence, RC has higher perioperative complication rates. The reported perioperative complication rates vary from 19% up to 99% within the first 90 postoperative days [1,2,3,4,5,6]. This big discrepancy may be explained by lack of standardized definitions of perioperative complications. Well-organized data collection with standard assessment is an important aspect of reporting outcomes. In 2002, Martin et al. proposed ten criteria for reporting complications following surgery to achieve a uniform and standardized approach [7]. This concept was adopted by the European Association of Urology (EAU) in their guideline for complication reporting [8]. Additionally, recently, the EAU panel released the Intraoperative Complications Assessment and Reporting with Universal Standards (ICARUS) criteria for reporting intraoperative adverse events [9]. However, in our knowledge, there is no study in the literature that reported complications accordingly fulfilled EAU intraoperative and postoperative quality criteria.
In this study, first, we aimed to evaluate the intraoperative and the 30-day and 90-day postoperative mortality and complications of open RC by using a standardized reporting methodology according to EAU guidelines. Second, we aimed to identify the associated independent risk factors for postoperative complications.
Materials and methods
Patient population
We conducted a retrospective analysis of perioperative complications operated in our tertiary referral institution. We identified 251 consecutive individuals who had undergone open standard RC, bilateral extended pelvic lymph node dissection, and urinary diversion (ileal loop or Studer pouch) for bladder cancer between 2009 and 2021. All patients had at least 90-day follow-up or died within 90 days. Patients who underwent an additional nephroureterectomy (n = 19), urinary diversion with ureterocolostomy (n = 3), and pelvic exenteration procedure (n = 2); who had previous radiotherapy (n = 6) and non-urothelial carcinoma (n = 5); and those who had two or more procedure simultaneously (n = 5) were excluded (Supplementary Fig. 1). The remaining 211 patients met the inclusion criteria. Surgery was performed by five surgeons, whereas two of them were trained during the study period. For perioperative care after RC, a protocol similar to the modified ERAS has been used in our clinic since 2015. In 2019, our protocol was revised to be more compatible with ERAS. The patients with full compliance to ERAS was 22.2% (47/211).
Data acquisition
In order to obtain a detailed perioperative 90-day complication follow-up, two urology residents (B. C., K. C.) screened our medical records for inpatient, outpatient, and emergency clinics. Patient demographics, all intraoperative and postoperative 30-day and 90-day complications and causes of readmission, reoperation, and mortality were noted. Comorbidities were also assessed with age-adjusted Charlson comorbidity index (ACCI) [10].
We calculated the following previously well-known inflammatory-nutritional markers reported in the literature, based on the blood tests on the day before surgery: (1) the platelet-to-lymphocyte ratio (PLR) [11]; (2) albumin-to-globulin ratio (AGR) [12]; (3) the systemic inflammatory response index (SIRI) (the product of neutrophils and monocytes, divided by lymphocyte count) [13,14,15,16,17]; and (4) the prognostic nutritional index (PNI) [(10 × blood albumin count) + 0.005 × blood lymphocyte count)] [18].
Assessment of complications
In order to adhere to the EAU guidelines for reporting and grading complications, the intraoperative complications were defined and reported based on ICARUS criteria [9] and the postoperative complications based on EAU proposal [8] and also the Martin criteria [7]. Intraoperative complications were graded according to EAU intraoperative adverse incident classification (EAUiaiC) [19]. Each postoperative complication was graded according to a modified version of the CDC [20].
The study was approved by the local ethics committee (approval number: KÜ GOKAEK-2022/05.06). The informed and signed consent has been obtained from all patients prior to surgery.
Statistical analysis
Statistical analyses were performed using SPSS Statistics 21 (IBM, Armonk, NY, USA). A p < 0.05 indicated statistical significance. The independent Student t-test and Mann-Whitney U were used for parametric and nonparametric values. Chi-square test was used for categorical values. Patient demographics were stratified and compared according to ACCI (< 6 vs. ≥ 6).
Univariable and multivariable logistic regression models were used to predict the most severe complication CDC ≥ grade IIIb (separately for 30 days and 90 days). All factors that could potentially influence the most severe complications were tested with simple logistic regression analyses. BMI was categorized as dichotomous variable according to the presence of obesity (< 25 kg/m2/≥ 25 kg/m2).
Receiver operating characteristic (ROC) curve analyses were used to assess the true-positive (sensitivity) and false-positive rates (1- specificity) of SIRI and ACCI with reference to the most severe complication binary outcome. ROC curve analyses were performed with the MedCalc 20.106 trial version. The areas under the curve (AUC) were calculated. The value with the highest sensitivity and specificity was selected as the cutoff value (Youden index J).
Results
Two-hundred eleven patients are included in the analyses. Demographics according to ACCI groups are given in Table 1. About 45% (94/211) of patients had ACCI ≥ 6 comorbidity. These patients had higher median age and incidence of prior pelvic surgery and had lower e-GFR and operation time; they were less smokers (p < 0.05). Statistically significant difference was found between ACCI groups according to pathological stages and characteristics.
Reporting of the intraoperative complications according to ICARUS criteria are given in Table 2. Overall, 196 intraoperative complications were recorded in 211 patients. Overall, 88.6% (187/211) patients experienced at least one intraoperative complication. Bleeding during cystectomy caused transfusion was the most common complication observed (81.5% [172/211]). Severe intraoperative complications (EAUiaiC grade > 2) were recorded in 8 patients. Two complications were observed due to malfunction of the surgical instruments (bowel stapler).
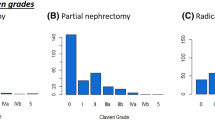
Detailed information about type, severity, and distribution of postoperative complications across different organ systems are given in Table 3. Overall, 521 postoperative complications were recorded in 211 patients (30 days n = 467 and 90 days n = 521). Overall, 69.6% (147/211) patients experienced at least one or more complications. Overall, 45.0% (95/211) of patients had > 1 complications. Gastrointestinal (26.9% at 30 days and 27.8% at 90 days) complications were most commonly observed, while thromboembolic complications were the least common (1.4% at 30 days and 1.7% at 90 days). Thirty-three (15.6%) and 39 (18.4%) patients suffered from most severe complications, requiring an intervention under general anesthesia at 30 days and 90 days, respectively. The distribution of complications according to the CDC is given in Fig. 1.
A total of 9 patients (4.2%) died of various non-cancer-related causes within 90 days of surgery, and these are specified in Table 3. Reasons for reoperations and readmissions are given in Supplementary Table 1. Reoperations related to the primary surgery were performed in 20 (9.4%) patients and were most commonly ileus (n = 19) related (Table 3). Thirty-five patients were readmitted to the hospital in 30 days and 42 patients in 90 days. Gastrointestinal causes are most common (Supplementary Table 1). The compliance of our postoperative complication recording with EAU quality criteria is given in Supplementary Table 2.
In the logistic regression analyses, in addition to preoperative clinical inflammatory-nutritional indexes, which are frequently encountered in the literature, we also evaluated other operative and preoperative factors for the prediction of most severe (CDC ≥ grade IIIb) complications (Table 4). For 30-day complications, ACCI (p < 0.001) and BMI ≥ 25 kg/m2 were significant independent factors in multivariable analysis, while at 90-day complications, the presence of neoadjuvant chemotherapy (NAC) and SIRI was also found to be independent predictive factors in addition to ACCI and BMI ≥ 25 kg/m2. It is obviously seen from the low OR in Table 4 (OR 0.342) that the absence of NAC increases complication rates. Additionally, SIRI was significantly higher in patients with pT4 disease and positive surgical margins (median SIRI 1.90 vs. 1.66; p = 0.037).
We further analyzed SIRI and ACCI in ROC curve analyses to determine a cutoff value to predict 90-day most severe CDC ≥ grade IIIb complication. We found SIRI cutoff value of > 2.35 (53.8% sensitivity, 76.7% specificity) and ACCI cutoff value of > 4 (89.7% sensitivity, 34.9% specificity) for accurate prediction of CDC ≥ grade IIIb complications (Fig. 2).
Discussion
RC is associated with high risk of complications. Especially, RC candidates tend to be elderly and have multiple comorbidities. In the current study, we reported meticulous and comprehensive evaluation of perioperative complications. Additionally, we paid attention to meet both the ICARUS criteria [9], Martin criteria [7], and the EAU guideline recommendations for complications [8]. Studies that reported complications in a standard manner are lacking in the literature. Maibom et al. conducted a systematic review of the prevalence of short-term (< 90 days) morbidity and mortality following RC [6]. They selected 66 out of 1957 articles that met the inclusion criteria. Only three of them are compatible with the 10/10 Martin criteria. The median number fulfilling the Martin criteria was 6 (range 2–10).
As a result of more detailed evaluation and reporting of the complications, reported number of complications in the studies is also increasing. In 2002, Meller et al. claimed that RC complications occur in 19% of patients [5]. Contrarily, Vetterlein et al. suggested that 99% of their patients experienced at least one complication [2]. The definition of surgical complication is generally defined as any deviation from the ideal postoperative course that is not inherent in the procedure [8]. However, in defining surgical complications, subjectivity cannot always be avoided. This could also be the reason for this inconsistency. Interestingly, Vetterlein et al. reported urinary tract infection in 62% of the patients (although they did not specify whether the infection was detected by urine culture or only by urinalysis), hematuria in 67% (not specified whether microscopic or macroscopic; probably most of them is not a deviation from an ideal course), and new-onset hydronephrosis in 41% (not specified) [2]. On the other hand, Haas et al. reported 85.7% complications for RC [1]. However, the largest part of this was related to intraoperative blood transfusion (21.2%), and it was incorrectly graded with CDC.
Mitroupolus et al. underlined that special attention should also be paid to proper use of the CDC because it has not been designed/validated to grade intraoperative complications, and any modifications and revisions can be confusing [8]. Recently, Biyani et al. published a new EAU guideline to report and grade intraoperative complications [19]. More recently, Cacciamani et al. proposed an EAU guideline to report intraoperative complications called ICARUS criteria [9]. In the current study, intraoperative complications were graded with Biyani et al. proposal (EAUiaiC). After that, we created a table to show full compatibility with ICARUS criteria. To our knowledge, this is the first time in the literature to publish intraoperative complications with full compliance with ICARUS criteria.
Surgical techniques for RC procedures or routines in postoperative care may cause differences, especially in the number and distribution of conservatively followed complications that do not require surgery. In our clinic, routine broad-spectrum antibiotic prophylaxis, use of antiemetics and bowel movement enhancers, active lung exercises, use of bronchodilators for the first 3 days, early mobilization, and low-molecular-weight heparin prophylaxis are performed for all patients. In our current study, the overall rate for any complication is 69.1%. GIS (27.5%) complications were the most frequent. Most severe postoperative complications requiring intervention (CDC ≥ grade IIIb) were found in 18.4%, compatible with the literature [1,2,3, 21, 22].
In the current study, SIRI, NAC, BMI ≥ 25 kg/m2, and ACCI were found to be independent predictors of most severe complications. Ornaghi et al. investigated the impact of preoperative nutritional factors (BMI, hypoalbuminemia, and sarcopenia) on complication and mortality rates after RC in a systematic review. They found that high BMI, hypoalbuminemia, and sarcopenia significantly increased the complication rates after RC [23]. Similarly, in the current study, BMI ≥ 25 kg/m2 was found to be a strong predictor of both 30-day and 90-day complications. We found that patients receiving NAC had fewer severe 90-day complication rates. This may be attributed to the fact that receiving NAC improves surgery-related outcomes. It is well known that NAC does not increase RC complications [24,25,26,27,28]. Additionally, Hoeh et al. reported complications in a detailed manner, and they found that patients who received NAC had significantly less surgical site, cardiac, pulmonary, and genitourinary complications than those who did not [26]. Similarly, Jerlström et al. found that RC patients who received NAC had significantly less GIS complications [27].
Vetterlein et al. found that ACCI and delta hemoglobin were independent predictors of most severe complications [2]. Hirobe et al. found that BMI ≥ 25 kg/m2, smoking history, and CCI ≥ 2 were independent risk factors for high-grade complications [22]. Zareba et al. reported that increased number of postoperative complications were observed with increasing ACCI [21]. We also found that ACCI ≥ 6 was a significant cutoff to predict most severe complications.
Previously, AGR, PLR, and PNI were found to be associated with oncological outcomes of RC [11, 12, 18]. In the current study, these markers were evaluated to predict complications, and no significant relationship was found. Recently, Claps et al. proposed another immune-nutritional marker called as CONUT score in a multi-institutional retrospective study. They were calculated the CONUT scores according to the serum albumin, lymphocyte count, and cholesterol levels of the patients. They found that high CONUT score (≥ 3) was independently predictive for both complications and oncologic outcomes [29]. Standard methods should be used in studies reporting complications [8]. This principle is the main topic of the current study. Very interestingly, Claps et al. did not clarify how they obtained and reported all complications from five different European centers in a standardized way. They also did not comment or report any limitation about this. Therefore, external validation is obviously needed for their notable results.
SIRI was found to be an independent predictor of 90-day most severe complications. In 2016, Qi et al. first described that SIRI showed good prognostic value in patients with pancreatic cancer [14]. Subsequently, SIRI has also been shown to be associated with various cancers [13, 15,16,17]. Urbanowicz et al. found SIRI to be associated with post-bypass mortality [30], Jin Z. et al. with ischemic stroke [31], and Lee L. E. et al. with all-cause mortality of vasculitis [32]. The precise mechanisms underlying the association between increasing SIRI levels and poor prognosis prevail to be uncovered. The high level of monocyte and neutrophil count represents a higher tumor burden as they play major roles on increasing the tumor cell migration, invasion, and angiogenesis and suppression of anti-tumor immune reaction [33, 34]. In contrast, lymphocytes have a vital function in anti-tumor defense via direct and antigen-dependent cytotoxic cell death and suppression of the tumor proliferation functions [35]. Therefore, higher level of SIRI indicates increased levels of the immunosuppressive monocytes and neutrophils and decreased levels of the immunogenic lymphocytes. Patients with high tumor burden may be more frail and prone for postoperative complications. In the current study, SIRI was found significantly higher in patients with pT4 stage and/or positive surgical margin. This might be responsible to some extent for severe complications in RC patients.
To our knowledge, first time in the literature, we found that SIRI is independent predictor for severe complications. However, very similar terminology of inflammatory markers causes confusion. For example, the marker obtained by multiplying the neutrophil-to-lymphocyte ratio with the platelet count was named systemic inflammatory index (SII) [36]. Despite that in the current study, SIRI obtained by multiplying neutrophil-to-lymphocyte ratio with monocytes count. Monocytes and platelets have very different and mostly unrelated functions in the human body. On the other hand, Ni et al. were using same terminology (SIRI) for hemoglobin × monocytes/lymphocytes [17], and they analyzed this marker for the cystectomy complications. Again, this study has very different methodology from the current study.
There are some important limitations of this study. Retrospective design of the study is the most important, although Andersen et al. showed that prospectively and retrospectively collected complication data were similar [4]. The current study analyzed the intraoperative complications of open RC. We reviewed the surgical records to investigate iAEs. In laparoscopic or robot-assisted surgery, retrospectively viewing surgical video data can provide additional advantage. Therefore, some EAUiaiC grade 0 complications may not have been mentioned in the surgical records of open RC. Another important limitation is caused from the nature of the CDC. For example, open wounds can be sutured under local anesthesia. However, it may also be performed under general anesthesia just because of patient preference; in this case, the complication grade increases to CDC ≥ IIIb. On the other hand, after the patient leaves the hospital, minor complications may be under-collected. There is also a possibility that complications may be over-looked (not recorded) because patient who experience complications after discharge may administer to local hospitals close to their homes. However, almost all of the patients are referred to our tertiary referral center due to the general health practice in our country. EAU muscle-invasive bladder cancer guideline noted that higher RC hospital volume is associated with lower postoperative mortality rates and higher quality of care [37]. And they also recommended performing at least 10, and preferably > 20, RCs per hospital/per year. Despite the fact that this study meets the minimum criteria, more is better. Cohorts with more cases could improve complications. On the other hand, a guideline was published on the use of the ERAS protocol in RC in late 2013 [38]. The fact that our study included patients before this date and also our ERAS compliance was 22% limits our results. This study was designed to assess all RCs performed by high- and low-volume surgeons and fellowship trained surgeons, and no case was excluded based on surgeon case volume alone. However, all RCs were performed under the supervision of a senior surgeon (OD).
Conclusions
In the current study, for the first time in the literature, detailed open RC complications were reported within a standardized manner, concordant with both the ICARUS and Martin criteria and EAU guideline recommendations. RC is surgery that has high complication rates. Both intraoperative and postoperative complications obviously increased with using standard methodology. ACCI, SIRI, BMI ≥ 25 kg/m2, and the absence of NAC were independent predictive factors for most severe complications (CDC ≥ IIIb). The cutoff values of > 2.35 for SIRI > 4 for ACCI could be predict of CDC ≥ grade IIIb complications.
Availability of data and materials
All of the material is owned by the authors, and/or no permissions are required.
References
Haas M, et al. The comprehensive complication index is associated with a significant increase in complication severity between 30 and 90 days after radical cystectomy for bladder cancer. Eur J Surg Oncol. 2021;47:1163–71.
Vetterlein MW, et al. Improving estimates of perioperative morbidity after radical cystectomy using the European Association of Urology Quality Criteria for Standardized Reporting and Introducing the Comprehensive Complication Index. Eur Urol. 2020;77:55–65.
Kowalewski KF, et al. The comprehensive complication index (CCI): proposal of a new reporting standard for complications in major urological surgery. World J Urol. 2021;39:1631–9.
Andersen CS, et al. Prospective versus retrospective recordings of comorbidities and complications in bladder cancer patients undergoing radical cystectomy - a randomized controlled trial. Scand J Urol. 2022;56:6–11.
Meller AE, et al. Complications in radical cystectomy performed at a teaching hospital. Int Braz J Urol. 2002;28:522–5.
Maibom SL, et al. Short-term morbidity and mortality following radical cystectomy: a systematic review. BMJ Open. 2021;11:e043266.
Martin RC 2nd, et al. Quality of complication reporting in the surgical literature. Ann Surg. 2002;235:803–13.
Mitropoulos D, et al. Reporting and grading of complications after urologic surgical procedures: an ad hoc EAU guidelines panel assessment and recommendations. Eur Urol. 2012;61:341–9.
Cacciamani GE, et al. The Intraoperative Complications Assessment and Reporting with Universal Standards (ICARUS) Global Surgical Collaboration Project: Development of Criteria for Reporting Adverse Events During Surgical Procedures and Evaluating Their Impact on the Postoperative Course. Eur Urol Focus. 2022;8:1847-58.
Koppie TM, et al. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer. 2008;112:2384–92.
Lei Y, et al. Prognostic values of preoperative neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and lymphocyte- to-monocyte ratio for patients with muscle- invasive bladder cancer undergoing radical cystectomy. Arch Esp Urol. 2022;75:287–94.
Wang K, et al. Combination of total psoas index and albumin-globulin score for the prognosis prediction of bladder cancer patients after radical cystectomy: a population-based study. Front Oncol. 2021;11:724536.
Zhao M, et al. Prognosis of hepatocellular carcinoma and its association with immune cells using systemic inflammatory response index. Future Oncol. 2022;18:2269-88.
Qi Q, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016;122:2158–67.
Jiang C, et al. The Pretreatment Systemic Inflammation Response Index as a Useful Prognostic Factor is Better Than Lymphocyte to Monocyte Ratio in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy. Clin Breast Cancer 2022;22:424-38.
Chen Z, et al. Systemic inflammation response index predicts prognosis in patients with clear cell renal cell carcinoma: a propensity score-matched analysis. Cancer Manag Res. 2019;11:909–19.
Ni J, et al. Prognostic value of the systemic inflammatory response index in patients undergoing radical cystectomy for bladder cancer: a population-based study. Front Oncol. 2021;11:722151.
Qi F, et al. Pre-treatment prognostic nutritional index may serve as a potential biomarker in urinary cancers: a systematic review and meta-analysis. Cancer Cell Int. 2018;18:207.
Biyani CS, et al. Intraoperative Adverse Incident Classification (EAUiaiC) by the European Association of Urology ad hoc Complications Guidelines Panel. Eur Urol. 2020;77:601–10.
Dindo D, et al. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Zareba A, et al. The comprehensive complication index. Proposed modification to improve estimates of perioperative morbidity after radical cystectomy. A pilot study. Cent European. J Urol. 2021;74:288–94.
Hirobe M, et al. Complications within 90 days after radical cystectomy for bladder cancer: results of a multicenter prospective study in Japan. Int J Clin Oncol. 2018;23:734–41.
Ornaghi PI, et al. The impact of preoperative nutritional status on post-surgical complication and mortality rates in patients undergoing radical cystectomy for bladder cancer: a systematic review of the literature. World J Urol. 2021;39:1045–81.
Johnson DC, et al. Neoadjuvant chemotherapy for bladder cancer does not increase risk of perioperative morbidity. BJU Int. 2014;114:221–8.
Arora A, et al. Neoadjuvant chemotherapy does not increase peri-operative morbidity following radical cystectomy. World J Urol. 2022;40:1697–705.
Hoeh B, et al. Effect of Neoadjuvant Chemotherapy on Complications, in-Hospital Mortality, Length of Stay and Total Hospital Costs in Bladder Cancer Patients Undergoing Radical Cystectomy. Cancers (Basel). 2022;14(5):1222.
Jerlström T, et al. No increased risk of short-term complications after radical cystectomy for muscle-invasive bladder cancer among patients treated with preoperative chemotherapy: a nation-wide register-based study. World J Urol. 2020;38:381–8.
Gandaglia G, et al. The effect of neoadjuvant chemotherapy on perioperative outcomes in patients who have bladder cancer treated with radical cystectomy: a population-based study. Eur Urol. 2014;66:561–8.
Claps F, et al. Impact of the controlling nutritional status (CONUT) score on perioperative morbidity and oncological outcomes in patients with bladder cancer treated with radical cystectomy. Urol Oncol. 2023;41:49.e13–22.
Urbanowicz T, et al. Neutrophil Counts, Neutrophil-to-Lymphocyte Ratio, and Systemic Inflammatory Response Index (SIRI) Predict Mortality after Off-Pump Coronary Artery Bypass Surgery. Cells. 2022;11(7):1124.
Jin Z, et al. Systemic inflammatory response index as an independent risk factor for ischemic stroke in patients with rheumatoid arthritis: a retrospective study based on propensity score matching. Clin Rheumatol. 2021;40:3919–27.
Lee LE, et al. Systemic inflammation response index predicts all-cause mortality in patients with antineutrophil cytoplasmic antibody-associated vasculitis. Int Urol Nephrol. 2021;53:1631–8.
Shibutani M, et al. The peripheral monocyte count is associated with the density of tumor-associated macrophages in the tumor microenvironment of colorectal cancer: a retrospective study. BMC Cancer. 2017;17:404.
Franklin RA, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–5.
Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–4.
Gu L, et al. Prognostic value of a systemic inflammatory response index in metastatic renal cell carcinoma and construction of a predictive model. Oncotarget. 2017;8:52094–103.
Bruins HM, et al. The importance of hospital and surgeon volume as major determinants of morbidity and mortality after radical cystectomy for bladder cancer: a systematic review and recommendations by the European Association of Urology Muscle-invasive and Metastatic Bladder Cancer Guideline Panel. Eur Urol Oncol. 2020;3:131–44.
Witjes JA, et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: summary of the 2020 guidelines. Eur Urol. 2021;79:82–104.
Funding
No financial funding was received.
Author information
Authors and Affiliations
Contributions
Study concepts: NBC. HY. Study design: NBC. HY. Data acquisition: NBC. KC. Quality control of data and algorithms: IEA. Data analysis and interpretation: HY. KC. Statistical analysis: HY. IEA. Manuscript preparation and prepared tables and figures: AQ8NBC. Manuscript editing: HY. KT. Manuscript review: OD. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the local ethics committee (approval number: KÜ GOKAEK-2022/05/06). The informed and signed consent has been obtained from all patients prior to surgery.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Fig. 1.
Flowchart illustrating the recruiting process.
Additional file 2: Supplementary Table 1.
Re-operations and Re-admissions causes of patients.
Additional file 3: Supplementary Table 2.
The fulfilment of EAU quality criteria.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cinar, N.B., Yilmaz, H., Avci, I.E. et al. Reporting perioperative complications of radical cystectomy: the influence of using standard methodology based on ICARUS and EAU quality criteria. World J Surg Onc 21, 58 (2023). https://doi.org/10.1186/s12957-023-02943-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-023-02943-9