Abstract
Background
A large number of Asian population studies examined the difference between the 6th and the 7th tumor, node, metastasis (TNM) while it is still poorly validated among Caucasian populations. This is a retrospective study aimed at investigating the efficacy of the 7th edition American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) staging system for gastric cancer focusing on the “N” parameter-related survival for prognostic assessment in gastric cancer patients of a single Western high-volume institution.
Methods
From January 2002 to December 2009, the data of 274 patients with gastric cancer who underwent gastric surgery at the 8th General and Gastrointestinal Surgical Centre of the Second University of Naples were analyzed retrospectively. We collected data for patient demographics, tumor characteristics, surgical characteristics, and TNM stage. Particularly, the nodal status, with the number of dissected nodes and metastatic nodes, was reviewed from the pathology records. The same patient dataset was used to stage patients according to both the 6th and 7th edition criteria.
Results
Age at surgery, tumor location, histological grade, Lauren’s classification subtypes, and 6th and 7th AJCC/UICC N categories were found to have statistically significant associations with overall survival on univariate analysis. In the 6th edition staging system, the Kaplan–Meier plot did not show significant overlapped survival curves: significant differences were found between N0 and N1, P < .001; N1 and N2, P = .04; and N2 and N3, P < .001. On the contrary, in the 7th edition, among all five substages, there were similar survival curves between N categories 2 and 3a (P = .98) with a statistically significant discriminatory ability only between N1 versus N3b and N2 versus N3b (P = .02 and .04, respectively).
Conclusions
Based on analysis, we found that several clinicopathological variables, especially histological grade and Lauren’s classification, were significant prognostic factors in our database. The 6th and 7th AJCC/UICC N classifications represent significantly independent prognostic factors, and the 6th AJCC/UICC N classification seems to be superior to the 7th AJCC/UICC N classification in terms of uniformity, differentiation, and monotonicity of gradients.
Similar content being viewed by others
Background
Gastric cancer is one of the most common malignant tumors in the world and the second leading cause of cancer-related death worldwide [1–5]. Although considerable progress has been made in the early gastric cancer diagnosis, so far, the prognosis of this tumor remains poor [6, 7].
Accurate categorization of the tumor stage, including the invasive depth and lymph node status, is crucial for prognostic assessment and decision-making of the stage-specific therapeutic strategy [8]. The American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) tumor, node, metastasis (TNM) staging system has been used widely for clinical practice and research in determining tumor stage for gastric cancers, representing the most important independent prognostic factor [9]. Several versions of this classification system have been used over the past 30 years [10, 11], and in 2010, the 7th edition of the AJCC/UICC gastric cancer staging manual was introduced, resulting in several changes from the 6th edition, particularly as regards the N categories [12]. According to the new TNM edition, N categories were so redefined in order to improve their reproducibility and prognostic validity: the previous N1 category (1–6 involved regional lymph nodes) is amended into a new N1 category (1–2 involved regional lymph nodes) and N2 category (3–6 involved regional lymph nodes), whereas the previous category N2 (7–15 involved regional lymph nodes) and the N3 category (>15 involved regional lymph nodes) are combined into the new N3 categories (N3a: 7–15 involved regional lymph nodes, and N3b: ≥16 involved regional lymph nodes) [2, 11]. In the medical literature, there are a large number of Asian population studies that examine the difference between the 6th and the 7th TNM [2, 11, 13–15]; however, gastric cancer in Western countries represents a different disease considering the pattern of presentation and pathophysiology [16–18]. Indeed, studies aimed at validating the new staging criteria focusing on the N category in the Italian population are poor [19]. Thus, the prognostic capability of this new classification in the Western remains ambiguous. In light of such evidences, we performed a retrospective study so-called NodUs study (Nodal statUs) to evaluate the efficacy and validity of the 7th edition AJCC/UICC “N” category for prognostic assessment and to compare the 6th and 7th editions of the AJCC/UICC “N” staging system focusing on the “N” parameter in a cohort of patients who underwent surgical therapy for gastric cancer from a single Western high-volume institution, providing reference for revision of a future edition of the AJCC/UICC for gastric cancer staging.
Methods
From January 2002 to December 2009, the data of 329 patients with gastric adenocarcinoma (ICD-O code 8140/3 according to the World Health Organization classification of tumors of the digestive system) [20] confirmed by histopathology who underwent gastric surgery at the 8th General and Gastrointestinal Surgical Centre of the Second University of Naples were analyzed retrospectively. Exclusion criteria were previous history of surgery for gastric cancer, gastric stump cancer, metastatic disease, non-curative resection (R1 or R2 resection), lymphadenectomy different from D2, inadequate lymph node dissection (<15 lymph nodes retrieved), preoperative radiotherapy, and/or chemotherapy. More specifically, in D1 dissections, only the perigastric nodes directly attached along the lesser curvature and greater curvatures of the stomach are removed (stations 1–6: right and left pericardial, lesser curvature, greater curvature, supra, and infrapyloric). D2 dissections add the removal of nodes along the left gastric artery (station 7), common hepatic artery (antero-superior group, station 8a), celiac trunk (station 9), splenic hilum and splenic artery (station 10 and 11), and hepatoduodenal ligament (along the proper hepatic artery, station 12a) [21].
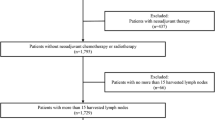
After applying the exclusion criteria, a total of 274 patients were enrolled in this study.
All patients underwent standardized total or partial gastrectomy (depending on the distance between the cardia and the tumor) by experienced surgeons, with spleen-preserving modified radical D2 lymphadenectomy according to the Japanese Classification of Gastric Carcinoma [21]. However, splenectomy was necessarily performed in a total of 19 patients (6.9 %) to ensure complete dissection of the splenic hilar lymph nodes in very difficult dissection cases. The surgical procedure of reconstruction was chosen according to the surgeon’s preference. We collected data for patient demographics, tumor characteristics, surgical characteristics, and TNM stage. Particularly, the nodal status, with the number of dissected and metastatic nodes, was reviewed from the pathology records. The same patient dataset was used to stage patients according to both the 6th and 7th edition criteria.
Surviving patients were followed at regular intervals at the outpatient clinic until 5 years after surgery. Outpatient clinic visits included history taking and physical examination. No routine imaging was performed. Overall survival, used as a prognostic parameter, was defined as the time between the date of operation and the date of death. Surviving patients were censored on the day of last follow-up. The last follow-up checkpoint was July 2014.
Ethics, consent, and permissions
The study was approved by the ethics committee of the Second University of Naples and conducted according to the ethical standards of the Helsinki Declaration. All patients gave informed consent to participate in this study.
Consent to publish
Consents to publish have been obtained from the participants (or legal parent when appropriate) to report individual patient data.
Statistical analysis
The observed data were normally distributed. Overall survival (OS) was calculated using the Kaplan–Meier method, and the log-rank test was employed to determine the significance.
Factors that were deemed of potential importance on univariate analysis, considering a P value of less than .05 as a statistically significant result, were included in multivariate analyses. Multivariate analysis was performed by the Cox proportional hazard model using the forward logistic regression stepwise procedure for variable selection. To measure homogeneity of the direct comparison of the two different edition stage systems, the likelihood ratio χ 2 test related to the Cox regression model was used. The discriminatory ability and monotonicity of gradient assessments were measured with the linear trend χ 2 test of survival curves according to the N classification of the 6th and 7th editions. The Akaike information criterion (AIC) was applied into the Cox proportional hazard regression model to correct for the potential bias in comparing prognostic systems with different numbers of stages. AIC was defined as follows: AIC = −2 log maximum likelihood + 2 × (the number of parameters in the model). A smaller AIC value indicated a better model for predicting outcome [22, 23]. Hazard ratios (HR) and 95 % confidence intervals (95 % CI) were generated. A statistical data analysis was performed using SPSS 20.0 software (SPSS Inc., Chicago, IL), and a P value of less than .05 was considered to be statistically significant.
Results
A consecutive series of 329 patients with diagnosis of gastric adenocarcinoma was analyzed retrospectively. In total, 20 patients were excluded because they had received neoadjuvant chemotherapy, 8 patients were excluded because of a non-curative (R1) resection, and 17 patients were excluded because of inadequate lymph node dissection and lymphadenectomy different from D2 (D1 lymphadenectomy). Ten patients had a metastatic disease and were also excluded from the current analysis. This resulted in a final study population of 274 patients. Mean follow-up was 53 months (median 39 months). The overall 5-year survival rate was 52.8 %. Age at surgery, tumor location, histological grade, Lauren’s classification subtypes, and 6th and 7th AJCC/UICC N parameters were found to have statistically significant associations with overall survival on univariate analysis. Patient characteristics and the effect of clinical features on survival are summarized in Table 1. No lymph node metastasis was found in 66 patients (23.9 %). The number of metastatic nodes was 1–6 (N1) in 103 patients (37.6 %), 7–15 (N2) in 61 patients (22.2 %), and more than 15 (N3) in 44 patients (16.2 %) according to the TNM 6th criteria. The number of metastatic nodes was 1–2 (N1) in 44 patients (16.2 %), 3–6 (N2) in 71 patients (26.1 %), 7–15 (N3a) in 60 patients (21.8 %), and more than 15 (N3b) in 33 patients (12 %) according to the new TNM 7th criteria. The total number of dissected lymph nodes was 7267, with an average of 26.5 ± 14.8 (mean ± SD) dissected nodes per case (median 24.0, range 0–87). The mean number of metastatic nodes was 6.5 ± 8.2 (median 4, range 0–55) in the overall series and 8.5 ± 8.4 (median 6, range 1–55) in lymph node-positive patients (data not shown). Figure 1 shows the 5-year survival rates for patients with N0 (66.0 %), N1 (52.1 %), N2 (50.0 %), and N3 (31.0 %) disease (P < .001) according to the AJCC/UICC 6th edition system. Also shown in Fig. 2 are the 5-year survival rates according to the AJCC/UICC 7th edition: N0 (66.0 %), N1 (66.7 %), N2 (48.1 %), N3a (51.2 %), and N3b (20.8 %) (P < .001). Indeed, in the 6th edition staging system, the Kaplan–Meier plot did not show significant overlapped survival curves: significant differences were found between N0 and N1, P < .001; N1 and N2, P = .04; and N2 and N3, P < .001. On the contrary, in the 7th edition, among all five substages, there were similar survival curves between N categories 2 and 3a (P = .98) with statistically significant discriminatory ability only between N1 versus N3b and N2 versus N3b (P < .001 and .04, respectively).
All six variables that resulted in statistical significance associated with overall survival on univariate analysis were included in a multivariate Cox proportional hazard model with forward logistic regression stepwise procedure to adjust for the effects of covariates. Therefore, two separate multivariate models, one with the 6th and the other with the 7th AJCC/UICC N classification, were run to avoid collinearity problems. In these models, we demonstrated that histological grade, the 6th AJCC N parameters, and the 7th AJCC/UICC N parameters were independent factors of prognosis of gastric cancer (Table 2). After that, a Cox regression model including both the 6th and 7th edition staging systems focusing on the “N” parameter showed that the 7th edition no longer significantly predicted survival, whereas the 6th edition remained a significant stratifier of prognosis (data not shown). Finally, the performance of the 6th and 7th edition N classification systems was quantified by the likelihood ratio chi-square, linear trend χ 2 test, and AIC. Predictive ability was best for the 6th AJCC/UICC N classification system (highest likelihood ratio χ 2) as well as the discriminatory ability and monotonicity of gradients (higher linear trend χ 2 score). Furthermore, the AIC value was smaller for the 6th edition compared to the 7th edition staging system, indicating that it has a better prognostic stratification (Table 2).
Discussion
The major finding of our investigation on 274 gastric cancer Italian patients who underwent primary surgical resection is that the 6th and 7th AJCC/UICC N classifications represent significantly independent prognostic factors, and the 6th AJCC/UICC N classification seems to be superior to the 7th AJCC/UICC N classification in terms of uniformity, differentiation, and monotonicity of gradients. Nowadays, the prognosis for gastric cancer patients remains poor, and the TNM stage system represents a prognostic factor that can effectively provide the means for appropriate treatment and predict the prognosis [23, 24]. Especially the extent of lymph node metastasis is proved to be the most important independent prognostic factor [25, 26]. The introduction of the new 7th AJCC/UICC TNM edition has brought several changes for gastric cancer classification, and 4 years later, there are still some debates about its prognostic power. Furthermore, in the literature are reported scientific contributions with controversial results about comparison between the 6th and the 7th AJCC/UICC N classification systems. Wang et al. [1] reported a better prognostic stratification of the latest TNM edition in 1503 gastric cancer patients who underwent primary surgical resection with an average per case of dissected lymph nodes less than 15, considering avoidable N3 substratification in N3a and N3b. Similarly, Deng et al. [27], Chae et al. [28], and Fang et al. [15] showed a more detailed classification of different prognostic groups with a high homogeneity rate in each TNM stage in R0-selected patients with more than 16 retrieved lymph nodes per case [29], assessing prognostic superiority for the 7th than for the 6th AJCC/UICC N classification system. From the Western point of view, McGhan et al. [25] evidenced a better survival discrimination and risk stratification of the 7th AJCC/UICC staging criteria in a retrospective review of 13,547 American gastric cancer patients. In order to evaluate the efficacy and validity of the 7th edition AJCC/UICC “N” classification system for prognostic assessment and to compare the 6th and 7th editions of the AJCC/UICC “N” classification system, we analyzed 274 patients who underwent curative surgery by experienced surgeons in this “NodUs” retrospective study.
Our data analysis, different from Asian surgeons’ results, showed that survivals according to the 6th AJCC/UICC N classification were more equally distributed than survivals according to the new AJCC/UICC classification. Moreover, in univariate analysis, significant prognostic factors were the age at surgery, tumor location, histological grade, Lauren’s classification, the 6th AJCC/UICC N category, and the 7th AJCC/UICC N category. However, the two multivariate analysis models showed that histological grade, the 6th AJCC/UICC N classification, and the 7th AJCC/UICC N classification were independent factors of prognosis of gastric cancer. Furthermore, when a Cox regression model including both the 6th and 7th edition staging systems focusing on the “N” parameter was run, only the 6th AJCC/UICC N classification remained a significant stratifier of prognosis with high prediction as well as the discriminatory ability and monotonicity of gradients. Similarly, some Eastern surgeons did not report the 7th AJCC/UICC N classification as having a more effective prognostic power compared to the 6th edition [2, 4, 30]. Likewise, Rausei et al. [31] in a retrospective unique Italian “real-world” comparative study on 224 non-metastatic gastric cancer patients who underwent surgery with curative intent and limited lymphadenectomy (D1 lymphadenectomy) did not find any prognostic superiority of the 7th AJCC/UICC TNM edition with respect to the N parameter in comparison to the 6th edition. As stated by Bickenbach et al. [16] and McGhan et al. [25], gastric cancer in Western countries represents a different disease as regards the pattern of presentation and pathophysiology making the classification according to the new 7th AJCC/UICC N classification system not better than the old 6th TNM for the prognostic stratification of staging in gastric cancer. Further nationwide studies with larger numbers of patients are necessary to evaluate the validity and effectiveness of the new classification from various angles.
Conclusions
Although our sample population comes from a single institution experience and is relatively small compared with the worldwide gastric cancer collaboration database, the surgical procedures, pathologic examinations, and patient follow-up were highly uniform throughout the entire study period. Moreover, it represents an original retrospective study on the Italian population. In our analysis, we found that several clinicopathological variables, especially histological grade and Lauren’s classification, were significant prognostic factors in our database worthy of further research. Overall, the 6th and 7th AJCC/UICC N classifications represent significantly independent prognostic factors, and the 6th AJCC/UICC N classification seems to be superior to the 7th AJCC/UICC N classification in terms of homogeneity, discriminatory ability, and monotonicity of gradients, providing reference for revision of a future edition of the AJCC/UICC for gastric cancer staging.
Abbreviations
- AIC:
-
Akaike information criterion
- AJCC:
-
American Joint Committee on Cancer
- CI:
-
confidence interval
- HR:
-
hazard ratio
- OS:
-
overall survival
- TNM:
-
tumor, node, metastasis
References
Wang W, Sun XW, Li CF, Lv L, Li YF, Chen YB, et al. Comparison of the 6th and 7th editions of the UICC TNM staging system for gastric cancer: results of a Chinese single-institution study of 1503 patients. Ann Surg Onc. 2011;18:1060–7.
Qiu MZ, Wang ZQ, Zhang DS, Liu Q, Luo HY, Zhou ZW, et al. Comparison of 6th and 7th AJCC TNM staging classification for carcinoma of the stomach in China. Ann Surg Oncol. 2011;18:1869–76.
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;10:2137–50.
Kikuchi S, Futawatari N, Sakuramoto S, Katada N, Yamashita K, Shibata T, et al. Comparison of staging system between the old (6th edition) and new (7th edition) TNM classifications in advanced gastric cancer. Anticancer Res. 2011;31:2361–5.
Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–62.
Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, et al. Histopathologic‐based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–8.
Peng CW, Wang LW, Zeng WJ, Yang XJ, Li Y. Evaluation of the staging systems for gastric cancer. J Surg Oncol. 2013;108:93–105.
Kim JP, Lee JH, Kim SJ, Yu HJ, Yang HK. Clinicopathologic characteristics and prognostic factors in 10.783 patients with gastric cancer. Gastric Cancer. 1998;1:125–33.
Zeng WJ, Hu WQ, Wang LW, Yan SG, Li JD, Zhao HL, et al. Lymph node ratio is a better prognosticator than lymph node status for gastric cancer: a retrospective study of 138 cases. Oncol Lett. 2013;6:1693–700.
Sobin LH, Wittekind C. TNM classification of malignant tumours. 6th ed. Hoboken: John Wiley & Sons; 2002.
Sun Z, Wang ZN, Zhu Z, Xu YY, Xu Y, Huang BJ, et al. Evaluation of the seventh edition of American Joint Committee on Cancer TNM staging system for gastric cancer: results from a Chinese monoinstitutional study. Ann Surg Oncol. 2012;19:1918–27.
Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–9.
Kim SS, Choi BY, Seo SI, Jung MY, Choi HS, Ahn SM, et al. The comparison between 6th and 7th International Union Against Cancer/American Joint Committee on Cancer classification for survival prognosis of gastric cancer. Korean J Gastroenterol. 2011;58:258–63.
Zhang P, Xu Y, Guo J, Xu H. Comparison of the predictive value of the sixth and seventh edition TNM pT classifications in prognosis for gastric cancer. Zhonghua Zhong Liu Za Zhi. 2015;37:190–4.
Fang WL, Huang KH, Chen JH, Lo SS, Hsieh MC, Shen KH, et al. Comparison of the survival difference between AJCC 6th and 7th editions for gastric cancer patients. World J Surg. 2011;35:2723–39.
Bickenbach K, Strong VE. Comparisons of gastric cancer treatments: East vs West. J Gastric Cancer. 2012;12:55–62.
Reim D, Loos M, Vogl F, Novotny A, Schuster T, Langer R, et al. Prognostic implications of the seventh edition of the International Union Against Cancer classification for patients with gastric cancer: the Western experience of patients treated in a single-center European institution. J Clin Oncol. 2013;31:263–71.
Warneke VS, Behrens HM, Hartmann JT, Held H, Becker T, Schwarz NT, et al. Cohort study based on the seventh edition of the TNM classification for gastric cancer: proposal of a new staging system. J Clin Oncol. 2011;29:2364–71.
Marrelli D, Morgagni P, de Manzoni G, Coniglio A, Marchet A, Saragoni L, et al. Prognostic value of the 7th AJCC/UICC TNM classification of non cardia gastric cancer: analysis of a large series from specialized Western centers. Ann Surg. 2012;255:486–91.
Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system, 4th edition (IARC WHO classification of tumours). OR Book News, Inc: Portland; 2011.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113–23.
Armitage P, Colton T. Encyclopedia of biostatistics. New York: Wiley; 1998. 6th ed.
Kee KM, Wang JH, Lee CM, Chen CL, Changchien CS, Hu TH, et al. Validation of clinical AJCC/UICC TNM staging system for hepatocellular carcinoma: analysis of 5,613 cases from a medical center in southern Taiwan. Int J Cancer. 2006;120:2650–5.
Ichikura T, Tomimatsu S, Uefuji K, Kimura M, Uchida T, Morita D, et al. Evaluation of the new American Joint Committee on Cancer/International Union Against Cancer Classification of lymph node metastasis from gastric carcinoma in comparison with the Japanese classification. Cancer. 1999;86:553–8.
McGhan LJ, Pockaj BA, Gray RJ, Bagaria SP, Wasif N. Validation of the updated 7th edition AJCC TNM staging criteria for gastric adenocarcinoma. J Gastrointest Surg. 2012;16:53–61.
Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer. Ten-year results of the German gastric cancer study. Ann Surg. 1998;228:449–61.
Deng J, Liang H, Sun D, Wang D, Pan Y. Suitability of 7th UICC N stage for predicting the overall survival of gastric cancer patients after curative resection in China. Ann Surg Oncol. 2010;17:1259–66.
Chae S, Lee A, Lee JH. The effectiveness of the new (7th) UICC N classification in the prognosis evaluation of gastric cancer patients: a comparative study between the 5th/6th and 7th UICC N classification. Gastric Cancer. 2011;14:166–71.
Sobin LH, Gospodarowicz MK, Wittekind C. International Union Against Cancer (UICC) TNM classification of malignant tumors. 7th ed. New York: Wiley-Liss; 2010.
Jung H, Lee HH, Song KY, Jeon HM, Park CH. Validation of the seventh edition of the American Joint Committee on Cancer TNM staging system for gastric cancer. Cancer. 2011;117:2371–8.
Rausei S, Dionigi G, Ruspi L, Proserpio I, Galli F, Tirotta F, et al. Lymph node staging in gastric cancer: new criteria, old problems. Int J Surg. 2013;11:S90–4.
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
LM, VB, BB, GE, MG, MaP, MoP, and GR performed the data extraction. LM, GI, AC, BB, VB, RP, and MS participated in the design of the study and performed the statistical analysis. LM, VB, and NDM conceived of the study, participated in its design and coordination, and drafted the manuscript. LM, VB, AC, GI, AR, BB, GE, RP, GR, and MS carried out the data analysis and interpretation. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Marano, L., Boccardi, V., Braccio, B. et al. Comparison of the 6th and 7th editions of the AJCC/UICC TNM staging system for gastric cancer focusing on the “N” parameter-related survival: the monoinstitutional NodUs Italian study. World J Surg Onc 13, 215 (2015). https://doi.org/10.1186/s12957-015-0633-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-015-0633-3