Abstract
Background
BRAF V600E-mutant colorectal cancers (CRCs) are associated with shorter survival than BRAF wild-type tumors. Therapeutic decision-making for colorectal liver metastases (CRLM) harboring this mutation remains difficult due to the scarce literature. The aim was to study a large cohort of BRAF V600E-mutant CRLM patients in order to see if surgery extend overall survival among others prognostic factors.
Methods
BRAF V600E-mutant CRCs diagnosed with liver-only metastases, resected or not, were retrospectively identified between April 2008 and December 2017, in 25 French centers. Clinical, molecular, pathological characteristics and treatment features were collected. Overall survival (OS) was defined as the time from CRLM diagnosis to death from any cause. Cox proportional hazard models were used for statistical analysis.
Results
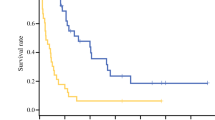
Among the 105 patients included, 79 (75%) received chemotherapy, 18 (17%) underwent upfront CRLM surgery, and 8 (8%) received exclusive best supportive care. CRLM surgery was performed in 49 (46.7%) patients. CRLM were mainly synchronous (90%) with bilobar presentation (61%). The median OS was 34 months (range, 28.9–67.3 months) for resected patients and 10.6 (6.7–12.5) months for unresected patients (P < 0.0001). In multivariate analysis, primary tumor surgery (hazard ratio (HR) = 0.349; 95% confidence interval (CI) 0.164–0.744, P = 0.0064) and CRLM resection (HR = 0.169; 95% CI 0.082–0.348, P < 0.0001) were associated with significantly better OS.
Conclusions
In the era of systemic cytotoxic chemotherapies, liver surgery seems to extend OS in BRAF V600E-mutant CRCs with liver only metastases historical cohort.
Similar content being viewed by others
Background
Approximately 50% of patients with colorectal cancer develop colorectal liver metastases (CRLM), and their outcomes are intimately related to CRLM resectability: the 5-year overall survival (OS) rate ranges from 30 to 50% after CRLM surgery, whereas it is lower than 10% for unresectable CRLM [1, 2]. However, 50 to 85% of patients experience relapse after CRLM resection, and the curative intent of metastasectomy is accomplished in approximately 20% of cases [3,4,5]. In the era of precision medicine, efforts are aimed at a better selection of patients who might benefit from metastasectomy. Several clinical scoring systems based on clinicopathological parameters have been proposed; but their clinical value is still questioned [6, 7].
Colorectal cancers (CRCs) harboring BRAF V600E mutations are aggressive cancers with rapid metastatic spread that more frequently involves peritoneal and nodal invasion than liver metastases. Until recently, their management was based on limited data, mainly from subgroup analysis of randomized clinical trials. This subgroup of patients is less responsive to standard chemotherapies. In the CALGB/SWOG 80405 trial assessing the addition of the targeted agent cetuximab or bevacizumab or both to doublet chemotherapy FOLFOX or FOLFIRI, the median OS for BRAF-mutant patients remained poor compared to that of BRAF wild-type patients: 13.5 months versus 30.6 months, respectively [8]. In addition, a recent meta-analysis of five randomized clinical trials demonstrated that intensive upfront chemotherapy with triplet FOLFOXIRI plus bevacizumab did not improve survival among BRAF V600E-mutant patients [9].
This gap in survival rates has been also observed after CRLM resection in several retrospective subgroup analyses. In the latest study, the 3-year OS rates for BRAF-mutant and wild-type patients were 54% and 82.9%, respectively [10]. However, these numbers must be interpreted with caution as BRAF-mutant CRCs with liver-only metastases represent a limited population, and only 5% of patients undergoing CRLM resection harbor these mutations [11,12,13].
Therefore, our knowledge about BRAF-mutant patients with CRLM is currently limited to those patients undergoing resection or with extra-hepatic metastases receiving chemotherapies. The aim of the study was to report outcomes of a large cohort of BRAF V600E-mutant patients with exclusive CRLM and to identify if surgery is a prognostic factor among others.
Methods
Study population and design
Data from 105 patients diagnosed with liver-limited CRC metastases harboring BRAF V600E mutations between April 1, 2008, and December 31, 2017, were retrospectively collected from 25 French hospitals. Exclusion criteria were as follows: presence of extra-hepatic metastases, date of CRLM diagnosis not available, and follow-up less than 12 months. Data from the majority of patients who underwent CRLM resection came from databases of the following four French scientific groups: Fédération de Recherche et Chirurgie (FRENCH), Association de Chirurgie Hépato-Bilio-Pancréatique et Transplantation (ACHBT), Association des Gastro-Enterologues Oncologues (AGEO), and the PRODIGE group (Partenariat de Recherche en Oncologie DIGEstive). BRAF V600E mutated-patients were identified from molecular biology platforms and each case was screened in order to identify and include patients with liver-only disease. Patients with synchronous extra-hepatic resectable disease metastases were excluded. The study was conducted according to the ethical standards in line with the French regulation. French Data Protection Authority (CNIL agreement n° DEC18-409 (2018_01)) provided a waiver of informed consent for this retrospective study and permitted the publication of anonymized data.
BRAF and RAS mutational statuses were determined from either primary CRC samples or CRLM tissues—as several studies have demonstrated a high molecular concordance between primary CRC and liver metastases [14, 15]—using PCR or next-generation sequencing according to the technology available at each hospital.
The following clinical, molecular, and pathological characteristics were collected at baseline: age at CRLM diagnosis, sex, KRAS and NRAS mutations, mismatch repair (MMR) status, primary tumor site, surgery of primary tumor, tumor and nodal stages according to the American Joint Committee on Cancer (AJCC), synchronous (<6 months) versus metachronous CRLM diagnosis, CRLM distribution and number, and initial resectability status. In addition, treatment features (CRLM surgery and systemic therapies) and survival were assembled.
MMR status was assessed by both immunohistochemical analysis of microsatellite instability-high (MSI-H) defined by loss of MLH1, MSH2, MSH6, or PMS2 expression and/or PCR. Right-sided tumors were defined as arising from the caecum to the transverse colon and left-sided tumors as arising from the splenic flexure to the rectosigmoid junction.
Treatment features and definitions
The treatment decision for each patient was made during multidisciplinary meetings in each institution. According to the CRLM resectability status and performance status, patients received preoperative chemotherapy, upfront liver surgery, palliative chemotherapy, or best supportive care. Patients were then followed-up every 2–3 months through physical examination, biological tests, and computed tomography scan. OS was defined as the time from CRLM diagnosis to the time of death or the date of last follow-up. Postoperative OS was defined as the time from CRLM resection. OS rate was determined at December 31, 2018.
Statistical analysis
For descriptive analysis, quantitative parameters are presented as median and quartiles and qualitative parameters as percentages. CRLM resected and unresected groups were compared using the χ2 or Fisher’s exact test, as appropriate. Survival rates were estimated by the Kaplan-Meier method and were compared using the log-rank test. After univariate analysis, variables with p < 0.2 and with less than 33% missing data were integrated in a backward selection for final multivariate Cox model. Boostrap methods were also used. The variables of interest were as follows: age, gender, primary tumor site, primary tumor surgery, synchronous CRLM, CRLM number, CRLM distribution, resectability status, metastasectomy, and the use of first-line chemotherapy and targeted therapies. All reported p values are two-sided, and P < 0.05 was considered statistically significant. Statistical analyses were performed using SAS V9.4 (Cary, NC, USA).
Results
Patient characteristics
One-hundred and five patients were identified with BRAF V600E-mutant CRLM diagnosed between April 2008 and December 2017. The median age at CRLM diagnosis was 67 years. CRLM were mainly synchronous (90%) with bilobar presentation (61%). One patient harbored co-KRAS mutation. MMR status was available for 69 patients (66%): 21 patients (30%) were identified with an MSI-H phenotype. Clinical, molecular, and pathological characteristics are summarized in Table 1.
Treatment features
The flow chart in Fig. 1 describes the treatments administrated. Forty-nine out of 105 patients (47%) underwent CRLM resection, of which 31 (63%) after chemotherapy. The details about surgical procedures were available for 37 out of 49 patients: major liver resection (≥3 segments) was performed in 38% of cases (14/37), two-stage liver resection in 24% of cases (9/37), and preoperative portal vein embolization in 11% of cases (4/37). Radiofrequency ablation was combined with liver surgery in 24% of patients (9/37). R1 parenchymal resections were present in 4 out of 36 cases (11%). Sixty-five percent received adjuvant chemotherapy.
Cytotoxic doublet chemotherapies (FOLFOX or FOLFIRI) represented the main first-line treatment (72%, n=57/79), followed by triplet chemotherapy (FOLFIRINOX) for 12 patients (15%). Twenty-seven patients (34%) received bevacizumab in combination with chemotherapy as the first-line treatment.
Among patients treated exclusively with chemotherapy (n = 48), 63% received a second line (n = 30) and 35% received a third line (n = 17). From the second line onward, targeted therapies were more frequently used. In total, 24 patients (50%) received concomitantly or successively the following cytotoxic drugs: fluoropyrimidine, oxaliplatin, and irinotecan. Of note, 54% of patients with unresected CRLM underwent primary tumor surgery (Table 1).
Finally, 10 out of 105 patients (10%) participated in clinical trials, four of which involved immune checkpoint inhibitors (ICIs) or targeted therapies.
Table 2 summarizes the results of descriptive and univariate survival analysis according to treatments.
Prognostic factors
The median OS was 16.2 months (95% confidence interval (CI): 13.2–20.7), with a 1-year OS rate of 65% and a 3-year rate of 16% (Fig. 2).
In univariate analysis, the following factors were associated with longer survival: CRLM resection, primary tumor surgery, CRLM less < 10, and initially resectable CRLM (Table 3).
In multivariate analysis, primary tumor surgery (hazard ratio (HR) = 0.349; 95% CI 0.164–0.744, P = 0.0064) and CRLM resection (HR = 0.169; 95% CI 0.082–0.348, P < 0.0001) were associated with significantly better OS.
CRLM resection was associated with a significantly longer OS, with a median of 34 months (range, 28.9–67.3 months) versus 10.6 (6.7–12.5) months for unresected patients, P < 0.0001 (Fig. 3). Patients who received preoperative chemotherapy had a median OS of 34 months (28.9–non-evaluable) versus 33 months (19.6–non-evaluable) for patients resected upfront (P = 0.3402). The median postoperative OS was 28 (19.8–53.5) months.
Primary tumor surgery was associated with a significantly longer OS, with a median of 23.7 (16.6–33) months versus 6.4 (2.9–11.1) months for unresected patients (P < 0.0001). The benefit of primary tumor surgery remained statistically significant in the unresected CRLM group (n = 30), with a median OS of 12.9 (9.4–16.1) months versus 6.4 (2.9–11.1) months in the unresected group (primary tumor and CRLM, n = 26) (P = 0.0002) (Fig. 4).
Exclusive chemotherapy treatment conferred a median OS of 11.5 months (7.1–13.2).
Comparison of resected and non-resected CRLM groups
Clinical and pathological characteristics according to CRLM treatment status are summarized in Table 1. Patients with resected CRLM (n = 49) were significantly more likely to present less than 10 metastases (P < 0.0001) with unilobar distribution (P = 0.0036) and initially resectable (P < 0.0001). Considering the missing data about MSI-H status, no significant difference between the two groups was observed (P = 0.7293). Concerning the radiologic responses, among the patients treated exclusively with chemotherapy, 29% (14/48) had an objective response, 27% (13/48) had stable disease, and 44% (21/48) were progressive. Data were available for 25 patients who received preoperative chemotherapy, with a stable disease rate of 28% (7/25), objective response rate of 64% (16/25), and progressive disease rate of 8% (2/25).
Sites of progression
In total, 95 out of 105 patients (90%) experienced disease relapse after liver surgery (n = 40) or disease progression (n = 55) by the end of follow-up. The liver was the main site of disease recurrences after liver surgery or progression after chemotherapy. Patients with unresected CRLM experienced more peritoneal progression than patients with CRLM resected (17.5% versus 7%).
Discussion
Data on patients with BRAF V600E-mutant CRC and liver-only metastases are scarce. Most of the published studies on BRAF mutated-CRLM included only patients who underwent surgery. This was the largest dedicated cohort (n = 105, regardless of treatments received) study to date, bringing an additional support that their resection is beneficial vs chemotherapy only.
The profit of liver resection is in line with findings in two recent retrospective studies. Johnson et al. showed that among 52 patients with BRAF V600E metastatic CRC, the median OS was significantly prolonged when liver resection with curative intent was performed: 29.1 versus 22.7 months, HR 0.33, 95% CI: 0.12–0.78, P = 0.01 [16]. In the second study, 43 out of 282 patients underwent surgery, with a median OS of 47.4 months versus 19.5 months for those who had no metastasectomy (HR 0.469, 95% CI: 0.288–0.765; P = 0.0024) [17]. In addition, a recent case-matched study demonstrated that BRAF mutations did not increase the risk of relapse after CRLM surgery compared to BRAF wild-type disease (HR 1.16, 95% CI 0.72–1.85; P = 0.547) [10]. The high proportion of patients undergoing resection in our cohort should reflect the fact that the assessment of mutational status was probably not performed in patients with poor prognosis.
To allow comparison with unresected patients, we defined OS as the time from CRLM diagnosis, whereas previous studies have reported OS from the date of liver surgery. Nonetheless, the median post-operative OS starting from the date of surgery in this cohort (28 months) was in line with those in previous studies: from 22.6 months reported by Schirripa et al. (n = 12) to 47.4 months reported by De la Fouchardière et al. (n = 35) [17,18,19,20]. OS results remain lower than the previous publication from Bachet et al. with a median OS of 52.7 months (n= 66). The exclusion of non-V600E BRAF mutated-patients in our study may explain this difference [10]. Relapse-free survival, progression-free survival, and disease-specific survival were not included in the present study as the definitions would differ for resected and unresected groups.
The positive results of primary tumor resection in the unresected CRLM group were surprising and should be interpreted with caution due to the small number of patients involved (30 versus 26). Indeed, a recent study showed that primary tumor resection followed by chemotherapy was not superior to chemotherapy alone (HR 1.10 [0.76–1.59], one-sided P = 0.69). The trial was terminated early for futility reason [21].
In this cohort, a small proportion of patients received an upfront triplet regimen with or without bevacizumab (12/79, 15%), with a median OS of 16.6 months (6.7–not reached). Of note, the majority of the cohort presented a good performance status (84% in the group treated exclusively with chemotherapy). An intensive regimen has been assumed beneficial in BRAF-mutant patients with unresectable liver-limited disease to date, based on a pooled analysis of a small number of patients (n = 20) [22]. However, a recent meta-analysis of five randomized trials comparing FOLFOXIRI plus bevacizumab with doublet plus bevacizumab in 105 BRAF V600E-mutant patients showed no increased benefit in the intensive therapeutic arm [9].
Among the patients treated with chemotherapy only, 63% received second-line treatment; this rate is superior to those reported in the COIN trial (33%) [23] and in a matched case-control study (51%) [24]. Beyond the second line, candidates for treatment decreased dramatically to 35%, and it is important to pinpoint that only 50% of patients received the three major cytotoxic drugs: 5-FU/leucovorin, oxaliplatin, and irinotecan. The present study showed that even if BRAF-mutant metastatic disease is confined to one organ, the prognosis remains poor when the patient is treated with chemotherapy only.
The study population mostly received standard chemotherapies, not new practice-changing therapies. Recently, mitogen-activated protein kinase pathway-targeted therapies have demonstrated better efficacy. In the BEACON trial, the combination of encorafenib, a BRAF inhibitor, and cetuximab (anti-EGFR) with or without binimetinib, a MEK inhibitor, was associated with a significantly longer OS than standard chemotherapy after at least one prior line in a large cohort of patients with BRAF V600E mutations [25]. The same regimen as a first-line is currently under investigation in a phase II trial (ANCHOR-CRC) [26].
ICIs represent another therapeutic option, especially for MSI-H mCRC. The phase III KEYNOTE-177 study demonstrated that first-line pembrolizumab was associated with significant progression-free survival improvement over chemotherapy in MSI-H mCRC (median progression-free survival of 16.5 versus 8.2 months, HR = 0.60; 95% CI 0.45–0.80, P = 0.0002). The benefit of pembrolizumab was consistent in the BRAF V600E-mutant subgroup [27]. In the present cohort, very few patients received ICI after chemotherapy failure (n = 3), explained by the period of inclusion. MSI-H status was not reliable for any conclusions due to insufficient data. In a recent study, MSI-H status was associated with significantly longer OS in a BRAF-mutant mCRC population (n = 194) treated with standard chemotherapies [17]. In the era of immunotherapy, the impact of immune cell infiltrations in BRAF-mutated colorectal cancers is questioned [28, 29].
The major weakness of the study is related to the differences between the resected and unresected CRLM groups with the bias of less aggressive disease in the resected group. However, this should be counterbalanced by increased liver surgery ability, and moreover, the initial resectability or unresectability status might have been subject to variability between the centers. The reasons for non-resectability were not specified, and patients could be considered unresectable solely based on the presence of BRAF mutations.
The missing data in this cohort represent an important limitation, and some known prognostic factors, such as MSI status, were not included in the statistical analysis. Therefore, a case-matched study (resected and unresected CRLM) was not feasible. A prospective study with current therapeutic strategies (ICI for MSI-H and anti-BRAF plus anti-MEK for non-MSI-H) should be considered.
With all the limitations of a retrospective study, this was conducted in the largest cohort of BRAF V600E mutant patients with CRLM reported to date. This will be considered therefore as a historical cohort of BRAF mutated patients who had a liver surgery before the advent of immunotherapies and combinations of anti-BRAF, anti-MEK, and anti-EGFR targeted therapies. A subgroup difficult to look at given its rarity, also prospective studies would be difficult to realize.
Conclusions
In BRAF V600E mutated patients, as long as systemic targeted therapies and immunotherapies are under development, liver resection is, with primary tumor resection, the only prognosis factor for overall survival. While our population is heterogeneous because of the lack of data about MMR phenotype, we show that those patients should not be excluded from liver surgery.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- CRC:
-
Colorectal cancer
- CRLM:
-
Colorectal liver metastases
- HR:
-
Hazard ratio
- ICI:
-
Immune checkpoint inhibitor
- MMR:
-
Mismatch repair
- MSI-H:
-
Microsatellite instability
- OS:
-
Overall survival
- PCR:
-
Polymerase chain reaction
References
Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240(4):644–57.
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208–15.
Viganò L, Ferrero A, Lo Tesoriere R, Capussotti L. Liver surgery for colorectal metastases: results after 10 years of follow-Up. Long-term survivors, late recurrences, and prognostic role of morbidity. Ann Surg Oncol. 2008;15(9):2458–64.
de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Trans Meet Am Surg Assoc. 2009;127:84–92.
Pulitanò C, Castillo F, Aldrighetti L, Bodingbauer M, Parks RW, Ferla G, et al. What defines « cure » after liver resection for colorectal metastases? Results after 10 years of follow-up. HPB. 2010;12(4):244–9.
Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77(7):1254–62.
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–18 discussion 318-321.
Innocenti F, Ou F-S, Qu X, Zemla TJ, Niedzwiecki D, Tam R, et al. Mutational analysis of patients with colorectal cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome. J Clin Oncol Off J Am Soc Clin Oncol. 2019;37(14):1217–27.
Cremolini C, Antoniotti C, Stein A, Bendell JC, Gruenberger T, Masi G, et al. FOLFOXIRI/bevacizumab (bev) versus doublets/bev as initial therapy of unresectable metastatic colorectal cancer (mCRC): A meta-analysis of individual patient data (IPD) from five randomized trials. J Clin Oncol. 2020;38(15_suppl):4015.
Bachet J-B, Moreno-Lopez N, Vigano L, Marchese U, Gelli M, Raoux L, et al. BRAF mutation is not associated with an increased risk of recurrence in patients undergoing resection of colorectal liver metastases. Br J Surg. 2019;106(9):1237–47.
Teng H-W, Huang Y-C, Lin J-K, Chen W-S, Lin T-C, Jiang J-K, et al. BRAF mutation is a prognostic biomarker for colorectal liver metastasectomy. J Surg Oncol. 2012;106(2):123–9.
Passiglia F, Bronte G, Bazan V, Galvano A, Vincenzi B, Russo A. Can KRAS and BRAF mutations limit the benefit of liver resection in metastatic colorectal cancer patients? A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2016;99:150–7.
Tosi F, Magni E, Amatu A, Mauri G, Bencardino K, Truini M, et al. Effect of KRAS and BRAF mutations on survival of metastatic colorectal cancer after liver resection: a systematic review and meta-analysis. Clin Colorectal Cancer. 2017;16(3):e153–63.
Kim K-P, Kim J-E, Hong YS, Ahn S-M, Chun SM, Hong S-M, et al. Paired primary and metastatic tumor analysis of somatic mutations in synchronous and metachronous colorectal cancer. Cancer Res Treat Off J Korean Cancer Assoc. 2017;49(1):161–7.
Bhullar DS, Barriuso J, Mullamitha S, Saunders MP, O’Dwyer ST, Aziz O. Biomarker concordance between primary colorectal cancer and its metastases. EBioMedicine. 2019;40:363–74.
Johnson B, Jin Z, Truty MJ, Smoot RL, Nagorney DM, Kendrick ML, et al. Impact of metastasectomy in the multimodality approach for BRAF V600E metastatic colorectal cancer: the mayo clinic experience. Oncologist. 2018;23(1):128–34.
de la Fouchardière C, Cohen R, Malka D, Guimbaud R, Bourien H, Lièvre A, et al. Characteristics of BRAFV600E mutant, deficient mismatch repair/proficient mismatch repair, metastatic colorectal cancer: a multicenter series of 287 patients. Oncologist. 2019;24(12):1331-40.
Schirripa M, Bergamo F, Cremolini C, Casagrande M, Lonardi S, Aprile G, et al. BRAF and RAS mutations as prognostic factors in metastatic colorectal cancer patients undergoing liver resection. Br J Cancer. 2015;112(12):1921–8.
Margonis GA, Buettner S, Andreatos N, Kim Y, Wagner D, Sasaki K, et al. Association of BRAF mutations with survival and recurrence in surgically treated patients with metastatic colorectal liver cancer. JAMA Surg. 2018;153(7):e180996.
Gagnière J, Dupré A, Gholami SS, Pezet D, Boerner T, Gönen M, et al. Is hepatectomy justified for BRAF mutant colorectal liver metastases?: a multi-institutional analysis of 1497 patients. Ann Surg. 2020;271(1):147-54.
Kanemitsu Y, Shitara K, Mizusawa J, Hamaguchi T, Shida D, Komori K, et al. A randomized phase III trial comparing primary tumor resection plus chemotherapy with chemotherapy alone in incurable stage IV colorectal cancer: JCOG1007 study (iPACS). J Clin Oncol. 2020;38(4_suppl):7.
Cremolini C, Casagrande M, Loupakis F, Aprile G, Bergamo F, Masi G, et al. Efficacy of FOLFOXIRI plus bevacizumab in liver-limited metastatic colorectal cancer: a pooled analysis of clinical studies by Gruppo Oncologico del Nord Ovest. Eur J Cancer. 2017;73:74–84.
Seligmann JF, Fisher D, Smith CG, Richman SD, Elliott F, Brown S, et al. Investigating the poor outcomes of BRAF-mutant advanced colorectal cancer: analysis from 2530 patients in randomised clinical trials. Ann Oncol Off J Eur Soc Med Oncol. 2017;28(3):562–8.
Kayhanian H, Goode E, Sclafani F, Ang JE, Gerlinger M, Gonzalez de Castro D, et al. Treatment and survival outcome of BRAF-mutated metastatic colorectal cancer: a retrospective matched case-control study. Clin Colorectal Cancer. 2018;17(1):e69–76.
Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E–mutated colorectal cancer. N Engl J Med. 2019;381(17):1632–43.
Grothey A, Yaeger R, Paez D, Tabernero J, Taïeb J, Yoshino T, et al. ANCHOR CRC: a phase 2, open-label, single arm, multicenter study of encorafenib (ENCO), binimetinib (BINI), plus cetuximab (CETUX) in patients with previously untreated BRAF V600E-mutant metastatic colorectal cancer (mCRC). Ann Oncol. 2019;30:iv109.
Andre T, Shiu K-K, Kim TW, Jensen BV, Jensen LH, Punt CJA, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer: The phase 3 KEYNOTE-177 Study. J Clin Oncol. 2020;38(18_suppl):LBA4.
Aasebø K, Bruun J, Bergsland C, Nunes L, Eide G, Pfeiffer P, et al. Prognostic role of tumour-infiltrating lymphocytes and macrophages in relation to MSI, CDX2 and BRAF status: a population-based study of metastatic colorectal cancer patients. Bri J Cancer. 2022 Jan;126(1):48–56.
El-Arabey A, Abdalla M, Rashad A-AA. SnapShot: TP53 status and macrophages infiltration in TCGA-analyzed tumors. Int Immunopharmacol. 2020;86:106758.
Acknowledgements
We would like to thank Julien Edeline, Farid El Hajbi, Marie-Pierre Galais, Claire Giraud, Vincent Hautefeuille, and Olivier Romano, who helped to get access to patients’ clinical data from their hospital.
We would like to thank Editage (www.editage.com) for English language editing
Funding
None.
Author information
Authors and Affiliations
Contributions
SJ, AP, and AT designed the study. SJ, SB, and CD collected the patient’s clinical data. PD performed the data processing and statistical analysis. SJ analyzed the data and wrote the paper under supervision of AP and AT. All authors revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the ethical standards in line with the French regulation. French Data Protection Authority (CNIL agreement n° DEC18-409 (2018_01)) provided a waiver of informed consent for this retrospective study and permitted the publication of anonymized data.
Non-deceased patients at the time of the study were informed and declared non-opposition to medical data collection and the data were anonymously analyzed.
Consent for publication
French Data Protection Authority (CNIL agreement n° DEC18-409 (2018_01)) provided a waiver of informed consent for this retrospective study and permitted the publication of anonymized data.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Javed, S., Benoist, S., Devos, P. et al. Prognostic factors of BRAF V600E colorectal cancer with liver metastases: a retrospective multicentric study. World J Surg Onc 20, 131 (2022). https://doi.org/10.1186/s12957-022-02594-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-022-02594-2