Abstract
Background
Recent researches have suggested that long noncoding RNA (lncRNA) is involved in the tumorigenesis and development of stomach cancer (SC). This meta-analysis aimed to identify the diagnostic performance of circulating lncRNAs in SC.
Methods
All relevant studies were systematically searched through PubMed, Web of Science, Cochrane Library, and EMBASE databases. The diagnostic values of lncRNAs were mainly assessed by pooled sensitivity, specificity, and summary receiver operating characteristic area under the curve (SROC AUC). Meta-DiSc 1.4, Review Manager 5.3, and STATA 12.0 were used for statistical analysis. The protocol for this systematic review was registered on INPLASY (INPLASY202120079) and is available in full on the inplasy.com (https://doi.org/10.37766/inplasy2021.2.0079).
Results
A total of 42 eligible studies were included in this meta-analysis. The pooled sensitivity, specificity, and SROC AUC were 0.78 (95%CI 0.75–0.81), 0.75 (95%CI 0.71–0.78), and 0.83 (95%CI 0.80–0.86), respectively, suggesting that the lncRNAs test had a high accuracy for the diagnosis of SC. Obvious heterogeneity might come from the type of lncRNA through subgroup and meta-regression analysis. Fagan diagram shows the clinical value of lncRNAs test in SC.
Conclusions
Abnormal expression of circulating lncRNAs exhibits a high efficacy for diagnosing SC, which is promising in clinical application.
Similar content being viewed by others
Background
Based on 2018 global cancer data, stomach cancer (SC) is the 5th most common neoplasm and the 3rd most deadly cancer, causing an estimated 783,000 deaths in 2018 [1]. Studies have shown that SC patients are often diagnosed at later stages due to the absence of typical early signs [2]. As a result, the overall survival in patients with advanced SC is poor; the 5-year survival rate ranges from approximately 10 to 30% [3]. The prognosis of SC is highly dependent on the timing of the diagnosis [4]. Blood-based cancer biomarkers are ideal for screening and early detection due to their convenience and low invasiveness. However, the low sensitivity and specificity of conventional blood biomarkers limit their application, such as carcinoembryonic antigen and carbohydrate antigen 19-9 [5]. Although considerable effort has been devoted to identifying the underlying mechanism of SC, the identification of new diagnostic markers for SC is still a considerable challenge.
In recent years, the regulation of gene expression by noncoding RNAs has been studied thoroughly. Long noncoding RNAs (lncRNAs) are RNA molecules greater than 200 nucleotides that modulate gene expression at the levels of transcription, posttranscription, and translation, but are not able to encode proteins [6]. An increasing body of evidence has suggested that lncRNAs play a major role during the processes of tumorigenesis and development, which may offer new ideas for the early diagnosis of SC. For instance, for distinguishing SC patients from normal subjects, the lncRNAs PCGEM1 and LOC80054 have higher area under the curve (AUC) values than other conventional tumor markers (AFP, CEA, CA12-5, CA19-9, and CA72-4) [7, 8]. Similarly, lncRNAs can also be detected in the blood, and circulating noncoding RNAs have become a new source of noninvasive cancer biomarkers [9], which can serve as new diagnostic biomarkers for SC.
However, considering the small sample size and limitations of the research design, there is insufficient evidence to confirm the diagnostic accuracy of circulating lncRNAs in SC patients. To address this shortcoming, a comprehensive systematic review and meta-analysis was conducted to explore the diagnostic accuracy of circulating lncRNAs in SC.
Methods
Search strategy
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [10]. The PubMed, Web of Science, Cochrane Library, and Embase databases were systematically searched for potentially relevant articles, which were independently screened by two authors (Cao F and Xu J). The reference lists of relevant meta-analyses and reviews were also searched to identify articles that were not included in the initial search. In addition, relevant articles in scientific congresses and conferences were reviewed. The search strategy and Participant, Index test, Comparison, Outcome, and Study (PICOS) design strategy are shown in Table 1. The publication search was updated regularly until July 9, 2020.
Selection criteria
The following inclusion criteria were used:
-
(i)
The expression of lncRNAs was determined in plasma or serum by quantitative reverse transcription-polymerase chain reaction or other molecular techniques;
-
(ii)
Studies evaluated the diagnosis value of lncRNA for SC;
-
(iii)
Sufficient data to determine false negatives, true negatives, false positives, and true positives.
The exclusion criteria were as follows:
-
(i)
Duplicate publications;
-
(ii)
Meta-analysis, correspondence, single case reports, review articles, and animal model studies.
Data extraction
The two authors (Cao F and Xu J) reviewed the full texts and independently extracted data from all included studies. The following data were extracted: first author, year of publication, race of participants, pathological type of experimental group/control group, sample size, specimen type, lncRNA type, dysregulated state of lncRNAs, sensitivity, and specificity.
Quality assessment
Two authors (Xu J and Cao F) independently evaluated the quality of each diagnostic study. The methodological quality and applicability of the included studies were examined using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) [11] tool in using Review Manager software version 5.3. The QUADAS-2 tool is used to assess the quality of diagnostic accuracy studies [11]. The QUADAS-2 tool contains 4 main areas: process and timing, index testing, reference standards, and patient selection. The risk of prejudice and apprehension was classified as “low,” “high,” or “unclear.” The differences were resolved through discussions among all the researchers.
Details of the protocol for this systematic review were registered on INPLASY (INPLASY202120079) and are available in full on the inplasy.com (https://doi.org/10.37766/inplasy2021.2.0079). This study is presented in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement.
Statistical analysis
Meta-analyses were performed using Meta-DiSc 1.4 (Romany Cajal Hospital, Madrid, Spain) [12], Review Manager 5.3 (Cochrane Collaboration, Oxford, England), and STATA 12.0 (Stata Corp LP, TX, USA).
For a meta-analysis of diagnostic accuracy, the sensitivity, specificity, negative likelihood ratio, positive likelihood ratio, diagnostic odds ratio, and the corresponding 95% CIs were used to determine the diagnostic value of lncRNAs. To quantitatively assess the accuracy of diagnosis, the area under the curves (AUCs) of summary receiver operating characteristic curves (SROCs) were determined. The SROC curve method is a meta-analysis of multiple different experiments of a certain detection index. According to the weight of their odds ratio, the diagnostic accuracy is comprehensively evaluated by fitting the SROC curve [13]. The hierarchical summary receiver operating characteristic (HSROC) model proposed by Rutter and Gatsonis in 2001 represents a general framework for the meta-analysis of diagnostic test studies that allows different parameters to be defined as random effects [14]. A HSROC model was adopted to extend the fixed-effects SROC model and evaluate the accuracy of multiple diagnostic tests.
The heterogeneity tests were carried out by the Q test and I2 statistics. P values of < 0.05 were regarded as statistically significant. An I2 value > 50% and a P value < 0.05 indicated significant heterogeneity between the included studies, and a random effects model was applied. Otherwise, if there was no obvious heterogeneity, the fixed effects model was applied to evaluate the aggregated results. The heterogeneity induced by the threshold effect was evaluated by the ROC plane. Galbraith Star charts and bivariate boxplots were employed to estimate the degree of heterogeneity. Subgroup analysis and meta-regression were used to assess the source of heterogeneity. Subgroup results were examined one at a time.
Sensitivity analysis was used to determine the stability of the results. Potential publication bias was examined by Deeks’ funnel plot. A P value of > 0.1 indicates that there is no publication bias. Fagan’s nomogram was applied to judge the clinical value of lncRNAs as a diagnostic method.
Results
Literature searching and study screening
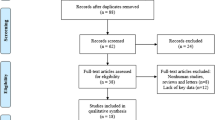
In total, 1867 articles were obtained from the four databases. After eliminating 639 duplicate articles, 1228 studies were further screened. After screening the titles, abstracts, and full texts, 42 eligible studies [2, 7, 15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54] were finally included based on the selection criteria (Fig. 1).
Quality evaluation and main characteristics of the eligible studies
The diagnostic meta-analysis analyzed 42 eligible studies [2, 7, 15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54] published between 2013 and 2020. Thirty-seven studies detected lncRNA expression in Asian population, while 5 studies detected lncRNA expression in Caucasian populations. Sample types included plasma, serum, and plasma/serum exosomes. All SC patients were pathologically confirmed, and the control groups consisted of healthy donor individuals and benign stomach disease patients. A total of 49 different lncRNAs were examined across all included studies; most of the lncRNAs were upregulated in SC (Table 2). The quality assessment is shown in Fig. 2.
Diagnostic accuracy of lncRNA
A total of 42 eligible diagnostic studies were meta-analyzed. As illustrated in Fig. 3, the pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio were 0.78 (95% CI 0.75–0.81), 0.75 (95% CI 0.71–0.78), 3.09 (95% CI 2.66–3.58), 0.29 (95% CI 0.25–0.33), and 10.67 (95% CI 8.34–13.65), respectively. As demonstrated in Fig. 4a, the AUC value of the SROC was 0.83 (95% CI 0.80–0.86). The SROC results were further evaluated through the HSROC model. As shown in Fig. 4b, the β estimate was 0.11 (95% CI −0.19−0.40) and the corresponding P value was 0.485. The lambda estimate was 2.38 (95% CI 2.13–2.63).
Heterogeneity analysis
As illustrated in Fig. 3, obvious heterogeneity was found in the pooled sensitivity (I2 = 88.93%, P < 0.01), specificity (I2 = 88.14%, P < 0.01), positive likelihood ratio (I2 = 88.49%, P < 0.01), negative likelihood ratio (I2 = 88.71%, P < 0.01), and diagnostic odds ratio (I2 = 100.00%, P < 0.01).
A nontypical shoulder arm appearance was observed in the ROC plane (Fig. 5a). Twenty out of the 63 studies of the Galbraith star chart and 10 out of 42 studies of the bivariate box plot fell outside the 95% CI (Fig. 5b, c). Figure 5d shows the meta-regression forest map. All studies were grouped according to race, pathological types of experimental groups, pathological types of control groups, sample size, specimen type, dysregulated state of lncRNAs, and lncRNA types. Table 3 shows the changes in sensitivity, specificity, and I2 values after meta-regression and subgroup analysis.
Sensitivity analysis and publication bias
First, sensitivity analysis was carried out to determine the stability of our results. The removal of individual studies exhibited no noticeable changes in pooled results (Additional file 1: Supplementary Figure 1, Fig. S1A). The P value of Deeks’ funnel plot asymmetry test was 0.12 (Additional file 1: Supplementary Figure 1, Fig. S1B).
Clinical values of lncRNAs for SC diagnosis
As shown in Fig. 6, Fagan’s nomogram revealed that if the patient had a positive lncRNA test result, the actual probability of suffering from SC was 76%, while the probability was 22% if a negative test result was obtained.
Discussion
In recent years, lncRNAs have been recognized as potential diagnostic biomarkers for different cancers [55]. As a diagnostic biomarker for cancer, lncRNAs have the following special advantages. First, the abundance of lncRNAs is relatively high. In the human genome, the number of lncRNAs is four times greater than that of coding RNAs [56]. Second, lncRNAs are highly expressed in the plasma, tissue, and exosomes of cancer cases [57]. Third, lncRNAs have complex biological functions and are closely related to tumorigenesis and development. Therefore, lncRNAs may be promising biomarkers for the early detection and prognosis of various cancers [58].
In the present meta-analysis, a total of 42 eligible studies were screened. The aggregated results of sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio, and SROC AUC indicated that the abnormal expression of circulating lncRNAs exhibits a high accuracy for the diagnosis of SC. The βestimate in the HSROC model indicated that the SROC is symmetrical. Meanwhile, the estimate of lambda reflected the diagnostic accuracy of lncRNAs. Sensitivity analysis verified the stability of the results, and the Deeks funnel chart asymmetry test showed that there was no obvious publication bias. The Fagan diagram also shows its advantages in clinical application, which was mainly due to its moderately high positive and negative predictive value.
For the obvious heterogeneity in the pooled estimates, many analyses have been applied to explore the source of heterogeneity. The ROC plane suggests the absence of a threshold effect, while the Galbraith star charts and bivariate boxplots suggest heterogeneity between studies. Meta-regression and subgroup analysis showed that the heterogeneity might come from the type of lncRNA: when lncRNA UCA1 was used as the grouping condition, the I2 of sensitivity was reduced to 83.60%, and the I2 of specificity was reduced to 45.20% (P = 0.161). In addition, the diagnostic value of lncRNA UCA1 was above average (AUC: 0.92 (95% CI 0.84–0.99) versus 0.83 (95% CI 0.80–0.86)). There was no evidence that race, pathological types of experimental groups, pathological types of control groups, sample size, specimen type, and dysregulated state of lncRNAs significantly affected the pooled results.
Although meta-analysis of lncRNAs in the diagnosis of SC has been reported before [59, 60], most of them focus on lncRNAs in SC tissues. Although lncRNAs in tissue also have high diagnostic accuracy (AUC= 0.755 [59]; 0.80 [60]), their clinical application value is limited for the following reasons: first, the diagnosis of SC after surgery depends on the pathological morphology and immunohistochemical analysis, and the auxiliary role of lncRNAs is optional; second, in regard to endoscopic biopsy specimens, the diagnosis of SC still depends on the pathological morphology, and no extra tumor tissue can be used to extract lncRNAs. In contrast, circulating lncRNAs are ideal biomarkers due to their convenience and low invasiveness. Therefore, the present study on the application of circulating lncRNAs in the diagnosis of SC has greater clinical significance.
Nevertheless, this meta-analysis possessed some limitations. First, this systematic review and meta-analysis lacks eligible non-Asian studies. Second, almost every study focuses on different lncRNAs, and it was difficult to perform subgroup analysis based on lncRNA types to explain the possible sources of heterogeneity. Third, obvious heterogeneity was found in the included studies. Although diagnostic meta-analysis suggested that the type of lncRNA was a source of heterogeneity through meta-regression and subgroup analysis, the heterogeneity of sensitivity and specificity were still high in each subgroup.
Conclusions
In conclusion, the findings of the diagnostic meta-analysis provide evidence that circulating lncRNA tests exhibit a high accuracy for diagnosing SC, which is promising in clinical application due to their high positive and negative predictive value. This study provides an important reference value for the application of circulating lncRNAs as biomarkers for the early diagnosis of SC. Due to potential limitations, further investigations are warranted to verify the diagnostic role of circulating lncRNAs in SC.
Availability of data and materials
All relevant data are within the paper and its additional files.
Abbreviations
- LncRNA:
-
Long noncoding RNA
- SC:
-
Stomach cancer
- SROC:
-
Summary receiver operating characteristic
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- CA-153:
-
Cancer antigen 153
- CEA:
-
Carcinoembryonic antigen
- QUADAS-2:
-
Quality Assessment of Diagnostic Accuracy Studies 2
- HSROC:
-
Hierarchical summary receiver operator characteristic
References
Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14(1):26–38. https://doi.org/10.5114/pg.2018.80001.
Zhang X, Liang W, Liu J, Zang X, Gu J, Pan L, Shi H, Fu M, Huang Z, Zhang Y, Qian H, Jiang P, Xu W. Long non-coding RNA UFC1 promotes gastric cancer progression by regulating miR-498/Lin28b. J Exp Clin Cancer Res. 2018;37(1):134. https://doi.org/10.1186/s13046-018-0803-6.
Jeddi F, Soozangar N, Sadeghi MR, Somi MH, Shirmohamadi M, Eftekhar-Sadat AT, Samadi N. Nrf2 overexpression is associated with P-glycoprotein upregulation in gastric cancer. Biomed Pharmacother. 2018;97:286–92. https://doi.org/10.1016/j.biopha.2017.10.129.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. https://doi.org/10.3322/caac.20107.
Kim DH, Oh SJ, Oh CA, Choi MG, Noh JH, Sohn TS, Bae JM, Kim S. The relationships between perioperative CEA, CA 19-9, and CA 72-4 and recurrence in gastric cancer patients after curative radical gastrectomy. J Surg Oncol. 2011;104(6):585–91. https://doi.org/10.1002/jso.21919.
McFadden EJ, Hargrove AE. Biochemical methods to investigate lncRNA and the influence of lncRNA: protein complexes on chromatin. Biochemistry. 2016;55(11):1615–30. https://doi.org/10.1021/acs.biochem.5b01141.
Jiang H, Guo S, Zhao Y, Wang Y, Piao HY, Wu Y, Zhang J. Circulating long non-coding RNA PCGEM1 as a novel biomarker for gastric cancer diagnosis. Pathol, Res Pract. 2019;215(10):152569.
Ren N, Jiang T, Wang C, Xie S, Xing Y, Piao D, Zhang T, Zhu Y. LncRNA ADAMTS9-AS2 inhibits gastric cancer (GC) development and sensitizes chemoresistant GC cells to cisplatin by regulating miR-233-3p/NLRP3 axis. Aging-Us. 2020;12(11):11025–41. https://doi.org/10.18632/aging.103314.
Hou J, Meng F, Chan LW, Cho WC, Wong SC. Circulating plasma microRNAs as diagnostic markers for NSCLC. Front Genet. 2016;7:193.
Walther S, Schuetz GM, Hamm B, Dewey M. Quality of reporting of systematic reviews and meta-analyses: PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses). Rofo. 2011;183(12):1106–10. https://doi.org/10.1055/s-0031-1281809.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36. https://doi.org/10.7326/0003-4819-155-8-201110180-00009.
Martindale JL, Wakai A, Collins SP, Levy PD, Diercks D, Hiestand BC, Fermann GJ, deSouza I, Sinert R. Diagnosing acute heart failure in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2016;23(3):223–42. https://doi.org/10.1111/acem.12878.
Macaskill P. Empirical Bayes estimates generated in a hierarchical summary ROC analysis agreed closely with those of a full Bayesian analysis. J Clin Epidemiol. 2004;57(9):925–32. https://doi.org/10.1016/j.jclinepi.2003.12.019.
Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20(19):2865–84. https://doi.org/10.1002/sim.942.
Liu W, Li Y, Zhang Y, Shen X, Su Z, Chen L, Cai W, Wang F, Ju S. Circulating long non-coding RNA FEZF1-AS1 and AFAP1-AS1 serve as potential diagnostic biomarkers for gastric cancer. Pathol, Res Pract. 2020;216(1):152757.
Yörüker EE, Keskin M, Kulle CB, Holdenrieder S, Gezer U. Diagnostic and prognostic value of circulating lncRNA H19 in gastric cancer. Biomed Rep. 2018;9(2):181–6. https://doi.org/10.3892/br.2018.1116.
Liu Y, Jiang M, Ni LN, Xu Z, Xu JZ. Diagnostic value of serum carcinoembryonic antigen and carbohydrate antigen 724 combined with long non-coding RNA MALAT1 detection in gastric cancer. Cancer Res Clin. 2019;31(2):88–92.
Hashad D, Elbanna A, Ibrahim A, Khedr G. Evaluation of the role of circulating long non-coding RNA H19 as a promising novel biomarker in plasma of patients with gastric cancer. J Clin Lab Anal. 2016;30(6):1100–5. https://doi.org/10.1002/jcla.21987.
Li Q, Shao Y, Zhang X, Zheng T, Miao M, Qin L, Wang B, Ye G, Xiao B, Guo J. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumour Biol. 2015;36(3):2007–12. https://doi.org/10.1007/s13277-014-2807-y.
Liu Z, Shao Y, Tan L, Shi H, Chen S, Guo J. Clinical significance of the low expression of FER1L4 in gastric cancer patients. Tumour Biol. 2014;35(10):9613–7. https://doi.org/10.1007/s13277-014-2259-4.
Liu J, Wang J, Song Y, Ma B, Luo J, Ni Z, Gao P, Sun J, Zhao J, Chen X, Wang Z. A panel consisting of three novel circulating lncRNAs, is it a predictive tool for gastric cancer? J Cell Mol Med. 2018;22(7):3605–13. https://doi.org/10.1111/jcmm.13640.
Lu Q, Yu T, Ou X, Cao D, Xie T, Chen X. Potential lncRNA diagnostic biomarkers for early gastric cancer. Mol Med Rep. 2017;16(6):9545–52. https://doi.org/10.3892/mmr.2017.7770.
Mohamed WA, Schaalan MF, Ramadan B. The expression profiling of circulating miR-204, miR-182, and lncRNA H19 as novel potential biomarkers for the progression of peptic ulcer to gastric cancer. J Cell Biochem. 2019;120(8):13464–77. https://doi.org/10.1002/jcb.28620.
Piao HY, Guo S, Wang Y, Zhang J. Exosomal long non-coding RNA CEBPA-AS1 inhibits tumor apoptosis and functions as a non-invasive biomarker for diagnosis of gastric cancer. Onco Targets Ther. 2020;13:1365–74. https://doi.org/10.2147/OTT.S238706.
Zhou H, Wang F, Chen H, Tan Q, Qiu S, Chen S, Jing W, Yu M, Liang C, Ye S, Tu J. Increased expression of long-noncoding RNA ZFAS1 is associated with epithelial-mesenchymal transition of gastric cancer. Aging (Albany NY). 2016;8(9):2023–38. https://doi.org/10.18632/aging.101048.
Cai C, Zhang H, Zhu Y, Zheng P, Xu Y, Sun J, Zhang M, Lan T, Gu B, Li S, Ma P. Serum Exosomal long noncoding RNA pcsk2-2:1 as a potential novel diagnostic biomarker for gastric cancer. Onco Targets Ther. 2019;12:10035–41. https://doi.org/10.2147/OTT.S229033.
Zhou X, Yin C, Dang Y, Ye F, Zhang G. Identification of the long non-coding RNA H19 in plasma as a novel biomarker for diagnosis of gastric cancer. Sci Rep. 2015;5(1):11516. https://doi.org/10.1038/srep11516.
Elsayed ET, Salem PE, Darwish AM, Fayed HM. Plasma long non-coding RNA HOTAIR as a potential biomarker for gastric cancer. Int J Biol Markers. 2018. Online ahead of print. https://doi.org/10.1177/1724600818760244.
Xian HP, Zhuo ZL, Sun YJ, Liang B, Zhao XT. Circulating long non-coding RNAs HULC and ZNFX1-AS1 are potential biomarkers in patients with gastric cancer. Oncol Lett. 2018;16(4):4689–98. https://doi.org/10.3892/ol.2018.9199.
Feng W, Zong W, Li Y, Shen X, Cui X, Ju S. Abnormally expressed long noncoding RNA B3GALT5-AS1 may serve as a biomarker for the diagnostic and prognostic of gastric cancer. J Cell Biochem. 2020;121(1):557–65. https://doi.org/10.1002/jcb.29296.
Fu M, Huang Z, Zang X, Pan L, Liang W, Chen J, Qian H, Xu W, Jiang P, Zhang X. Long noncoding RNA LINC00978 promotes cancer growth and acts as a diagnostic biomarker in gastric cancer. Cell Prolif. 2018;51(1):e12425. https://doi.org/10.1111/cpr.12425.
Gao J, Cao R, Mu H. Long non-coding RNA UCA1 may be a novel diagnostic and predictive biomarker in plasma for early gastric cancer. Int J Clin Exp Pathol. 2015;8(10):12936–42.
Ghaedi H, Mozaffari MAN, Salehi Z, Ghasemi H, Zadian SS, Alipoor S, Hadianpour S, Alipoor B. Co-expression profiling of plasma miRNAs and long noncoding RNAs in gastric cancer patients. Gene. 2019;687:135–42. https://doi.org/10.1016/j.gene.2018.11.034.
Guo X, Lv X, Ru Y, Zhou F, Wang N, Xi H, Zhang K, Li J, Chang R, Xie T, Wang X, Li B, Chen Y, Yang Y, Chen L, Chen L. Circulating exosomal gastric cancer-associated long noncoding RNA1 as a biomarker for early detection and monitoring progression of gastric cancer: a multiphase study. JAMA Surg. 2020;155(7):572–9. https://doi.org/10.1001/jamasurg.2020.1133.
Arita T, Ichikawa D, Konishi H, Komatsu S, Shiozaki A, Shoda K, Kawaguchi T, Hirajima S, Nagata H, Kubota T, Fujiwara H, Okamoto K, Otsuji E. Circulating long non-coding RNAs in plasma of patients with gastric cancer. Anticancer Res. 2013;33(8):3185–93.
Ji B, Huang Y, Gu T, Zhang L, Li G, Zhang C. Potential diagnostic and prognostic value of plasma long noncoding RNA LINC00086 and miR-214 expression in gastric cancer. Cancer Biomark. 2019;24(2):249–55. https://doi.org/10.3233/CBM-181486.
Lin LY, Yang L, Zeng Q, Wang L, Chen ML, Zhao ZH, Ye GD, Luo QC, Lv PY, Guo QW, Li BA, Cai JC, Cai WY. Tumor-originated exosomal lncUEGC1 as a circulating biomarker for early-stage gastric cancer. Mol Cancer. 2018;17(1):84. https://doi.org/10.1186/s12943-018-0834-9.
Pan L, Liang W, Fu M, Huang ZH, Li X, Zhang W, Zhang P, Qian H, Jiang PC, Xu WR, Zhang X. Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J Cancer Res Clin Oncol. 2017;143(6):991–1004. https://doi.org/10.1007/s00432-017-2361-2.
Jin C, Shi W, Wang F, Shen X, Qi J, Cong H, Yuan J, Shi L, Zhu B, Luo X, Zhang Y, Ju S. Long non-coding RNA HULC as a novel serum biomarker for diagnosis and prognosis prediction of gastric cancer. Oncotarget. 2016;7(32):51763–72. https://doi.org/10.18632/oncotarget.10107.
Zhao R, Zhang Y, Zhang X, Yang Y, Zheng X, Li X, Liu Y, Zhang Y. Exosomal long noncoding RNA HOTTIP as potential novel diagnostic and prognostic biomarker test for gastric cancer. Mol Cancer. 2018;17(1):68. https://doi.org/10.1186/s12943-018-0817-x.
Burock S, Herrmann P, Wendler I, Niederstrasser M, Wernecke KD, Stein U. Circulating metastasis associated in colon cancer 1 transcripts in gastric cancer patient plasma as diagnostic and prognostic biomarker. World J Gastroenterol. 2015;21(1):333–41. https://doi.org/10.3748/wjg.v21.i1.333.
Ke D, Li H, Zhang Y, An Y, Fu H, Fang X, Zheng X. The combination of circulating long noncoding RNAs AK001058, INHBA-AS1, MIR4435-2HG, and CEBPA-AS1 fragments in plasma serve as diagnostic markers for gastric cancer. Oncotarget. 2017;8(13):21516–25. https://doi.org/10.18632/oncotarget.15628.
Liu Y, Zhang YM, Ma FB, Pan SR, Liu BZ. Long noncoding RNA HOXA11-AS promotes gastric cancer cell proliferation and invasion via SRSF1 and functions as a biomarker in gastric cancer. World J Gastroenterol. 2019;25(22):2763–75. https://doi.org/10.3748/wjg.v25.i22.2763.
Shan L, Liu C, Ma C. High expression of serum UCA1 may be a potential biomarker for clinical diagnosis of gastric cancer. Clin Lab. 2019;65(9). Online ahead of print. https://doi.org/10.7754/Clin.Lab.2019.190317.
Shao Y, Ye M, Li Q, Sun W, Ye G, Zhang X, Yang Y, Xiao B, Guo J. LncRNA-RMRP promotes carcinogenesis by acting as a miR-206 sponge and is used as a novel biomarker for gastric cancer. Oncotarget. 2016;7(25):37812–24. https://doi.org/10.18632/oncotarget.9336.
Yang Z, Sun Y, Liu R, Shi Y, Ding S. Plasma long noncoding RNAs PANDAR, FOXD2-AS1, and SMARCC2 as potential novel diagnostic biomarkers for gastric cancer. Cancer Manag Res. 2019;11:6175–84. https://doi.org/10.2147/CMAR.S201935.
Xu H, Zhou J, Tang J, Min X, Yi T, Zhao J, Ren Y. Identification of serum exosomal lncRNA MIAT as a novel diagnostic and prognostic biomarker for gastric cancer. J Clin Lab Anal. 2020;34(8):e23323. https://doi.org/10.1002/jcla.23323.
Xu Y, Zhang G, Zou C, Gong Z, Wang S, Liu J, Ma G, Liu X, Zhang W, Jiang P. Long noncoding RNA DGCR5 suppresses gastric cancer progression by acting as a competing endogenous RNA of PTEN and BTG1. J Cell Physiol. 2019;234(7):11999–2010. https://doi.org/10.1002/jcp.27861.
Xu Y, Zhang G, Zou C, Qi W, Gong Z, Zhang G, Ma G, Zhang W, Jiang P. Long non-coding RNA LINC01225 promotes proliferation, invasion and migration of gastric cancer via Wnt/β-catenin signalling pathway. J Cell Mol Med. 2019;23(11):7581–91. https://doi.org/10.1111/jcmm.14627.
Yang T, Zeng H, Chen W, Zheng R, Zhang Y, Li Z, Qi J, Wang M, Chen T, Lou J, Lu L, Zhou T, Dai S, Cai M, You W, Pan K. Helicobacter pylori infection, H19 and LINC00152 expression in serum and risk of gastric cancer in a Chinese population. Cancer Epidemiol. 2016;44:147–53. https://doi.org/10.1016/j.canep.2016.08.015.
Zheng P, Zhang H, Gao H, Sun J, Li J, Zhang X, Gao L, Ma P, Li S. Plasma exosomal long noncoding RNA lnc-SLC2A12-10:1 as a novel diagnostic biomarker for gastric cancer. Onco Targets Ther. 2020;13:4009–18. https://doi.org/10.2147/OTT.S253600.
Zheng R, Liang J, Lu J, Li S, Zhang G, Wang X, Liu M, Wang W, Chu H, Tao G, Zhao Q, Wang M, Du M, Qiang F, Zhang Z. Genome-wide long non-coding RNAs identified a panel of novel plasma biomarkers for gastric cancer diagnosis. Gastric Cancer. 2019;22(4):731–41. https://doi.org/10.1007/s10120-018-00915-7.
Zhou Q, Li H, Jing J, Yuan Y, Sun L. Evaluation of C5orf66-AS1 as a potential biomarker for predicting early gastric cancer and its role in gastric carcinogenesis. Onco Targets Ther. 2020;13:2795–805. https://doi.org/10.2147/OTT.S239965.
Tan L, Yang Y, Shao Y, Zhang H, Guo J. Plasma lncRNA-GACAT2 is a valuable marker for the screening of gastric cancer. Oncol Lett. 2016;12(6):4845–9. https://doi.org/10.3892/ol.2016.5297.
Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer cell. 2016;29(4):452–63. https://doi.org/10.1016/j.ccell.2016.03.010.
Carninci P, Kasukawa T, Katayama S, Gough J, Frith M, Maeda N, Oyama R, Ravasi T, Lenhard B, CJS W. The transcriptional landscape of the mammalian genome. Science. 2005;309(5740):1559–63.
Liu L, Meng T, Yang XH, Sayim P, Lei C, Jin B, Ge L, Wang HJ. Prognostic and predictive value of long non-coding RNA GAS5 and mircoRNA-221 in colorectal cancer and their effects on colorectal cancer cell proliferation, migration and invasion. Cancer Biomark. 2018;22(2):283–99. https://doi.org/10.3233/CBM-171011.
Yang M, Wei W. SNHG16: a novel long-non coding RNA in human cancers. Onco Targets Ther. 2019;12:11679–90. https://doi.org/10.2147/OTT.S231630.
Hu QY, Zhao ZY, Li SQ, Li L, Li GK. A meta-analysis: the diagnostic values of long non-coding RNA as a biomarker for gastric cancer. Mol Clin Oncol. 2017;6(6):846–52. https://doi.org/10.3892/mco.2017.1227.
Cui Z, Chen Y, Xiao Z, Hu M, Lin Y, Chen Y, Zheng Y. Long noncoding RNAs as auxiliary biomarkers for gastric cancer screening: a pooled analysis of individual studies. Oncotarget. 2016;7(18):25791–800. https://doi.org/10.18632/oncotarget.8268.
Acknowledgements
The current study was supported by grants from 2019 Kunshan Key R&D Plan (Ecological Agriculture and Social Development)-Social Development Science and Technology Project (KS1941), Suzhou Science, Education and Health Project (KJXW2015053), Kunshan City Social Development Science and Technology Plan Project (KS1532), Kunshan City Social Development Science and Technology Plan Project (KS1420) and Medical Clinical Science and Technology Development Fund Project of Jiangsu University (JLY20160040).
Funding
The current study was supported by grants from 2019 Kunshan Key R&D Plan (Ecological Agriculture and Social Development)-Social Development Science and Technology Project (KS1941) , Suzhou Science, Education and Health Project (KJXW2015053), Kunshan City Social Development Science and Technology Plan Project (KS1532), Kunshan City Social Development Science and Technology Plan Project (KS1420) and Medical Clinical Science and Technology Development Fund Project of Jiangsu University (JLY20160040).
Author information
Authors and Affiliations
Contributions
Investigation: Yongwei Hu. Methodology: Jianhao Xu, Fang Cao. Supervision: Houzhong Ding, Xiaojun Shen. Writing – original draft: Fang Cao, Zaichang Chen. Writing – review & editing: Wei Han, Weijie Lu. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Fig S1. Sensitivity analysis and publication bias. (A) Sensitivity analysis of the pooled studies. (B) Deeks’ funnel plot of the pooled studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cao, F., Hu, Y., Chen, Z. et al. Circulating long noncoding RNAs as potential biomarkers for stomach cancer: a systematic review and meta-analysis. World J Surg Onc 19, 89 (2021). https://doi.org/10.1186/s12957-021-02194-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-021-02194-6