Abstract
Background
Primary liver cancer is the second-most commonly occurring cancer and has resulted in numerous deaths worldwide. Hepatic resection is of two main types, i.e., anatomic resection (AR) and non-anatomic resection (NAR). The oncological outcomes of hepatocellular carcinoma (HCC) patients after AR and NAR are still considered controversial. Therefore, we aimed to compare the impact of AR and NAR on the oncological outcomes of HCC patients with tumor diameters ≤ 5 cm using the propensity score matching method and research-based evidence.
Method
A systematic literature search was conducted. The main outcomes were disease-free survival (DFS), overall survival (OS), intrahepatic recurrence rate, and extrahepatic metastasis rate. Relative risk (RR) was calculated from forest plots and outcomes using random-effects model (REM).
Result
AR significantly improved DFS at 1, 3. and 5 years after surgery, compared to NAR (RR = 1.09, 95% CI = 1.04–1.15, P = 0.0003; RR = 1.16, 95% CI = 1.07–1.27, P = 0.0005; RR = 1.29, 95% CI = 1.07–1.55, P = 0.008). However, both of the difference in DFS at 7 years and OS at 1 and 3 years after AR versus that after NAR were not statistically significant. Nevertheless, the long-term OS associated with AR (5, 7, and 10 years) was superior to that associated with NAR (RR = 1.12, 95% CI = 1.03–1.21, P = 0.01; RR = 1.19, 95% CI = 1.04–1.36, P = 0.01; RR = 1.18, 95% CI = 1.05–1.34, P = 0.008). The difference in the intrahepatic recurrence rate after AR versus that after NAR was not statistically significant, but the extrahepatic metastasis rate after AR was significantly lower than that observed after NAR (RR = 0.61, 95% CI = 0.40–0.94, P = 0.03).
Conclusion
Therefore, AR should be the preferred surgical approach for HCC patients with tumor diameters ≤ 5 cm.
Trial registration
PROSPERO registration number CRD42022330596.
Similar content being viewed by others
Introduction
Primary liver cancer is the second-most commonly occurring cancer and has resulted in numerous deaths worldwide [1]. According to the latest GLOBOCAN statistics, approximately 841,000 new cases of liver cancer were reported worldwide in 2018 (4.7% of all tumors), and these resulted in 781,631 patient deaths (8.2% of all tumor deaths). Histological subtypes of liver cancer include hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), mixed hepatocellular-cholangiocarcinoma, and fibrolamellar carcinoma (FLC). Of these, HCC accounts for approximately 90% of all primary liver cancer cases [2]. Currently, the main treatment modalities for HCC include surgical resection, liver transplantation, transhepatic artery chemoembolization, local ablation, targeted therapy, and immunotherapy [3].
As with other solid tumors, surgical resection is recognized as the treatment of choice for resectable HCC because it is associated with a relatively good prognosis [4, 5]. Hepatic resection is of two main types, i.e., anatomic resection (AR) and non-anatomic resection (NAR). The former is a regular segmental resection process based on the anatomical structure of the liver. The most significant advantage of AR is that it facilitates systematic distribution to the portal vein, which allows for a more complete resection of the lesion with controlled bleeding. In NAR, the extent of resection depends entirely on the tumor distribution, irrespective of the intrahepatic anatomy. In principle, only the complete resection of the tumor (negative margins) is required, thus allowing tumor-free liver tissues to be preserved more effectively [6]. Both AR and NAR are widely used as surgical approaches for the treatment of primary liver cancer.
However, controversies centered on the oncological outcomes of AR and NAR for HCC have been ongoing since several years. Some scholars have argued that AR maximizes tumor resection and improves postoperative survival and thus prefer AR [7,8,9,10,11,12,13]. Others argue that there is no difference in postoperative survival after the two procedures [14]. Clinically, AR results in a larger liver section and smaller residual liver volume than NAR. The time required to perform AR is longer, and a tendency of increase in the extent of intraoperative bleeding is observed subsequently. In addition, most patients with liver cancer have different degrees of chronic liver disease and cirrhosis; thus, the incidence of postoperative small liver syndrome or liver failure is higher than that observed with NAR. Because of certain limitations and shortcomings of AR, NAR has become important for the treatment of HCC patients with tumor diameters ≤ 5 cm [15]. In the current case-control study of HCC patients with tumor diameters ≤ 5 cm, it was difficult to obtain a convincing result after comparing the prognosis of the two procedures. The clinical characteristics of the AR and NAR groups, including basic preoperative characteristics, liver function status, tumor number, and vascular invasion were somewhat different, and these variables may affect outcomes. In observational studies, the propensity score matching method (PSM) is frequently used to ensure balanced comparability, to reduce the effects of data bias and confounding variables and to conduct a more reasonable comparison of experimental and control groups [16]. However, no systematic evaluation has been conducted using PSM to date, to address the above-mentioned issues.
Taking these unresolved issues in the clinic into consideration, the current study, which includes an analysis of the most recent retrospective studies, provides evidence-based support that is applicable to clinical practice, [7,8,9,10,11,12,13,14] and uses PSM and meta-analysis methods to systematically evaluate the oncological outcomes of HCC patients with tumor diameters ≤ 5 cm after treatment with AR and NAR.
Materials and methods
Our work has been conducted in line with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement standards and registered in the PROSPERO database (CRD42022330596, https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=330596).
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) patients were diagnosed with primary HCC and the study included a comparison between AR and NAR, (2) tumor diameters were ≤ 5 cm, (3) overall survival (OS) and disease-free survival (DFS) after surgery were reported, (4) the findings were reported in the English language, and (5) propensity-score matched studies.
The exclusion criteria were as follows: (1) patients underwent secondary surgery or had metastatic HCC, and HCC patients had tumor diameters >5 cm; (2) valid data such as outcome indicators could not be extracted; (3) animal experiments, reviews, guidelines, meta-analyses, case reports, abstracts, conference proceedings, or discussion of expert opinions; and (4) studies contained repeatedly published information.
Literature search strategy
The PubMed, Embase, and Cochrane databases were searched until 30 April 2022. The literature search was performed using a combination of subject-related terms and keywords. The search terms included hepatocellular carcinoma, liver cell carcinoma, liver neoplasms, anatomic resection, non-anatomic resection, hepatectomy, and propensity score matching.
Literature screening and data extraction
Two investigators independently screened the literature and then extracted and cross-checked the data. The literature was screened by first reading the title and abstract, and after excluding irrelevant literature, the full text was read further, to decide whether to include or exclude the study. The extracted data included (1) basic information about the included studies, such as the name of the first author, type of study, and year of publication; (2) baseline characteristics of the study population and interventions; (3) key elements of risk of bias evaluation; and (4) outcome indicators and outcome measurement data of interest.
Evaluation of literature quality
The risk of bias of included literature was evaluated independently by two investigators and the results were cross-checked using the New Castle-Ottawa scale (NOS), [17] to evaluate the risk of bias. The total score was 9, with a score ≥ 7 being indicative of high-quality literature and a score ≥ 6 required for inclusion.
Statistical processing
All statistics have been performed using the RevMan 5.3 software. Dichotomous variables were assessed using the relative risk (risk ratio, RR), and their 95% CIs were calculated to determine each effect size. Meta-analysis was performed for all outcomes of interest using the random-effects model (REM). The extent of heterogeneity between studies was determined quantitatively using the I2 value. Heterogeneity was considered to be present if the I2 statistic was > 50%. If there was significant heterogeneity (I2 > 50%) between the results of the studies, the source of heterogeneity was further assessed using sensitivity analysis. Publication bias was assessed with funnel plots. All tests were two-tailed, and differences were considered statistically significant if P < 0.05.
Results
Literature screening process
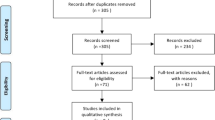
The search strategy and successive steps used during the selection process are shown in Fig. 1. Of the 77 retrieved records, 27 were duplicate studies; 15 were considered irrelevant to the content of the study after reading the title and abstract; the remaining 35 studies were read in their entirety, after which 27 studies that did not meet the inclusion criteria were excluded. Finally, 8 retrospective studies [7,8,9,10,11,12,13,14] with an NOS score ≥ 7 were found to be eligible (Table 1). A total of 1861 patients, including 984 (53%) in the AR group and 877 (47%) in the NAR group, were matched, to assess propensity scores in the literature.
Basic characteristics of included studies and baseline characteristics of patients
Basic characteristics
The basic characteristics of included studies were recorded. These included the first author, year of publication, selection of subjects, and variables used for matching while performing propensity analysis, as shown in Table 1.
Baseline characteristics
Within the included literature, study subjects were classified by gender, liver function, hepatitis B and/or C infection, isolated nodules, microvascular infiltration, and perioperative morbidity contributing to within-study variations (see Table 2). Overall, in 8 studies, the percentages of female patients who underwent AR and NAR were 21.4% and 20.6%, respectively (P = 0.40; I2 = 4%). The vast majority of patients presented with Child-Pugh class A liver function (7 studies with 99.3% AR and 98.9% NAR; P = 0.72; I2 = 0%) and single nodules (8 studies with 95.0% AR and 96.7% NAR; P = 0.51, I2 = 0%); a lower percentage of patients exhibited combined microvascular invasion in 8 studies (AR, 24.0%, NAR, 26.2%; P = 0.98; I2 = 0%); 6 studies reported on patients with a history of hepatitis, with 46.5% and 43.3% of them undergoing AR and NAR, respectively, for hepatitis B testing; P = 0.79, I2 = 0%; 32.1% AR and 33.7% NAR for hepatitis C; P = 0.99, I2 = 0%; a total of 4 studies reported perioperative complications, of which 3 reported overall complications (AR, 20.0%, NAR, 18.1%; P = 0.41; I2 = 0%) and 2 reported major complications (AR, 5.7%, NAR, 4.6%; P = 0.34; I2 = 0%).
Results of meta-analysis
Disease-free survival
A total of 8 studies [7,8,9,10,11,12,13,14] reported 1-year, 3-year, and 5-year DFS; of these, 3 studies [8, 10, 11] reported a 7-year DFS. The heterogeneity tests performed in studies reporting 5-year DFS showed that there was heterogeneity between studies (P = 0.01, I2 = 61%). A sensitivity analysis, after omitting one study at the time, did not change the risk estimate or the level of significance for the outcome measures. The publication bias was evaluated by constructing a funnel plot, and the symmetry of the funnel plot suggested the absence of any publication bias (Fig. 2a). Meta-analysis using REM showed that AR significantly improved patients’ DFS postoperatively at 1, 3, and 5 years, compared to that observed with NAR (RR = 1.09, 95% CI = 1.04–1.15, P = 0.0003; RR = 1.16, 95% CI = 1.07–1.27, P = 0.0005; RR = 1.29, 95% CI = 1.07–1.55, P = 0.008) (Fig. 3a–c). However, the differences in DFS at 7 years after AR versus those after NAR were not statistically significant (RR = 1.13, 95% CI = 0.87–1.47, P = 0.35) (Fig. 3d).
Overall survival
A total of 8 studies [7,8,9,10,11,12,13,14] reported the OS at year 1, 3 and 5; of these, 3 studies [8, 10, 11] reported a 7-year OS and 2 studies reported [8, 11] a 10-year OS. The 3-year and 5-year OS heterogeneity test showed that there was heterogeneity among studies (P = 0.0006, I2 = 73%; P = 0.04, I2 = 52%). A sensitivity analysis, after omitting one study at the time, did not change the results. The related funnel plots for publication bias are shown in Fig. 2b, c. The results of a meta-analysis performed using REM showed that the differences in OS at 1 and 3 years after AR and NAR were not statistically significant (RR = 1.01, 95% CI = 0.99–1.02, P = 0.50; RR = 1.06, 95% CI = 0.99–1.14, P = 0.10) (Fig. 4a, b). However, AR was superior to NAR in terms of long-term OS (5, 7, and 10 years) (RR = 1.12, 95% CI = 1.03–1.21, P = 0.01; RR = 1.19, 95% CI = 1.04–1.36, P = 0.01; RR = 1.18, 95% CI = 1.05–1.34, P = 0.008) (Fig. 4c–e).
Intrahepatic and extrahepatic metastasis rates
A total of 3 studies [11, 12, 14] reported intrahepatic recurrence rates and 2 studies reported [11, 14] extrahepatic metastasis rates, and the results of the heterogeneity test, performed using REM, showed that there was heterogeneity among studies (P = 0.007, I2 = 80%). Sensitivity analysis did not significantly change the results. The results of the meta-analysis showed that the difference in intrahepatic recurrence rates after AR versus that observed after NAR was not statistically significant (RR = 0.67, 95% CI = 0.40–1.11, P = 0.12) (Fig. 5a), but the incidence of extrahepatic metastasis after AR was significantly lower than that observed after NAR (RR = 0.61, 95% CI = 0.40–0.94, P = 0.03) (Fig. 5b).
Discussion
Liver surgery techniques have been developed over several stages, via techniques such as wedge resection, regular lobectomy, irregular partial resection, and anatomical segmental hepatectomy [18]. In the mid-1950s, Goldsmith and Woodbume [19] emphasized that lobectomy should be performed strictly in accordance with the internal anatomy of the liver, thus introducing the concept of a regular lobectomy. In 1985, Makuuchi et al. [20] used AR for the first time for the treatment of HCC, which resulted in the currently accepted concept of AR. From the anatomical point of view alone, AR has many advantages over NAR. First, AR can simultaneously remove tumors and intrahepatic disseminated micrometastases [21]. In addition, several previous studies have shown that HCC patients exhibit lower rates of recurrent metastases, higher survival rates, and better prognoses after AR [7, 22, 23].
However, in practical clinical work, the application of AR is limited by several factors. For perioperative safety reasons, surgeons prefer AR for patients exhibiting good liver function (Child A), small tumor size (e.g., tumor diameter ≤ 5 cm), and no cirrhosis, leading to an unavoidable selection bias [22]. Therefore, there is a long-standing debate on whether the choice of AR or NAR is more beneficial and results in a better long-term prognosis for HCC patients. On one hand, separate case-control studies addressing these issues have led to large discrepancies in results due to the use of different criteria for patient selection (because of differences in clinical characteristics, such as basic preoperative characteristics, liver function classification, tumor number, and vascular invasion). On the other hand, though some meta-analyses have addressed these issues, there is a natural inherent cohort bias that affects the conclusiveness of the results [24, 25]. This suggests that there is an urgent need for a more scientific approach for the analysis of the above issues. The concept of PSM was first proposed by Rosenbaum and Rubin in 1983, and the method reduces bias and ensures that baseline information is balanced between groups [16]. In the absence of RCTs, PSM can reasonably match two groups of patients to achieve an approximation of a randomized controlled clinical study, thus ensuring balanced comparability. Therefore, we used PSM to reduce the differences in variables between groups, and again compared the OS, DFS, and recurrent metastasis rates of HCC patients with a tumor diameter ≤ 5 cm who underwent AR and NAR. Our study reduced the heterogeneity of the included studies to some extent, and analyzed more distantly related indicators such as 7-year DFS and OS; thus, our study is more convincing than previous meta-analyses.
Patients with HCC have a high mortality rate and poor prognosis, with an overall 5-year survival rate of only 18% [26] and a cumulative recurrence rate of 70-80% even 5 years after undergoing radical surgery [27]. Studies have shown that microvascular invasion (MVI) is an independent risk factor affecting the prognosis of HCC patients [28]. Before performing liver resection in HCC patients with combined MVI, tumor cells may already be present in the remnant liver and exhibit microscopic portal infiltration and the formation of satellite foci, which in turn might allow early tumor recurrence and metastasis [29, 30]. In this study, we found that the DFS was higher in the AR group than in the NAR group at 1, 3, and 5 years after surgery, probably because AR was based on the systematic distribution of the portal vein, which reduced the spread of tumor cells in the portal system and decreased the possibility of tumor metastasis. In addition, postoperative extrahepatic metastases usually occur later and are more lethal than intrahepatic recurrence for HCC patients [31,32,33]. In this study, we found that the rate of extrahepatic metastasis was higher in NAR than in AR, which could be attributable to the fact that there were no significant differences in the DFS for the procedures at 7 years after surgery.
It was found that the probability of intrahepatic metastasis in the short term (within 2 years) after liver cancer surgery was about 0.68, while extrahepatic metastasis was predominant over the long term [34]. The cumulative survival rate associated with intrahepatic recurrence was significantly higher than that associated with extrahepatic metastasis [35]. In this study, we found that there was no significant difference in the rate of intrahepatic recurrence after AR and NAR, which may explain the lack of significant difference in the short-term OS (1, 3 year) between the two groups. The long-term OS (5, 7 and 10 years) was significantly higher for the AR group than the NAR group, which may be attributed to the higher rate of extrahepatic metastasis associated with NAR, compared to AR. Therefore, this study suggests that in HCC patients with tumor diameters ≤ 5 cm, AR can effectively reduce the rate of postoperative extrahepatic metastasis and thus improve the postoperative long-term OS.
Nevertheless, there are some limitations to this study. First, the selection criteria of patients were varied; important indicators such as the ICG15 level, the occurrence of MVI, and cutting-edge condition did not help us develop uniform criteria. Some previous studies [6, 36] have shown that the cutting-edge condition did not influence the recurrence rates after hepatectomy for HCC. Most intrahepatic recurrences were considered to arise from intrahepatic metastasis by venous dissemination, which a wide resection margin could not prevent. However, recent studies [37,38,39,40,41] suggest that HCC patients with narrow resection margin were associated with a higher tumor recurrence rate and a shorter DFS. A multicenter retrospective study [42] concluded that AR with a negative 0-mm surgical margin may be acceptable in patients with a single hepatocellular carcinoma. NAR with a negative 0-mm margin was associated with a less favorable survival outcome. For better oncologic outcomes, surgeons should endeavor in keeping the surgical resection margin widths ≥1 cm during NAR. Furthermore, there are differences in prognostic outcomes due to the different etiologies of patients in the East and West. Finally, even if we use PSM as a scientific method, an intergroup error was still observed between the included studies. Therefore, a multi-center, randomized controlled study needs to be performed, to further investigate the impact of these two procedures on the long-term prognosis of HCC patients.
In conclusion, AR is superior to NAR in terms of both the short-term DFS and long-term OS of HCC patients with tumor diameters ≤ 5 cm. Therefore, whenever feasible, AR should be the preferred surgical approach for HCC patients with tumor diameters ≤ 5 cm.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–79.
Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018.
Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74.
Yamazaki O, Matsuyama M, Horii K, Kanazawa A, Shimizu S, Uenishi T, et al. Comparison of the outcomes between anatomical resection and limited resection for single hepatocellular carcinomas no larger than 5 cm in diameter: a single-center study. J Hepatobiliary Pancreat Sci. 2010;17:349–58.
Dahiya D, Wu TJ, Lee CF, Chan KM, Lee WC, Chen MF. Minor versus major hepatic resection for small hepatocellular carcinoma (HCC) in cirrhotic patients: a 20-year experience. Surgery. 2010;147:676–85.
Poon RT, Fan ST, Ng IO, Wong J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal. Ann Surg. 2000;231:544–51.
Cucchetti A, Qiao GL, Cescon M, Li J, Xia Y, Ercolani G, et al. Anatomic versus nonanatomic resection in cirrhotic patients with early hepatocellular carcinoma. Surgery. 2014;155:512–21.
Haruki K, Furukawa K, Fujiwara Y, Taniai T, Hamura R, Shirai Y, et al. Effectiveness of Anatomical Resection for Small Hepatocellular Carcinoma: a Propensity Score-Matched Analysis of a Multi-institutional Database. J Gastrointest Surg. 2021;25:2835–41.
Hokuto D, Nomi T, Yasuda S, Yoshikawa T, Ishioka K, Yamada T, et al. Does anatomic resection improve the postoperative outcomes of solitary hepatocellular carcinomas located on the liver surface? Surgery. 2018;163:285–90.
Ishii M, Mizuguchi T, Kawamoto M, Meguro M, Ota S, Nishidate T, et al. Propensity score analysis demonstrated the prognostic advantage of anatomical liver resection in hepatocellular carcinoma. World J Gastroenterol. 2014;20:3335–42.
Kaibori M, Kon M, Kitawaki T, Kawaura T, Hasegawa K, Kokudo N, et al. Comparison of anatomic and non-anatomic hepatic resection for hepatocellular carcinoma. J Hepato Biliary Pancreatic Sci. 2017;24:A41.
Minagawa M, Mise Y, Omichi K, Ichida H, Mizuno T, Yoshioka R, et al. Anatomic resection for hepatocellular carcinoma: prognostic impact assessed from recurrence treatment. Ann Surg Oncol. 2022;29:913–21.
Kwon JH, Lee JW, Lee JW, Lee YJ. Effects of Anatomical or Non-Anatomical Resection of Hepatocellular Carcinoma on Survival Outcome. J Clin Med. 2022;11:1369.
Cho CW, Choi GS, Kim JM, Kwon CHD, Joh JW. Long-term oncological outcomes of laparoscopic liver resection for solitary hepatocellular carcinoma: comparison of anatomical and nonanatomical resection using propensity score matching analysis. J Laparoendosc Adv Surg Tech A. 2019;29:752–8.
Huang ZY, Chen G, Hao XY, Cai RY, Zhao YF, Chen XP. Outcomes of non-anatomic liver resection for hepatocellular carcinoma in the patients with liver cirrhosis and analysis of prognostic factors. Langenbeck's Arch Surg. 2011;396:193–9.
Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–61.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5.
McClusky DA 3rd, Skandalakis LJ, Colborn GL, Skandalakis JE. Hepatic surgery and hepatic surgical anatomy: historical partners in progress. World J Surg. 1997;21:330–42.
Goldsmith NA, Woodburne RT. The surgical anatomy pertaining to liver resection. Surg Gynecol Obstet. 1957;105:310–8.
Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 1985;161:346–50.
Ochiai T, Sonoyama T, Kikuchi S, Okayama T, Konishi H, Kitagawa M, et al. Anatomic wide hepatectomy for treatment of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2007;133:563–9.
Cho YB, Lee KU, Lee HW, Cho EH, Yang SH, Cho JY, et al. Anatomic versus non-anatomic resection for small single hepatocellular carcinomas. Hepatogastroenterology. 2007;54:1766–9.
Jing-Dong L, Yun-Hong T, Kanduri HK, Quan-Lin L, De-Xing L, Qiang L. Prognosis in patients with small hepatocellular carcinoma: a meta-analysis. Hepatogastroenterology. 2011;58:1708–12.
Moris D, Tsilimigras DI, Kostakis ID, Ntanasis-Stathopoulos I, Shah KN, Felekouras E, et al. Anatomic versus non-anatomic resection for hepatocellular carcinoma: a systematic review and meta-analysis. Eur J Surg Oncol. 2018;44:927–38.
Jiao S, Li G, Zhang D, Xu Y, Liu J, Li G. Anatomic versus non-anatomic resection for hepatocellular carcinoma, do we have an answer? A meta-analysis. Int J Surg. 2020;80:243–55.
Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450–62.
Shindoh J, Kawamura Y, Kobayashi Y, Akuta N, Kobayashi M, Suzuki Y, et al. Time-to-interventional failure as a new surrogate measure for survival outcomes after resection of hepatocellular carcinoma. J Gastrointest Surg. 2020;24:50–7.
Huang X, Lu S. A Meta-analysis comparing the effect of anatomical resection vs. non-anatomical resection on the long-term outcomes for patients undergoing hepatic resection for hepatocellular carcinoma. HPB (Oxford). 2017;19:843–9.
Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Kakisaka T, et al. Analysis of the risk factors for early death due to disease recurrence or progression within 1 year after hepatectomy in patients with hepatocellular carcinoma. World J Surg Oncol. 2012;10:107.
Kim JM, Yi NJ, Kwon CHD, Lee KW, Suh KS, Joh JW. Early disseminated recurrence after liver resection in solitary hepatocellular carcinoma. Ann Surg Treat Res. 2018;94:129–34.
Uchino K, Tateishi R, Shiina S, Kanda M, Masuzaki R, Kondo Y, et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer. 2011;117:4475–83.
Mao K, Wang J. Present treatment situation of hepatocellular carcinoma with extrahepatic metastasis. Zhonghua Wai Ke Za Zhi. 2019;57:466–70.
Ochiai T, Ikoma H, Okamoto K, Kokuba Y, Sonoyama T, Otsuji E. Clinicopathologic features and risk factors for extrahepatic recurrences of hepatocellular carcinoma after curative resection. World J Surg. 2012;36:136–43.
Cheng Z, Yang P, Qu S, Zhou J, Yang J, Yang X, et al. Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB (Oxford). 2015;17:422–7.
Hashimoto M, Kobayashi T, Ishiyama K, Ide K, Ohira M, Tahara H, et al. Predictive independent factors for extrahepatic metastasis of hepatocellular carcinoma following curative hepatectomy. Anticancer Res. 2017;37:2625–31.
Valenzuela A, Ha NB, Gallo A, Bonham C, Ahmed A, Melcher M, et al. Recurrent hepatocellular carcinoma and poorer overall survival in patients undergoing left-sided compared with right-sided partial hepatectomy. J Clin Gastroenterol. 2015;49:158–64.
Su CM, Chou CC, Yang TH, Lin YJ. Comparison of anatomic and non-anatomic resections for very early-stage hepatocellular carcinoma: the importance of surgical resection margin width in non-anatomic resection. Surg Oncol. 2021;36:15–22.
Tsilimigras DI, Sahara K, Moris D, Hyer JM, Paredes AZ, Bagante F, et al. Effect of surgical margin width on patterns of recurrence among patients undergoing r0 hepatectomy for T1 hepatocellular carcinoma: an international multi-institutional analysis. J Gastrointest Surg. 2020;24:1552–60.
Han J, Li ZL, Xing H, Wu H, Zhu P, Lau WY, et al. The impact of resection margin and microvascular invasion on long-term prognosis after curative resection of hepatocellular carcinoma: a multi-institutional study. HPB (Oxford). 2019;21:962–71.
Lim C, Goumard C, Casellas-Robert M, Lopez-Ben S, Lladó L, Busquets J, et al. Impact on oncological outcomes and intent-to-treat survival of resection margin for transplantable hepatocellular carcinoma in all-comers and in patients with cirrhosis: a multicenter study. World J Surg. 2020;44:1966–74.
Liu L, Shui Y, Yu Q, Guo Y, Zhang L, Zhou X, et al. Narrow-margin hepatectomy resulted in higher recurrence and lower overall survival for r0 resection hepatocellular carcinoma. Front Oncol. 2020;10:610636.
Aoki T, Kubota K, Hasegawa K, Kubo S, Izumi N, Kokudo N, et al. Significance of the surgical hepatic resection margin in patients with a single hepatocellular carcinoma. Br J Surg. 2020;107:113–20.
Acknowledgements
This study was supported by research grants from Natural Science Foundation of Hunan Province (Grant No. 2021JJ70120) and Clinical Medicine Technological Innovation Leading Project of Hunan Province (Grant No. 2020SK51818).
Funding
This study was funded by Natural Science Foundation of Hunan Province (Grant No. 2021JJ70120) and Clinical Medicine Technological Innovation Leading Project of Hunan Province (Grant No. 2020SK51818).
Author information
Authors and Affiliations
Contributions
Xiao-ming Dai contributed to the conception of the study; Zhi-qiang Xiang, and Zhu Zhu performed the data analyses and wrote the manuscript; Qian Wang and Hua-jian Li helped perform the analysis with constructive discussions. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Xiao-ming Dai, Zhi-qiang Xiang, Qian Wang, Hua-jian Li, and Zhu Zhu declare they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dai, Xm., Xiang, Zq., Wang, Q. et al. Oncological outcomes of anatomic versus non-anatomic resections for small hepatocellular carcinoma: systematic review and meta-analysis of propensity-score matched studies. World J Surg Onc 20, 299 (2022). https://doi.org/10.1186/s12957-022-02770-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-022-02770-4