Abstract
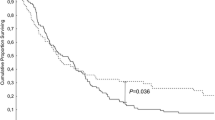
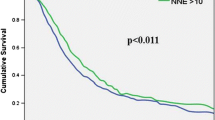
The impact of lymphadenectomy in prognosis and staging in periampullary malignancies remains largely undefined. We examined all pancreaticoduodenectomies for periampullary carcinomas in the SEER cancer registry from 1993 through 2003. Overall, 5465 pancreaticoduodenectomies for nonmetastatic periampullary carcinomas were identified. The cohort was comprised of 62.5% pancreatic, 18.9% ampullary, 11.6% distal bile duct, and 7.0% duodenal cancers. A linear association between the number of lymph nodes (LNs) examined and overall survival was observed overall and for pancreas and ampullary cancers for node-negative (N0) disease. Median survival for all patients with localized, N0 disease improved from 24 to 31 months, with sampling of a minimum of 10 LNs, whereas 2 and 5-year survival improved from 52 and 29%, with <10 nodes examined to 58 and 37% with 10+ nodes examined (P < 0.001). A 1-month median survival advantage was seen in patients with node-positive disease when more than 10 lymph nodes examined (15 versus 16 months, P < 0.001). Significantly better median survival and cure rates are observed after pancreaticoduodenectomy for localized periampullary adenocarcinoma when a minimum of 10 lymph nodes are examined. This benefit likely represents more accurate staging. To optimize the prognostic accuracy and prevent stage migration errors in multicenter trials a minimum of 10 lymph nodes should be obtained and examined before the determination of node-negative disease.



Similar content being viewed by others
References
Cameron JL. Current surgical therapy, 8th ed. Philadelphia: Elsevier Mosby, 2004.
Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin 2006;56(2):106–130.
Buchler MW, Wagner M, Schmied BM, Uhl W, Friess H, Z’Graggen K. Changes in morbidity after pancreatic resection: Toward the end of completion pancreatectomy. Arch Surg 2003;138(12):1310–1314; discussion 1315.
Lillemoe KD, Yeo CJ, Cameron JL. Pancreatic cancer: State-of-the-art care. CA Cancer J Clin 2000;50(4):241–268.
Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN, Dooley WC, Coleman J, Pitt HA. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg 1995;221(6):721–731; discussion 731–733.
Diener MK, Knaebel HP, Heukaufer C, Antes G, Buchler MW, Seiler CM. A systematic review and meta-analysis of pylorus-preserving versus classical pancreaticoduodenectomy for surgical treatment of periampullary and pancreatic carcinoma. Ann Surg 2007;245(2):187–200.
Fortner JG. Regional resection of cancer of the pancreas: a new surgical approach. Surgery 1973;73(2):307–320.
Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, Coleman J, Abrams RA, Hruban RH. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: Part 2. Randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg 2002;236(3):355–366; discussion 366–368.
Satake K, Nishiwaki H, Yokomatsu H, Kawazoe Y, Kim K, Haku A, Umeyama K, Miyazaki I. Surgical curability and prognosis for standard versus extended resection for T1 carcinoma of the pancreas. Surg Gynecol Obstet 1992;175(3):259–265.
Kayahara M, Nagakawa T, Ueno K, Ohta T, Tsukioka Y, Miyazaki I. Surgical strategy for carcinoma of the pancreas head area based on clinicopathologic analysis of nodal involvement and plexus invasion. Surgery 1995;117(6):616–623.
Manabe T, Ohshio G, Baba N, Miyashita T, Asano N, Tamura K, Yamaki K, Nonaka A, Tobe T. Radical pancreatectomy for ductal cell carcinoma of the head of the pancreas. Cancer 1989;64(5):1132–1137.
Pedrazzoli S, DiCarlo V, Dionigi R, Mosca F, Pederzoli P, Pasquali C, Kloppel G, Dhaene K, Michelassi F. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg 1998;228(4):508–517.
Henne-Bruns D, Vogel I, Luttges J, Kloppel G, Kremer B. Ductal adenocarcinoma of the pancreas head: Survival after regional versus extended lymphadenectomy. Hepatogastroenterology 1998;45(21):855–866.
Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: Information from a large US population database. Ann Surg Oncol 2006;13(9):1189–1200.
Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas—616 patients: Results, outcomes, and prognostic indicators. J Gastrointest Surg 2000;4(6):567–579.
Khanna A, Walker GR, Livingstone AS, Arheart KL, Rocha-Lima C, Koniaris LG. Is adjuvant 5-FU-based chemoradiotherapy for resectable pancreatic adenocarcinoma beneficial? A meta-analysis of an unanswered question. J Gastrointest Surg 2006;10(5):689–697.
White RR, Kattan MW, Haney JC, Clary BM, Pappas TN, Tyler DS, Brennan MF. Evaluation of preoperative therapy for pancreatic cancer using a prognostic nomogram. Ann Surg Oncol 2006;13(11):1485–1492.
Surveillance E, and End Results (SEER) Program http://www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER 17 Regs Limited-Use, Nov 2005 Sub (1973–2003), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2006, based on the November 2005 submission.
Fritz AG. International classification of diseases for oncology: ICD-O, 3rd ed. Geneva: World Health Organization, 2000.
Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: Data from a large US-population database. J Clin Oncol 2005;23(28):7114–7124.
Siewert JR, Bottcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: Ten-year results of the German Gastric Cancer Study. Ann Surg 1998;228(4):449–461.
Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, Morimoto T, Kato T, Kito T. The number of metastatic lymph nodes: A promising prognostic determinant for gastric carcinoma in the latest edition of the TNM classification. J Am Coll Surg 1998;187(6):597–603.
Roukos DH, Lorenz M, Karakostas K, Paraschou P, Batsis C, Kappas AM. Pathological serosa and node-based classification accurately predicts gastric cancer recurrence risk and outcome, and determines potential and limitation of a Japanese-style extensive surgery for Western patients: A prospective with quality control 10-year follow-up study. Br J Cancer 2001;84(12):1602–1609.
Lee HY, Choi HJ, Park KJ, Shin JS, Kwon HC, Roh MS, Kim C. Prognostic significance of metastatic lymph node ratio in node-positive colon carcinoma. Ann Surg Oncol 2007;14(5):1712–1717.
Le Voyer TE, Sigurdson ER, Hanlon AL, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG. Colon cancer survival is associated with increasing number of lymph nodes analyzed: A secondary survey of intergroup trial INT-0089. J Clin Oncol 2003;21(15):2912–2919.
Swanson RS, Compton CC, Stewart AK, Bland KI. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol 2003;10(1):65–71.
Goldstein NS, Sanford W, Coffey M, Layfield LJ. Lymph node recovery from colorectal resection specimens removed for adenocarcinoma. Trends over time and a recommendation for a minimum number of lymph nodes to be recovered. Am J Clin Pathol 1996;106(2):209–216.
Sosa JA, Diener-West M, Gusev Y, Choti MA, Lange JR, Dooley WC, Zeiger MA. Association between extent of axillary lymph node dissection and survival in patients with stage I breast cancer. Ann Surg Oncol 1998;5(2):140–149.
Polednak AP. Survival of lymph node-negative breast cancer patients in relation to number of lymph nodes examined. Ann Surg 2003;237(2):163–167.
Acknowledgment
This study was funded by the James and Esther King Biomedical Research Program of the Florida Department of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gutierrez, J.C., Franceschi, D. & Koniaris, L.G. How Many Lymph Nodes Properly Stage a Periampullary Malignancy?. J Gastrointest Surg 12, 77–85 (2008). https://doi.org/10.1007/s11605-007-0251-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-007-0251-7