Abstract
Background
Most pancreatoduodenectomy resections do not meet the minimum of 12 lymph nodes recommended by the American Joint Committee on Cancer for accurate staging of periampullary malignancies. The purpose of this study was to investigate factors affecting the likelihood of adequate nodal yield in pancreatoduodenectomy specimens subject to routine pathological assessment.
Methods
Six hundred sixty-two patients subject to pancreatoduodenectomy between 1990 and 2013 for pancreatic, ampullary, and common bile duct cancers were reviewed. Predictors of yielding at least 12 lymph nodes were evaluated with a logistic regression model, and a survival analysis was carried out to verify the prognostic implications of nodal counts.
Results
The median number of evaluated nodes was 17 (interquartile range 11 to 25), and less than 12 lymph nodes were reported in surgical specimens of 179 (27 %) patients. Tumor diameter ≥20 mm (odds ratio [OR] 2.547, 95 % confidence interval [CI] 1.225 to 5.329, P = 0.013), lymph node metastases (OR 2.642, 95 % CI 1.378 to 5.061, P = 0.004), and radical lymphadenectomy (OR 5.566, 95 % CI 2.041 to 15.148, P = 0.01) were significant predictors of retrieving 12 or more lymph nodes. Lymph node counts did not influence the overall prognosis of the patients. However, a subgroup analysis carried out for individual cancer sites demonstrated that removing at least 12 lymph nodes is associated with better prognosis for pancreatic cancer.
Conclusions
Few variables affect adequate nodal yield in pancreatoduodenectomy specimens subject to routine pathological assessment. Considering the ambiguities related to the only modifiable factor identified, appropriate pathology training should be considered to increase nodal yield rather than more aggressive lymphatic dissection.
Similar content being viewed by others
Background
Precise pathologic information is essential for clinical decision-making in patients with solid tumors, including those in the pancreaticoduodenal area (i.e., pancreatic, common bile duct, and ampullary cancers). Therefore, minimizing the risk of misclassification by harvesting an adequate number of lymph nodes is important not only for prognostic stratification but also for implementation of adjuvant therapy when indicated.
The accuracy of staging lymph node status is directly proportional to the number of lymph nodes retrieved and the optimum cutoff value minimizing the stage migration phenomenon reported previously for pancreatic cancer varies from 10 to 15 [1, 2]. Moreover, many studies suggested that removing at least ten lymph nodes is significantly associated with improved survival regardless of the presence of nodal metastases [1, 3, 4]. Others suggested that pathologic assessment of more than 12 lymph nodes may provide more accurate survival estimates for patients with node-negative disease [5, 6]. Based on these observations, at least 12 lymph nodes are required for adequate staging for pancreatoduodenectomy specimens of pancreatic, distal bile duct, and ampullary cancer according to the most recent edition of the American Joint Committee on Cancer (AJCC) TNM classification [7]. Contrary to these recommendations only about seven to eight nodes are dissected in many institutions worldwide [2, 3, 8–10]. This carries the risk of understaging, as an inadequate assessment of regional lymph nodes may erroneously identify node-positive patients as node negative.
Several previous reports demonstrated marked improvements in lymph node counts by adopting adequate methods of specimen processing by dedicated pathologists [11–14]. Surprisingly, there are hardly any studies that discuss other factors affecting retrieval of the optimal 12 lymph nodes according to the current recommendations of AJCC in patients undergoing pancreatoduodenectomy for cancers of the periampullary area. As understaging may have important therapeutic implications in routine clinical practice, the aim of this study was to investigate the impact of clinical and pathological factors on the likelihood of identifying the appropriate number of lymph nodes for cancers of the pancreatic head, ampulla of Vater, and common bile duct.
Methods
Patients
All patients undergoing pancreatic resections between 1990 and 2013 at our academic tertiary surgical center were reviewed to identify pancreatoduodenectomies carried out for malignancy of the pancreatic head, distal bile duct, and ampulla of Vater. Patients operated for non-malignant conditions were excluded. All data were prospectively collected and recorded in a dedicated database. Variables potentially affecting the number of lymph nodes identified in surgical specimens were retrieved from the database and analyzed retrospectively, including demographic data, pathologic features of the tumor, and therapeutic interventions. Follow-up data was collected based on clinical examinations performed every 3–6 months after discharge and dates of death from the census registry office. The study was approved by the Bioethics Committee of Jagiellonian University.
Surgical procedures and pathological evaluation
All procedures were carried out by senior consultant surgeons experienced in pancreato-biliary surgery and using a similar technique of dissection. Primary tumors were resected en bloc with pancreaticoduodenal lymph nodes (groups 13 and 17 according to the Japanese Society of Biliary Surgery (JSBS) [15] and Japan Pancreas Society (JPS) [16]), whereas all other nodal stations were dissected separately. The extent of lymphadenectomy was described by the operating surgeon and classified as defined by the recent guidelines [17, 18]. Briefly, standard lymphadenectomy included resection of the following lymph node groups: anterior and posterior pancreaticoduodenal (nos. 13 and 17), hepatoduodenal ligament (no. 12), nodes to the right side of the superior mesenteric artery from its origin at the aorta to the inferior pancreaticoduodenal artery (nos. 14a and 14b), lymph nodes around the common hepatic artery (no. 8a), and celiac trunk (no. 9), suprapyloric (no. 5), and infrapyloric (no. 6) lymph nodes. Radical lymphadenectomy included removal of lymph node groups described for standard pancreatoduodenectomy along with para-aortic lymph nodes (nos. 16a2 and 16b1) located between the level of coeliac trunk and inferior mesenteric artery. The choice of surgical technique and the extent of lymphadenectomy was made at the discretion of the operating surgeon without any preoperative allocation. Lymph nodes were identified and retrieved from formalin-fixed surgical specimens by the pathologists without any specific techniques aimed at increasing nodal retrieval. In patients subject to total pancreatectomy, groups of lymph nodes located around the pancreatic body and tail (i.e., nos. 10, 11, and 18) were not included in nodal counts for the purpose of this study as they are not dissected at pancreatoduodenectomy.
Statistical analysis
Mann-Whitney U and χ 2 tests were used where appropriate to identify the significant factors predictive of retrieving at least 12 lymph nodes. Predictors significantly associated with nodal count were used for the development of a multivariate logistic regression model. The probability for entering the model was 0.05 and for removal from the model 0.100. Survival data was analyzed according to the Kaplan-Meier method and included postoperative mortality. The log-rank test was used to detect differences between groups. All tests were two-sided and P < 0.050 was considered statistically significant. Statistical analysis was performed using the IBM® SPSS® Statistics v.21 software package (IBM Corporation, NY).
Results
Study population
Among 842 pancreatoduodenectomies identified in our database between 1990 and 2013, 662 were carried out for pancreatic, ampullary, and common bile duct cancers. A group of 180 patients were excluded due to the final diagnosis of benign pancreatic disorders (n = 103) or other malignancies (n = 77). Clinical and demographic data of the selected population are summarized in Table 1.
Evaluation of lymph nodes
During the study period, 444 (67 %) resection specimens were assessed onsite by one senior gastrointestinal pathologist (KN) and the remaining 218 (37 %) at a cooperating university pathology center. The median number of evaluated nodes was 17 (interquartile range 11 to 25, range 2 to 92), and less than 12 lymph nodes were reported in surgical specimens of 179 (27 %) patients. Table 2 shows detailed pattern of lymph node distribution. The highest median nodal yield was found for the pancreaticoduodenal lymph nodes (group nos. 13 and 17), followed by para-aortic (no. 16) and hepatoduodenal ligament nodes (no. 12). Overall, positive lymph nodes were identified in 396 (60 %) patients, including 264 patients with pancreatic cancer, 113 with ampullary cancer, and 19 with common bile duct cancer. There was no significant variability over time in the number of identified lymph nodes (correlation coefficient r = 0.086, P = 0.741) or the proportion of patients with 12 or more nodes examined (r = 0.124, P = 0.660). Median node counts were comparable among all operating surgeons and were not associated with the type of the primary tumor. There was a highly significant negative correlation between patients’ body mass index (BMI) and the proportion of patients with ≥12 lymph nodes examined (correlation coefficient r = –0.679, P = 0.002), but no such association was found for the absolute node count (correlation coefficient r = –0.056, P = 0.349).
Predictive factors for lymph node yield
Table 3 shows results of a univariate analysis of factors associated with removal of at least 12 lymph nodes. Subsequent regression analysis, summarized in Table 4, identified only three independent predictors for adequate nodal yield, i.e., tumor diameter ≥20 mm (odds ratio [OR] 2.547, 95 % confidence interval [CI] 1.225 to 5.329), lymph node metastases (OR 2.642, 95 % CI 1.378 to 5.061), and radical lymphadenectomy (OR 5.566, 95 % CI 2.041 to 15.148).
Lymph node count and survival in patients with malignancies
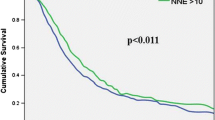
A group of 247 patients was alive after a median follow-up of 94 months (range 24–295 months, final follow-up December 2015). The overall median survival for patients with pancreatic, ampullary, and common bile duct cancers were 15 months (95 % CI 10.6–21.2), 52 months (95 % CI 34.9–68.9), and 18 months (95 % CI 10.6–25.4). A subgroup analysis carried out for individual cancer sites demonstrated that removing at least 12 lymph nodes is associated with better 3- and 5-year survival rates among patients with pancreatic cancer (Fig. 1). The survival benefit of higher nodal counts was maintained also in patients subject to standard lymphadenectomy (Fig. 2). No such effects were found for other malignancies. A subgroup analysis was also carried out for the effects of lymphadenectomy on patients’ survival, but the extent of lymph node dissection (standard vs radical) did not influence prognosis or perioperative complications.
Discussion
Appropriate evaluation of lymph nodes in patients with solid tumors has obvious implications for more accurate staging. This study has demonstrated that the adequate lymph node yield with standard pathologic processing of pancreatoduodenectomy specimens in patients with suspected periampullary malignancy is influenced by only three factors, i.e., tumor diameter, metastases to lymph nodes and extent of lymphadenectomy. Moreover, evaluation of 12 or more nodes was associated with survival benefit in patients with pancreatic cancer.
The amount of lymphatic tissue and numbers of lymph nodes in the upper abdomen vary among individuals [19]. However, the yield of lymph nodes in all surgical specimens, including pancreatoduodenectomy, is mostly influenced by three main groups of variables, i.e., those related to the patient and underlying pathology, to surgical intervention, and to pathologic assessment of the specimen. Although tissue processing and thoroughness of the pathologic examination are the key factors for identifying lymph nodes in surgical specimens, there is no general agreement regarding the appropriate pathological evaluation of pancreatoduodenectomy specimens, leading to ambiguities in defining R1 resections and marked variability in counts of lymph nodes identified in the peripancreatic tissue [20, 21]. The average number of lymph nodes identified in such specimens with standard techniques of pathology sampling is five to seven [20]. Rarely, the number reaches 15 to 29 in some studies examining anatomic distribution of peripancreatic lymph nodes or those applying meticulous processing of the specimen [12, 22, 23]. The use of a standardized protocol for harvesting lymph nodes in our department and the fact that almost 70 % of the specimens were examined by a single pathologist minimize the risk that pathologist-related variability could bias results of the current study and give the opportunity to evaluate the influence of other factors on nodal yield.
There are very few studies reporting variables affecting the number of lymph nodes dissected in pancreatic surgery. Govindarajan et al. in a population of 2111 patients subject to pancreatoduodenectomy or total pancreatectomy for pancreatic head cancer from 1998 through 2003 and identified from the Surveillance, Epidemiology and End Results (SEER) registry found that younger age, female sex, tumor diameter >2 cm, and node-positive status increased the overall nodal count by 10 to 18 % [3]. Another analysis of the same database, but covering the period from 1993 through 2003, demonstrated that the likelihood of removing ten or more lymph nodes among 5465 pancreatoduodenectomies for periampullary carcinomas was higher in females, tumor diameter ≥2 cm, pancreatic head cancers, and metastases to regional lymph nodes [1]. However, the use of SEER data carries several disadvantages, including the unavailability of some variables potentially affecting lymph node counts such as BMI or the extent of lymphadenectomy. Another important issue that has not been addressed before is variability in lymph node counts among different surgeons and pathologists. In contrast with both previous studies, our database provides much more detailed information necessary to better characterize potential predictors of nodal yield.
The association between the lymph node yield and node positivity, as found in this study, is somewhat controversial. Besides the SEER studies, two previous reports suggested that in patients with pancreatic cancer subject to various pancreatic resections there was a significant difference in the total lymph node count in cases with or without nodal metastases of 19 vs 13 (P = 0.02) [5] and 15 vs 10 (P < 0.001) [24]. However, some other studies failed to confirm such a relationship [6, 25–27]. These discrepancies may derive from two potential aspects. First, the presence of enlarged, metastatic nodes may force the operating surgeon to a more extended dissection, and second, metastatic lymph nodes are usually larger and thus easier to identify by the pathologist. The proportion of patients subject to radical lymphadenectomy in our study was not influenced by the presence of metastatic lymph nodes, but the overall median number of nodes was significantly higher in this group (18 vs 15, P < 0.001). As a subsequent analysis in individual nodal stations revealed that the median count among subjects with metastatic nodes was higher only for pancreaticoduodenal stations (9 vs 7, P = 0.040), we may assume that lymph node metastases did not affect the extent of surgery.
As reasonably expected, our study revealed that performing radical lymph node dissection, including para-aortic nodes, is the most significant factor and the only surgeon-dependent one to achieve the recommended nodal yield. Although none of the prospective randomized clinical trials on the extent of lymphadenectomy in periampullary malignancies analyzed variables that potentially affect nodal yield, median numbers of lymph nodes dissected in these studies during standard pancreatoduodenectomy was 13 to 17 and for extended 20 to 36 [23, 25, 28, 29]. The idea of radical dissection is further supported by data accumulated over recent years suggesting that the incidence of lymph node metastasis to para-aortic nodal stations in periampullary malignancies is relatively high and an appropriate degree of lymphadenectomy is necessary to achieve an R0 resection [30–35]. Although a recent meta-analysis of sixteen studies comprising 1909 patients comparing outcomes of standard and extended pancreatoduodenectomy showed similar perioperative morbidity and mortality rates, it also emphasized no improved survival after the latter procedure (hazard ratio 0.77, P = 0.100) [36]. Therefore, the only benefit of the latter procedure seems to be associated with more accurate staging of nodal disease even if some controversies still exist about station 16b1 considered as one of the major lymphatic drainage routes for pancreatic head cancer [18].
Removal of the recommended number of lymph nodes was not associated with any clear survival benefit in the whole population of patients with periampullary malignancies. However, if the tumors were analyzed separately, pancreatic cancer demonstrated better survival among patients with ≥12 lymph nodes resected regardless the extent of lymphadenectomy. This is similar to some observations using cutoff values of 10 or 12 lymph nodes; however, the relationship between node counts and survival is not clear as previous studies on periampullary malignancies reported conflicting results [1, 3–6, 37].
The limitations of the present study are related to its retrospective design and potential bias resulting from such analyzes. In particular, we were unable to account for the premises for performing a more extensive lymph node dissection, such as finding suspicious nodes intraoperatively or decisions made a priori, even though data analysis showed no such correlation. Nevertheless, the lack of major changes observed over time in the absolute number of lymph nodes harvested and the proportion of patients with ≥12 nodes support the assumption that the surgical technique remained unchanged over the study period.
Conclusions
In conclusion, this study demonstrated that only few factors were associated with the likelihood of removing at least 12 lymph nodes in surgical specimens of patients subject to pancreatoduodenectomy for suspected periampullary malignancy, i.e., tumor diameter, lymph node metastases, and radical lymphadenectomy. However, the latter and the only modifiable factor offered no clear survival benefit in previous randomized clinical trials and potentially may increase postoperative morbidity. Therefore, appropriate pathology training should be considered to increase nodal yield rather than more aggressive lymphatic dissection.
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- ASA:
-
American Society of Anesthesiologists
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- IQR:
-
Interquartile range
- JPS:
-
Japan Pancreas Society
- JSBS:
-
Japanese Society of Biliary Surgery
- OR:
-
Odds ratio
- PD:
-
Pancreatoduodenectomy
- SEER:
-
Surveillance, Epidemiology, and End Results
- TNM:
-
Tumor, lymph nodes, distant metastases
References
Gutierrez JC, Franceschi D, Koniaris LG. How many lymph nodes properly stage a periampullary malignancy? J Gastrointest Surg. 2008;12(1):77–85.
Tomlinson JS, Jain S, Bentrem DJ, Sekeris EG, Maggard MA, Hines OJ, Reber HA, Ko CY. Accuracy of staging node-negative pancreas cancer: a potential quality measure. Arch Surg. 2007;142(8):767–23.
Govindarajan A, Tan JC, Baxter NN, Coburn NG, Law CH. Variations in surgical treatment and outcomes of patients with pancreatic cancer: a population-based study. Ann Surg Oncol. 2008;15(1):175–85.
Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol. 2006;13(9):1189–200.
House MG, Gonen M, Jarnagin WR, D’Angelica M, DeMatteo RP, Fong Y, Brennan MF, Allen PJ. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg. 2007;11(11):1549–55.
Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, Wolfgang C, Hruban RH, Schulick RD, Yeo CJ, Choti MA. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141(5):610–8.
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual (7th edn). New York: Springer; 2010.
Slidell MB, Chang DC, Cameron JL, Wolfgang C, Herman JM, Schulick RD, Choti MA, Pawlik TM. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2008;15(1):165–74.
Westgaard A, Laronningen S, Mellem C, Eide TJ, Clausen OP, Moller B, Gladhaug IP. Are survival predictions reliable? Hospital volume versus standardisation of histopathologic reporting for accuracy of survival estimates after pancreatoduodenectomy for adenocarcinoma. Eur J Cancer. 2009;45(16):2850–9.
Bilimoria KY, Talamonti MS, Wayne JD, Tomlinson JS, Stewart AK, Winchester DP, Ko CY, Bentrem DJ. Effect of hospital type and volume on lymph node evaluation for gastric and pancreatic cancer. Arch Surg. 2008;143(7):671–8.
Jeyarajah DR, Khithani A, Siripurapu V, Liu E, Thomas A, Saad AJ. Lymph node retrieval in pancreaticoduodenectomy specimens: does educating the pathologist matter? HPB (Oxford). 2014;16(3):263–6.
Liszka L, Pajak J, Zielinska-Pajak E, Golka D, Mrowiec S, Lampe P. Different approaches to assessment of lymph nodes and surgical margin status in patients with ductal adenocarcinoma of the pancreas treated with pancreaticoduodenectomy. Pathology. 2010;42(2):138–46.
Rowsell CH, Hanna S, Hsieh E, Law C, Khalifa MA. Improved lymph node retrieval in Whipple specimens as a result of implementation of a new uncinate margin protocol. HPB (Oxford). 2007;9(5):388–91.
Staley CA, Cleary KR, Abbruzzese JL, Lee JE, Ames FC, Fenoglio CJ, Evans DB. The need for standardized pathologic staging of pancreaticoduodenectomy specimens. Pancreas. 1996;12(4):373–80.
Japanese Society of Biliary Surgery. Classification of biliary tract carcinoma (Second English edn). Tokyo: Kanehara & Co., Ltd; 2004.
Japanese Pancreas Society. Classification of pancreatic carcinoma (Second English edn). Tokyo: Kanehara & Co., Ltd; 2003.
Pedrazzoli S, Beger HG, Obertop H, Andren-Sandberg A, Fernandez-Cruz L, Henne-Bruns D, Luttges J, Neoptolemos JP. A surgical and pathological based classification of resective treatment of pancreatic cancer. Summary of an international workshop on surgical procedures in pancreatic cancer. Dig Surg. 1999;16(4):337–45.
Tol JA, Gouma DJ, Bassi C, Dervenis C, Montorsi M, Adham M, Andren-Sandberg A, Asbun HJ, Bockhorn M, Buchler MW, Conlon KC, Fernandez-Cruz L, Fingerhut A, Friess H, Hartwig W, Izbicki JR, Lillemoe KD, Milicevic MN, Neoptolemos JP, Shrikhande SV, Vollmer CM, Yeo CJ, Charnley RM, International Study Group on Pancreatic S. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery. 2014;156(3):591–600.
Wagner PK, Ramaswamy A, Ruschoff J, Schmitz-Moormann P, Rothmund M. Lymph node counts in the upper abdomen: anatomical basis for lymphadenectomy in gastric cancer. Br J Surg. 1991;78(7):825–7.
Adsay NV, Basturk O, Altinel D, Khanani F, Coban I, Weaver DW, Kooby DA, Sarmiento JM, Staley C. The number of lymph nodes identified in a simple pancreatoduodenectomy specimen: comparison of conventional vs orange-peeling approach in pathologic assessment. Mod Pathol. 2009;22(1):107–12.
Verbeke CS. Resection margins and R1 rates in pancreatic cancer—are we there yet? Histopathology. 2008;52(7):787–96.
Cubilla AL, Fortner J, Fitzgerald PJ. Lymph node involvement in carcinoma of the head of the pancreas area. Cancer. 1978;41(3):880–7.
Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, Coleman J, Abrams RA, Hruban RH. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236(3):355–66.
Konstantinidis IT, Deshpande V, Zheng H, Wargo JA, Fernandez-del Castillo C, Thayer SP, Androutsopoulos V, Lauwers GY, Warshaw AL, Ferrone CR. Does the mechanism of lymph node invasion affect survival in patients with pancreatic ductal adenocarcinoma? J Gastrointest Surg. 2010;14(2):261–7.
Farnell MB, Pearson RK, Sarr MG, DiMagno EP, Burgart LJ, Dahl TR, Foster N, Sargent DJ. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery. 2005;138(4):618–28.
Massucco P, Ribero D, Sgotto E, Mellano A, Muratore A, Capussotti L. Prognostic significance of lymph node metastases in pancreatic head cancer treated with extended lymphadenectomy: not just a matter of numbers. Ann Surg Oncol. 2009;16(12):3323–32.
Malleo G, Maggino L, Capelli P, Gulino F, Segattini S, Scarpa A, Bassi C, Butturini G, Salvia R. Reappraisal of nodal staging and study of lymph node station involvement in pancreaticoduodenectomy with the standard international study group of pancreatic surgery definition of lymphadenectomy for cancer. J Am Coll Surg. 2015;221(2):367–79.
Pedrazzoli S, DiCarlo V, Dionigi R, Mosca F, Pederzoli P, Pasquali C, Kloppel G, Dhaene K, Michelassi F. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg. 1998;228(4):508–17.
Nimura Y, Nagino M, Kato H, Miyagawa S, Yamaguchi A, Kinoshita T. Regional versus extended lymph node dissection in radical pancreaticoduodenectomy for pancreatic cancer: a multicenter, randomized controlled trial. HPB. 2004;6 Suppl 1:2.
Kanda M, Fujii T, Nagai S, Kodera Y, Kanzaki A, Sahin TT, Hayashi M, Yamada S, Sugimoto H, Nomoto S, Takeda S, Morita S, Nakao A. Pattern of lymph node metastasis spread in pancreatic cancer. Pancreas. 2011;40(6):951–5.
Kayahara M, Nagakawa T, Kobayashi H, Mori K, Nakano T, Kadoya N, Ohta T, Ueno K, Miyazaki I. Lymphatic flow in carcinoma of the head of the pancreas. Cancer. 1992;70(8):2061–6.
Kayahara M, Nagakawa T, Ohta T, Kitagawa H, Ueno K, Tajima H, Elnemr A, Miwa K. Analysis of paraaortic lymph node involvement in pancreatic carcinoma: a significant indication for surgery? Cancer. 1999;85(3):583–90.
Matsuno S, Egawa S, Fukuyama S, Motoi F, Sunamura M, Isaji S, Imaizumi T, Okada S, Kato H, Suda K, Nakao A, Hiraoka T, Hosotani R, Takeda K. Pancreatic Cancer Registry in Japan: 20 years of experience. Pancreas. 2004;28(3):219–30.
Nagakawa T, Kobayashi H, Ueno K, Ohta T, Kayahara M, Miyazaki I. Clinical study of lymphatic flow to the paraaortic lymph nodes in carcinoma of the head of the pancreas. Cancer. 1994;73(4):1155–62.
Nakao A, Harada A, Nonami T, Kaneko T, Murakami H, Inoue S, Takeuchi Y, Takagi H. Lymph node metastases in carcinoma of the head of the pancreas region. Br J Surg. 1995;82(3):399–402.
Iqbal N, Lovegrove RE, Tilney HS, Abraham AT, Bhattacharya S, Tekkis PP, Kocher HM. A comparison of pancreaticoduodenectomy with extended pancreaticoduodenectomy: a meta-analysis of 1909 patients. Eur J Surg Oncol. 2009;35(1):79–86.
Falconi M, Crippa S, Dominguez I, Barugola G, Capelli P, Marcucci S, Beghelli S, Scarpa A, Bassi C, Pederzoli P. Prognostic relevance of lymph node ratio and number of resected nodes after curative resection of ampulla of Vater carcinoma. Ann Surg Oncol. 2008;15(11):3178–86.
Acknowledgements
Not applicable.
Funding
This study was financially supported by the Polish National Science Centre, grant no. N N403 084939 and the Leading National Research Centre (KNOW) of the Medical Faculty, Jagiellonian University Medical College. The provider of the financial support was not involved in the study design, collection, analysis, and interpretation of the data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Availability of data and materials
The manuscript does not refer to any new software, application, or tool. The authors do not wish to share data analyzed in this manuscript as no such consent was provided by the patients treated and no approval of the Bioethics Committee was obtained.
Authors’ contributions
MS, AM, and JK were responsible for the conception of the work. MS and ŁB helped in the data collection. MS contributed to the data analysis and interpretation. MS helped in drafting the article. AM and JK did the critical revision of the article. MS, ŁB, AM, and JK gave final approval of the version to be published.
Competing interests
The authors declare that they have no competing interest.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All the patients gave informed consent for collection and processing of clinical and pathology related data throughout the period covered by this study. The study was approved by the Bioethics Committee of Jagiellonian University for a retrospective analysis of the collected data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sierzega, M., Bobrzyński, Ł., Matyja, A. et al. Factors predicting adequate lymph node yield in patients undergoing pancreatoduodenectomy for malignancy. World J Surg Onc 14, 248 (2016). https://doi.org/10.1186/s12957-016-1005-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-016-1005-3