Abstract
Background
Following the establishment of the anti-cancer effect of immune checkpoint inhibitors, lymphopenia has attracted attention as a parameter of preexisting cancer-related immune tolerance. Although the pretreatment absolute lymphocyte count (ALC) has been reported as a prognostic factor in gastric cancer patients, the impact of perioperative changes in the ALC remains unknown. The aim of the present study was to explore the relationship between surgery-induced lymphopenia and outcome.
Methods
Database entries for 584 patients who underwent curative resections for pathological Stage IB-III gastric cancer were reviewed. We retrospectively compared clinicopathological factors including pretreatment ALC (pre-ALC) and ALC at first visit after discharge (post-ALC) with the survival. The low ALC was defined as < 1000/μL.
Results
The ALC decreased significantly at 1 and 3 days after surgery and then recovered to the baseline value. A low pre-ALC (p < 0.001) and a low post-ALC (p < 0.001) were both correlated with a poor relapse-free survival (RFS). A multivariate analysis of RFS identified a low post-ALC (hazard ratio 1.875, 95% CI 1.156–3.402, p = 0.01), age, gender, BMI, T disease, N disease, severe vessel invasion, type of gastrectomy and postoperative morbidity as independent factors. The low post-ALC group had a poor RFS among patients with Stage II (p = 0.04) and Stage III (p = 0.04) disease, but not among patients with Stage IB disease (p = 0.13). Consistently, the overall survival (OS) rate was significantly lower among patients with a low post-ALC for all stage (p < 0.001), stage II (p = 0.02) and stage III (p = 0.01) disease, not for stage IB (p = 0.09). A low post-ALC was identified as an independent factor for predicting OS by multivariate analysis (hazard ratio: 2.275, 95% CI 1.373–3.769, p = 0.01).
Conclusions
A decrease in post-ALC was correlated with both of RFS and OS after curative resection in patients with locally advanced gastric cancer.
Highlights
Postoperative lymphopenia was a poor prognostic factor for gastric cancer.
Similar content being viewed by others
Background
Gastric cancer is common as a cause of cancer-related death worldwide [1]. Though the curative resection is the most promising treatment for a cure, patients with locally advanced gastric cancer often die of recurrence after surgery [2]. The pathological tumor-node-metastasis (TNM) stage is reliable indicator of the possibility of residual foci of cancer during potentially curative resection, from which the recurrence is supposed to arise. Adjuvant chemotherapy is established under the concept to treat remnant lesions and prevent the recurrence, then randomized controlled trials (RCTs) clearly showed the efficacy [3, 4]. However, the process by which residual micro-metastases develop into recurrences requires clarification for the further improvement of multimodal treatments.
The host inflammatory response is thought to play an important role in cancer development and progression, and host immunocytes are an essential component of the tumor microenvironment [5, 6]. Lymphopenia is considered a parameter of preexisting cancer-related immune tolerance. Several studies have reported that the pretreatment absolute lymphocyte count (ALC), which can be estimated by performing a peripheral blood examination at baseline, was significantly correlated with the prognosis of patients with solid cancers [7,8,9,10].
Surgical trauma is known to induce an inflammatory cascade composed of systemic inflammatory response syndrome (SIRS) and a subsequent anti-inflammatory response known as compensatory anti-inflammatory response syndrome (CARS) [11,12,13]. The ALC is known to decrease temporarily after surgery, reflecting the degree of CARS [14]. This series of responses might influence the development of recurrences. Practically, the postoperative complication accompanying with excessive inflammatory response after gastrectomy for gastric cancer reported to impair survival [15, 16]. On the other hand, the relationship between postoperative CARS and the recurrence has been unknown.
Recently, the potent efficacy of immune checkpoint inhibitors (ICI) for the treatment of advanced gastric cancer, with the aim of regulating immune tolerance, has been established [17,18,19]. The ALC [20,21,22] and the neutrophil-to-lymphocyte ratio (NLR) [23,24,25] have been the focus of attention as prognostic biomarkers for ICI treatment.
The aim of the present study was to investigate the impact of the perioperative ALC on the outcomes of patients who underwent curative resections for locally advanced gastric cancer.
Methods
Patients
This study was conducted as a retrospective analysis of clinical data from a prospectively maintained database of Niigata Cancer Center Hospital. Patients with pathologically diagnosed Stage IB-III gastric cancer who underwent gastrectomy with curative intent between January 2006 and December 2019 were enrolled. The exclusion criteria were as follows: (1) use of preoperative chemotherapy, (2) remnant gastric cancer, (3) any evidence of residual tumor, (4) simultaneous active malignancy in another organ, (5) simultaneous surgery for other disease, (6) postoperative hospital death, and 7) unavailability of blood examination data collected during a period corresponding to postoperative day (POD) 15–60.
Data collection
Data on clinical variables including age, sex, BMI, tumor location, representative histological feature, surgical findings, postoperative morbidity, pathological findings, pathological TNM stage, presence or absence of postoperative chemotherapy, and compliance with postoperative chemotherapy were collected. The TNM stage was defined according to the Japanese classification of gastric carcinoma, 3rd English edition [26], and the Union for International Cancer Control TNM classification of malignant tumors, 8th edition [27]. ALC was appraised at baseline and on POD 1, POD 3, and POD 7 as well as at the time of the first clinical visit after hospital discharge. In cases with hospitalization for 30 days or more, data obtained at around POD 30 was substituted for that of the first visit date. The median (range) duration from surgery until the day of the first clinical visit or the substituted examination date was 31 (17–60) days. After discharge, patients visited the outpatient clinic every 1–3 months for the first 2 years and every 3–6 months thereafter. The date on which the first recurrence after surgery was diagnosed and the site of the recurrence as determined using relevant imaging was retrieved from the medical records.
Statistical analysis
All continuous variables were presented as medians and ranges. The ALC was compared in relation to the category and postoperative period using the Mann–Whitney U test and the Wilcoxon’s test, respectively. Relapse-free survival (RFS) was defined as the number of months from surgery until relapse or death from any cause. Overall survival (OS) was defined as the number of months from surgery until death from any cause. RFS and OS were assessed using a Kaplan–Meier analysis, respectively. The log-rank test was used for comparisons of survival between two groups. Variables that were significantly correlated with the survival in a univariate analysis were further applied in a multivariable Cox model and subgroup analyses. A p value < 0.05 was considered to denote statistical significance. The statistical analyses were performed using a statistical analysis software package (SPSS 9.0, SPSS, Inc., Chicago, IL).
Declarations
All procedures were in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. The study was approved by the institutional review board of Niigata Cancer Center Hospital (2020–231). Informed consent was obtained from all individual participants in the form of opt-out.
Results
Clinicopathological characteristics
A total of 584 patients were enrolled in this study. The baseline characteristics, tumor-related factors, and perioperative findings are shown in Table 1. The median age (range) was 67 (21–92) years, and the study population was predominantly male (67.5%). The pathological stage was diagnosed as pStage IB in 163 (27.9%) patients, pStage II in 236 (40.4%) patients, and pStage III in 185 (31.6%) patients. Postoperative adjuvant chemotherapy was administered in 361 (61.8%) patients. Ninety-four (16.1%) patients developed recurrences during the observation period. The median follow-up period was 59.2 months.
Perioperative ALC values
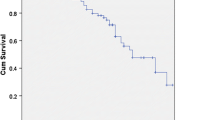
The perioperative change in ALC is shown in Fig. 1. The postoperative ALCs were significantly lower than the baseline ALC (pre-ALC). The decline in ALC bottomed out on POD 3 and then began to recover, returning to near baseline. We used representative ALC data obtained after discharge to evaluate the impact of the postoperative ALC (post-ALC) on the survival outcome. The pre- and post-ALC values assessed for patients in each clinicopathological variable category are shown in Table 2. While the pre-ALC values were correlated with body mass index (BMI) and vessel invasion, the representative post-ALC values were correlated with BMI alone.
A low ALC was defined as < 1000/μL in accordance with the findings of previous reports [28]. While 42 (7.2%) patients were categorized as having a low pre-ALC, 54 (9.2%) patients were categorized as having a low post-ALC.
Survival analysis
The RFS and OS curves stratified according to pathological stage are shown in Fig. 2. The 3-year RFS rates in patients with pStage IB, II and III were 96.2%, 85.9%, and 69.2%, respectively. The 5-year OS rates in patients with pStage IB, II and III were 90.2%, 84.8%, and 71.6%, respectively. The frequency of postoperative chemotherapy in the low post-ALC group was significantly lower (25/54; 46.3%) than that in the normal post-ALC group (337/530; 63.6%) (p = 0.01). The median time from surgery until the start of chemotherapy was similar: 38 days in the regular post-ALC group, and 35 days in the low post-ALC group. The treatment completion rate in the low post-ALC group (21/25; 84.0%) was higher than that in the normal post-ALC group (266/337; 78.9%; p = 0.02).
The RFS rate was significantly lower among patients with a low post-ALC for all stage (Fig. 3a), stage II (Fig. 3c), and stage III (Fig. 3d) disease, but not for patients with stage IB disease (Fig. 3b). The results of the univariate and multivariate analyses of RFS are shown in Table 3. Several covariates including age, sex, BMI, tumor size, pT disease, pN disease, vessel invasion, type of gastrectomy, blood loss on surgery, postoperative morbidity (≥ Grade II), low pre-ALC and low post-ALC were significantly correlated with RFS. Among these parameters, age, sex, BMI, tumor size, T disease, N disease, vessel invasion, total gastrectomy, postoperative morbidity (≥ Grade II), and low post-ALC were identified as independent factors predicting relapse.
Consistent with RFS, the OS rate was significantly lower among patients with a low post-ALC for all stage (Fig. 4a), Stage II (Fig. 4c) and Stage III (Fig. 4d) disease, not for Stage IB (Fig. 4b). The results of the univariate and multivariate analyses of RFS are shown in Table 4. The age, sex, tumor size, pT disease, pN disease, vessel invasion, type of gastrectomy, postoperative morbidity (≥ Grade II), low pre-ALC and low post- ALC were significantly correlated with OS, then age, sex, tumor size, T disease, N disease, vessel invasion, total gastrectomy, postoperative morbidity (≥ Grade II), and low post-ALC were identified as independent factors.
Discussion
Surgically induced inflammation has been shown to serve as a trigger for the development of distant metastasis, the outgrowth of which had been successfully suppressed preoperatively [29, 30]. The immune escape prompted by the postoperative downregulation of the adaptive immune response is one plausible explanation for this phenomenon. Since lymphocytes play a pivotal role in eradicating cancer cells through the immunological reaction of the host against cancer [31], postoperative lymphopenia is thought to be related to the immune suppressive response of the host, which can encourage the development of recurrence. In the present study, we investigated the effect of postoperative immunosuppression, known as CARS, on the outcomes of patients with Stage IB-III gastric cancer who were suspected of having residual micro-metastases of cancer after surgery.
An assessment of perioperative changes in the ALC (Fig. 1) showed a reduction in ALC values between POD 1 and POD 7, after which the value gradually recovered to the baseline value. Mokart, et al. demonstrated the presence of CARS during the early postoperative period by measuring cytokine levels after surgery in patients with cancer [12]. Rubinkiewicz, et al. reported that the lymphopenia at POD2 after surgery for colorectal cancer occurred in parallel with the decrease of CD4 + lymphocyte, CD8 + lymphocyte and Th17 lymphocyte [32]. Zheng et al. assessed the alteration of lymphocyte subpopulations at POD 3 after gastrectomy for gastric cancer, and an increase in regulatory T cells and the plasma level of TGF-β1, in addition to a decrease in Th17 lymphocytes and a plasma level of IL-17, was observed [33]. A postoperative transient decrease in ALC, which reflected the magnitude of postoperative SIRS and CARS, was consistent with these previous reports.
We focused on the post-ALC measured on around POD 30. It has been reported that sepsis-induced immunosuppressive dysregulation persisted for 28 days [34], and the decrease in this value was considered to be due to the delayed recovery of CARS. The results of the survival analysis showed that the post-ALC was a statistically significant predictor of recurrence that was independent of other known predictive factors and that was more reliable than the pre-ALC. When survival was examined according to each pathological stage, a low post-ALC was significantly correlated with a poor outcome among patients with stage II and III disease, but not among patients with Stage IB disease; this result can probably be attributed to an insufficient number of relapse or death events. Several reports have suggested that the postoperative ALC is related to the long-term outcomes of patients with gastric cancer [35, 36], and the designs of previous studies are not suitable for evaluating patients with remnant cancer or postoperative immunosuppression. Furthermore, survival analyses that include quite a few patients with Stage IA disease have relatively low recurrence rates [35], and the ALC at months after surgery is thought to reflect post-surgery nutrition, rather than the surgery-related immune status [36]. The results of the present study suggested that a 1-month postoperative reduction in ALC was a promising parameter reflecting the dysfunction of the lymphocyte-mediated immune response, which is correlated with the immune tolerance to residual cancer.
The negative effect of surgical morbidity on the survival of gastric cancer patients [15, 16] is also thought to be influenced by the immune status of the patient. The present study identified postoperative morbidity (≥ Grade 2) as another independent factor predicting both the RFS and OS. An excessive elevation of the serum CRP value [37] and a prolonged inflammatory response [38] after a gastrectomy were reportedly associated with a poor prognosis. These results suggest that inflammatory cytokines released by overstimulation of systemic inflammation activated the growth of residual cancerous lesions. However, high-magnitude SIRS enhances subsequent CARS, so postoperative recurrence might develop in response to CARS as well as SIRS.
Following the establishment of the clinical efficacy of ICIs for the treatment of advanced gastric cancer, the additive use of ICIs in perioperative chemotherapy is now being tested [39]. Several reports of treatment with ipilimumab in patients with melanoma have revealed that an increase in the ALC after treatment was correlated with an improved survival outcome [20, 22]. Thus, surgery-induced lymphopenia has the potential to become a treatment target, and recovery of ALC with perioperative treatment may improve survival.
The present study had several limitations. First, as the study was designed retrospectively and was performed at a single institution, the certainty of the evidence remains inadequate. Second, post-ALC was speculated to be an indirect parameter of the immunosuppressive status of the patients, but supportive data was not available. The measurement of lymphocyte subpopulations or the levels of cytokines that act as immunosuppressants in the tumor microenvironment is required. Third, the optimal cut-off value for ALC and the optimal period from surgery until the measurement of immunosuppressive parameters also needs to be elucidated. Fourth, the observational period used to assess long-term survival was insufficient for some of the patients. Fifth, because patients receiving preoperative chemotherapy were excluded from the present study, the value of the post-ALC parameter in this setting remains unclear.
Conclusions
A decrease in the post-ALC was correlated with both of the RFS and OS after curative resection in patients with locally advanced gastric cancer, regardless of other clinicopathological factors. Low post-ALC may help complement TNM stage in determining adjuvant chemotherapy indications and regimens to further improve the prognosis of stage II and III gastric cancer patients. The future development of treatments focused on postoperative lymphopenia may improve the outcomes of multimodal therapy.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due to the institutional privacy policy on clinical data but are available from the corresponding author on reasonable request.
Abbreviations
- TNM:
-
Tumor-node-metastasis
- RCT:
-
Randomized controlled trial
- ALC:
-
Absolute lymphocyte count
- pre-ALC:
-
Preoperative ALC
- Post-ALC:
-
Postoperative ALC
- SIRS:
-
Systemic inflammatory response syndrome
- CARS:
-
Compensatory anti-inflammatory response syndrome
- ICI:
-
Immune checkpoint inhibitors
- NLR:
-
Neutrophil-to-lymphocyte ratio
- POD:
-
Postoperative day
- TNM:
-
Tumor-node-metastasis
- RFS:
-
Relapse-free survival
- OS:
-
Overall survival
- BMI:
-
Body mass index
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Katai H, Ishikawa T, Akazawa K, Isobe Y, Miyashiro I, Oda I, Tsujitani S, Ono H, Tanabe S, Fukagawa T, et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001–2007). Gastric Cancer. 2018;21:144–54.
Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, Martenson JA. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–30.
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44.
Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, Tredan O, Verweij J, Biron P, Labidi I, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69:5383–91.
Ceze N, Thibault G, Goujon G, Viguier J, Watier H, Dorval E, Lecomte T. Pre-treatment lymphopenia as a prognostic biomarker in colorectal cancer patients receiving chemotherapy. Cancer Chemother Pharmacol. 2011;68:1305–13.
De Giorgi U, Mego M, Scarpi E, Giuliano M, Giordano A, Reuben JM, Valero V, Ueno NT, Hortobagyi GN, Cristofanilli M. Relationship between lymphocytopenia and circulating tumor cells as prognostic factors for overall survival in metastatic breast cancer. Clin Breast Cancer. 2012;12:264–9.
Saroha S, Uzzo RG, Plimack ER, Ruth K, Al-Saleem T. Lymphopenia is an independent predictor of inferior outcome in clear cell renal carcinoma. J Urol. 2013;189:454–61.
Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med. 1996;24:1125–8.
Mokart D, Capo C, Blache JL, Delpero JR, Houvenaeghel G, Martin C, Mege JL. Early postoperative compensatory anti-inflammatory response syndrome is associated with septic complications after major surgical trauma in patients with cancer. Br J Surg. 2002;89:1450–6.
Munteanu A, Munteanu D, Iancu M, Lupan I, Samasca G, Aldea C, Mocan T, Iancu C. Assessing immunological surgical stress markers in patients undergoing digestive surgery for pancreatic, hepatic and gastric tumors. J BUON. 2018;23:1655.
Hogan BV, Peter MB, Shenoy HG, Horgan K, Hughes TA. Surgery induced immunosuppression. Surgeon. 2011;9:38–43.
Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol. 2013;20:1575–83.
Tokunaga M, Kurokawa Y, Machida R, Sato Y, Takiguchi S, Doki Y, Yabusaki H, Watanabe M, Hato S, Nakamori M, et al. Impact of postoperative complications on survival outcomes in patients with gastric cancer: exploratory analysis of a randomized controlled JCOG1001 trial. Gastric Cancer. 2021;24:214–23.
Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–26.
Kang Y-K, Boku N, Satoh T, Ryu M-H, Chao Y, Kato K, Chung HC, Chen J-S, Muro K, Kang WK, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. 2017;390:2461–71.
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013.
Martens A, Wistuba-Hamprecht K, Yuan J, Postow MA, Wong P, Capone M, Madonna G, Khammari A, Schilling B, Sucker A, et al. Increases in absolute lymphocytes and circulating CD4+ and CD8+ T cells are associated with positive clinical outcome of melanoma patients treated with ipilimumab. Clin Cancer Res. 2016;22:4848–58.
Ho WJ, Yarchoan M, Hopkins A, Mehra R, Grossman S, Kang H. Association between pretreatment lymphocyte count and response to PD1 inhibitors in head and neck squamous cell carcinomas. J Immunother Cancer. 2018;6:84.
Postow MA, Chasalow SD, Kuk D, Panageas KS, Cheng ML, Yuan J, Wolchok JD. Absolute lymphocyte count as a prognostic biomarker for overall survival in patients with advanced melanoma treated with ipilimumab. Melanoma Res. 2020;30:71–5.
Maymani H, Hess K, Groisberg R, Hong DS, Naing A, Piha-Paul S, Janku F, Fu S, Tsimberidou AM, Pant S, et al. Predicting outcomes in patients with advanced non-small cell lung cancer enrolled in early phase immunotherapy trials. Lung Cancer. 2018;120:137–41.
Soyano AE, Dholaria B, Marin-Acevedo JA, Diehl N, Hodge D, Luo Y, Manochakian R, Chumsri S, Adjei A, Knutson KL, Lou Y. Peripheral blood biomarkers correlate with outcomes in advanced non-small cell lung cancer patients treated with anti-PD-1 antibodies. J Immunother Cancer. 2018;6:129.
Suh KJ, Kim SH, Kim YJ, Kim M, Keam B, Kim TM, Kim DW, Heo DS, Lee JS. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother. 2018;67:459–70.
Association JGC: Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011, 14:101–112.
JD Brierley MG, C Wittekind, (Ed.). TMN Classification of Malignant Tumours. Chichester, UK: John Willey & Sons, Ltd; 2017.
Menetrier-Caux C, Ray-Coquard I, Blay JY, Caux C. Lymphopenia in cancer patients and its effects on response to immunotherapy: an opportunity for combination with cytokines? J Immunother Cancer. 2019;7:85.
Tohme S, Simmons RL, Tsung A. Surgery for cancer: a trigger for metastases. Can Res. 2017;77:1548–52.
Krall JA, Reinhardt F, Mercury OA, Pattabiraman DR, Brooks MW, Dougan M, Lambert AW, Bierie B, Ploegh HL, Dougan SK, Weinberg RA: The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Science translational medicine 2018, 10.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7.
Rubinkiewicz M, Sieminska I, Malczak P, Major P, Baran J, Budzynski A, Pedziwiatr M. Perioperative changes in lymphocyte subpopulations in patients undergoing surgery for colorectal cancer. Acta Clin Croat. 2019;58:337–42.
Zheng X, Dong L, Wang K, Zou H, Zhao S, Wang Y, Wang G. MiR-21 participates in the PD-1/PD-L1 pathway-mediated imbalance of Th17/Treg cells in patients after gastric cancer resection. Ann Surg Oncol. 2019;26:884–93.
Mathias B, Delmas AL, Ozrazgat-Baslanti T, Vanzant EL, Szpila BE, Mohr AM, Moore FA, Brakenridge SC, Brumback BA, Moldawer LL, et al. Human myeloid-derived suppressor cells are associated with chronic immune suppression after severe sepsis/septic shock. Ann Surg. 2017;265:827–34.
Saito H, Kono Y, Murakami Y, Shishido Y, Kuroda H, Yamamoto M, Fukumoto Y, Osaki T, Ashida K, Fujiwara Y. Prognostic significance of pre- and postoperative lymphocyte counts in patients with gastric cancer. Dig Surg. 2019;36:137–43.
Oh SE, Choi MG, Seo JM, An JY, Lee JH, Sohn TS, Bae JM, Kim S. Prognostic significance of perioperative nutritional parameters in patients with gastric cancer. Clin Nutr. 2019;38:870–6.
Kurokawa Y, Yamashita K, Kawabata R, Fujita J, Imamura H, Takeno A, Takahashi T, Yamasaki M, Eguchi H, Doki Y. Prognostic value of postoperative C-reactive protein elevation versus complication occurrence: a multicenter validation study. Gastric Cancer. 2020;23:937–43.
Okumura Y, Hiki N, Kumagai K, Ida S, Nunobe S, Ohashi M, Sano T. Postoperative prolonged inflammatory response as a poor prognostic factor after curative resection for gastric cancer. World J Surg. 2017;41:2611–8.
Bang YJ, Van Cutsem E, Fuchs CS, Ohtsu A, Tabernero J, Ilson DH, Hyung WJ, Strong VE, Goetze TO, Yoshikawa T, et al. KEYNOTE-585: Phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol. 2019;15:943–52.
Acknowledgements
None
Funding
There was no funding for this study.
Author information
Authors and Affiliations
Contributions
Aizawa made substantial contributions to the conception and design, data analysis and interpretation, and article drafting. Matsuki and Bamba contributed to the acquisition of the data. Yabusaki participated in critically revising the article with regard to important intellectual content. Nakagawa approved the final version of the submitted manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures were in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. The study was approved by the institutional review board of Niigata Cancer Center Hospital (2020–231). Informed consent was obtained from all individual participants in the form of opt-out.
Consent for publication
This manuscript does not include any individual person’s data in any form.
Competing interests
Drs. Masaki Aizawa, Hiroshi Yabusaki, Atsushi Matsuki, Takeo Bamba, and Satoru Nakagawa declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Aizawa, M., Yabusaki, H., Matsuki, A. et al. Predictive significance of surgery-induced lymphopenia on the survival after curative resection for locally advanced gastric cancer: a retrospective cohort analysis. World J Surg Onc 21, 7 (2023). https://doi.org/10.1186/s12957-023-02887-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-023-02887-0