Abstract
Background
A Task Force from the International Society of Pharmacoeconomics and Outcomes Research (ISPOR) provides recommendations on how to systematically identify and appraise health state utility (HSU) weights for cost-effectiveness analyses. We applied these recommendations to conduct a systematic review (SR) to identify HSU weights for different stages of chronic kidney disease (CKD), renal replacement therapy (RRT) and complications.
Methods
MEDLINE® and Embase were searched for interventional and non-interventional studies reporting HSU weights for patients with CKD stages 1–5 or RRT. As per ISPOR Task Force Guidance, study quality criteria, applicability for Health Technology Assessment (HTA) and generalisability to a broad CKD population were used to grade studies as either 1 (recommended), 2 (to be considered if there are no data from grade 1 studies) or 3 (not recommended).
Results
A total of 17 grade 1 studies were included in this SR with 51 to 1767 participants, conducted in the UK, USA, Canada, China, Spain, and multiple-countries. Health related quality of life (HRQL) instruments used in the studies included were EQ-5D-3L (10 studies), SF-6D (4 studies), HUI2/HUI3 (1 study), and combinations (2 studies). Although absolute values for HSU weights varied among instruments, HSU weights decreased with CKD severity in a consistent manner across all instruments.
Conclusions
This SR identified HSU weights for a range of CKD states and showed that HRQL decreases with CKD progression. Data were available to inform cost-effectiveness analysis in CKD in a number of geographies using instruments acceptable by HTA agencies.
Similar content being viewed by others
Background
Chronic kidney disease (CKD) has a substantial impact on patients’ health and life expectancy. CKD has been estimated to affect between 10 and 15% of the population in the U.S. and Canada [1, 2]. CKD can be a progressive disease and the leading causes include diabetes (38%), high blood pressure (26%), and glomerulonephritis (16%) [3]. Progression to end-stage renal disease (ESRD) leaves the patients reliant on dialysis or a kidney transplant [4]. CKD also leads to substantial healthcare resource use. The total Medicare spending on both CKD and ESRD was over $114 billion in 2016 [5].
The KDIGO (Kidney Disease: Improving Global Outcomes) 2012 guidelines recommended that CKD patients should be categorised based on cause, glomerular filtration rate (GFR) category, and albuminuria category in order to aid in predicting CKD prognosis.
Despite guideline directed management of risk factors and use of renin angiotensin aldosterone system inhibitors (RAASi), disease progression, adverse clinical outcomes and mortality rates remain high in patients with CKD, particularly in those patients at risk such as those with moderately or severely increased albuminuria, highlighting a clinical need for new treatments to delay renal disease progression and improve health related quality of life (HRQL).
Since the introduction of health technology assessment (HTA) agencies across the world, the decision to adopt new treatments is becoming more frequently based on the results of cost-effectiveness analyses. The cost-effectiveness of new treatments is influenced by HRQL weights (referred to as health state utility [HSU] weights). HSU weights range between 0 and 1, with 1 representing the valuation of perfect health and 0 representing the valuation of death and are used to estimate quality adjusted life years (QALYs). A systematic review (SR) reported that most cost-effectiveness models in CKD utilised a framework based on disease progression defined by a worsening in GFR stage or albuminuria category [6].
A Task Force from the International Society of Pharmacoeconomics and Outcomes Research (ISPOR) led by Brazier and colleagues (2019) provided recommendations on how to systematically identify and appraise HSU weights for cost-effectiveness analysis. The recommendations were divided into four sections which describe: (1) iterative search strategy; (2) review process to include studies based on inclusion criteria and data quality; (3) data to be extracted from each study; (4) basis for selecting HSUs to inform a cost-effectiveness analysis (e.g. data availability for a country of interest or data availability using a specific instrument) (Fig. 1).
The impact of dialysis and renal transplantation on HSU weights has been reported in previous SRs, however, it remains uncertain how the magnitude of HSU weights change as CKD progresses between stages 1 and 5 [7,8,9,10,11].
The aim of this SR was to identify HSU weights to inform cost-effectiveness modelling in CKD applying current best practices, and the review was conducted to provide an international perspective.
Methods
Search strategy
This SR was based on a prespecified protocol and conducted in accordance with the standards prescribed by the ISPOR Task Force (but also reflects best practice at the Centre for Reviews and Dissemination, National Institute for Health and Care Excellence (NICE), and the Cochrane Collaboration) [12,13,14,15]. The search was conducted in both MEDLINE (PubMed) and Embase (OVID) in August 2019. The full search strategy is provided in Additional file 1. Grey literature searches included conference proceedings of three major nephrology congresses and one health economics congress held between 2017 and 2019, and reports from four major HTA agencies (Additional file 2). The bibliographies of relevant published SRs and cost-effectiveness analyses were hand-searched to find additional articles that were not identified in the electronic database searches.
Two independent reviewers (JC, JGS) screened the title and abstract of each record (stage 1), as well as the full texts of all potentially eligible records identified in stage 1 (stage 2). A third independent reviewer (AL) resolved any disagreements.
Study inclusion criteria are shown in Table 1 and are based on the PICOS (population, intervention, comparator, outcome, study) framework.
Critical appraisal
Each study was assessed against the following criteria:
-
The study was conducted in a CKD population
-
The study reports original empirical HSU weights
-
Data were collected using a generic HRQL measure (i.e. EQ-5D, short-form 6-dimention [SF-6D] or a mappable equivalent such as short-form 36 [SF-36] or short-form 12 [SF-12]; or the Health Utility Index [HUI])
-
The study sample size was at least 25 patients
-
The study was conducted in a country of interest (i.e., USA, Canada, Australia, China, UK, Spain, Italy, France or Germany)
-
HSU weights were presented in a comprehensive way that is useful to inform cost-effectiveness analysis (e.g. HSU weights were available by CKD stage)
To weigh both data quality and data appropriateness as recommended by Brazier and colleagues (2019), each study that met the critical appraisal at stage 1 was then reviewed in full in stage 2 and graded from 1 to 3 with consideration to the presence of bias, alignment with HTA criteria, and general compliance with our initial selection criteria (Table 2). To assess bias, each study’s methodology was examined for selection bias, bias in data analysis or interpretation, drop out or missing data, or bias in study execution such as unblinding in randomised control trials.
Grade 1 studies were considered most appropriate for HTA. If data for a specific health state was not available using Grade 1 studies, then, Grade 2 studies would be reviewed to identify a missing value following the iterative approach recommended by Brazier and colleagues (2019). Grade 3 studies were considered to be inappropriate.
Relevant data, as recommended by Brazier and colleagues (2019), was extracted from the included studies into a prespecified extraction grid.
Results
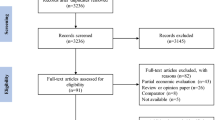
Electronic database searches identified 1091 records. After title/abstract screening, 150 studies were selected for full-text review, of which 52 met the final inclusion criteria. The grey literature identified 83 studies, although no new studies met our inclusion criteria (Additional file 2). The article selection process is displayed in Fig. 2.
Of the 52 included studies, the grading process identified 17 Grade 1, 30 Grade 2, and 5 Grade 3 studies (Additional file 3). Data were extracted for the Grade 1 studies (Additional file 4). Fourteen of the studies reported more than one CKD HSU weight, resulting in a total of 58 CKD HRQL estimates across different health states (i.e., CKD stages, haemodialysis (HD), peritoneal dialysis (PD) and renal transplant (Trx)).
Ten studies (59%) used the EQ-5D-3L, four (24%) used the SF-6D, one (6%) used the HUI3, and two (12%) used multiple instruments (HUI2 and HUI3; EQ-5D-3L and HUI3). Of the reported HSU weights, 18 (41%) described dialysis patients, 17 (39%) described transplant patients, and 9 (21%) described cohorts by CKD stages. Studies were reported from Canada (n = 4; 29%), the UK (n = 3; 18%), the US (n = 3; 28%), Spain (n = 2; 12%), and China (n = 2; 12%), and two studies (12%) were multinational. A summary of key study characteristics is reported in Table 3.
HSU weights for the different CKD health states are reported in Table 4. HRQLs for haemodialysis and post-transplant patients were the most common. There is a scarcity of data describing HRQL for patients in CKD stages 1–5; only one study was identified that reported an HSU value for stage 2 patients and no studies reported HSUs for stage 1 patients.
Four longitudinal studies reported HSU weights. Limited data were available describing HRQL changes with disease progression. Regarding HRQL in patients undergoing RRT, HSU weights increased with time (Table 5).
Mean weighted HSU weights for the different CKD health states according to instrument are reported in Fig. 3. There is clear variation in utility values across instruments. However, there is an overall consistent trend with each instrument showing a reduction in HRQL with CKD progression. HRQL is lowest with dialysis. HSU weights reported using SF-6D indicate no difference between haemodialysis and peritoneal dialysis while HSU weights are lower for peritoneal dialysis when using EQ-5D-3L. HRQL increases after renal transplantation.
Mean HSU weights by state presented by instrument. HSU values are weighted averages calculated using subgroup population sizes; Error bars represent standard error. Abbreviations: CKD, chronic kidney disease; HSU, health state utility; HUI2, Health Utilities Index Mark 2; HUI3, Health Utilities Index Mark 3; SF-6D, Short Form questionnaire-6 Dimensions
Only one study identified in our SR reported the impact of adverse events or complications on CKD patients on dialysis [18]. The HSU weights reported are shown in Table 6. No studies reported the impact of adverse events or complications on patients with CKD stage 1–5 or after a renal transplant.
Study quality is reported in Additional file 5. Since all analysed studies met our grade 1 screening requirements, overall study quality was high. Quality assessment reported a lack of clarity in 7 studies regarding drop out or missing data rates. Lee et al. reported a low 33% response rate but was retained due to the questionnaire administration method (survey packets were mailed to patients’ houses) [19].
Discussion
This SR was designed to identify HSU weights for a range of CKD health states using methods promoted by Brazier and colleagues (2019) and other best guidance available. To our understanding, this is the first SR to report HSU weights for CKD stages 2–5, as well as RRT, as previous SRs focused on RRT only [7, 10, 33,34,35,36,37].
The review identified a large number of published studies that reported HSU weights for CKD populations. By focusing to the most generalisable and reliable Grade 1 studies, we hope to present the most accurate summary of HSU weights in CKD.
This is also the first SR to have been undertaken in the area of HSU weights since the Brazier and colleagues (2019) guidance on SR methods for the identification of HSU weights for cost-effectiveness analysis was released [12]. Based on our experience of implementing the guidance, we found that the recommended approach worked well and the guidance provided a very good rationale and set of methods for identifying the most relevant data.
According to the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices, conducting a meta-analysis on this topic may have been appropriate. However, the Brazier and colleagues (2019) guidance for identification of HSU weights in particular does not specify the need for this type of analysis. We believe this SR more directly addresses the needs of decision-making entities in different countries, as ideal data for decision-making would be country-specific with a relevant presence of comorbidities, settings, HSU instruments, and date-of-publication ranges - entities that may be lost in meta-analysis.
The review found an overall trend across studies for a decline in HSU scores as CKD deteriorated, (based on GFR). This fits with clinical expectation, but it is a point worth making because we believe that it provides some justification or validation of the identified HSU weights. Different factors may affect HRQL decline at different CKD stages. For instance, reductions in HRQL in early CKD stages may be driven by the presence of comorbidities such as diabetes, while a decline in HRQL in more advanced CKD stages may also be driven by an increase in the incidence of heart failure, and cardiovascular complications such as myocardial infarction or stroke which could also have a substantial impact on HRQL [38,39,40,41]. However, it is difficult to determine the cause of any decline in HRQL when exploring published data because we are limited to the data that have been included in the publication. This is one important limitation of the published data and of this SR. The studies included varied in terms of their design (cross-sectional survey, randomised trial, prospective observational study) and used different instruments which made comparisons between them challenging. Although a similar declining trend was observed with CKD progression across instruments, absolute HSU values were different across instruments with EQ-5D-3L reporting the highest values. This could reflect that sensitivity to capture the impact of CKD progression on HRQL may be different between the instruments reported in this systematic review. This could also present a challenge when estimating QALYs gained in cost-effectiveness analyses of new treatments for CKD. As a consequence, incremental cost-effectiveness ratios could be different depending on the instrument used and, potentially, this could result in different recommendations for the adoption of new treatments for CKD by HTA agencies. Variability in HSU weights between instruments remains a source of bias when combining results from studies using different instruments. This could be avoided by only including studies which use one specific instrument. While the number of patients assessed differed substantially between studies, by HSU instruments used and CKD stages, this did not seem to influence HSUs reported as they seemed aligned for each instrument and CKD stage regardless of sample size. While HSU weights were lowest for dialysis, it was not clear if HSU differs between haemodialysis and peritoneal dialysis as different trends were noted between the instruments used. Further research should be conducted to increased the understanding of these differences.
A number of aspects may affect the mix of patients included in the studies reported in this SR and, therefore, the eventual HSU weights reported. For example, patients receiving in-centre dialysis may have more comorbidities and complications than patients that are good candidates for peritoneal dialysis, dialysis at home or nocturnal dialysis. Patients with less severe kidney disease or higher HRQL may also be more likely to respond to voluntary questionnaires or participate in trials, potentially skewing the data. Further, it is possible that geographical variations may arise due to differences in clinical practice but also how people interpret HRQL questionnaires.
Regression methods such as those applied by Briggs and colleagues (2012) provide a way to estimate HSUs from longitudinal studies for different CKD stages improving the precision of the effects and understand their origin. Regression analyses of large datasets allow us to understand the impact of CKD related events on HRQL as well as understanding the influence of covariates and so this offers advantages over SR methods. It may also be possible to explore some of these issues with meta-regression type techniques. However, the studies are not consistent in the information that they present which makes it difficult to compare these variables systematically. Alternatively, it could be assumed that a ‘true’ score for a specific health state lies within the range of scores that have been identified from the review for a specific health state. Therefore, cost-effectiveness analysis could be informed by the range of scores as opposed to a single point estimate.
All studies used generic instruments of health rather than disease-specific instruments, but despite this the HSU weight varied substantially between different instruments (Fig. 3). This figure showed that the HUI3 questionnaire produces lower HRQL scores in comparison to the SF-6D and EQ-5D-3L. Higher HSU weights were reported with the SF-6D. SF-6D values for dialysis patients in particular, (0.76, and 0.78 for haemodialysis and peritoneal dialysis, respectively) seemed high considering that these applied to patients receiving dialysis. If the measures are not in agreement, then this could be explored (and perhaps controlled for) using a meta-regression approach. Where possible, HSU weights used in a cost-effectiveness analysis could be limited to a single instrument relevant to the specific research question for a cost-effectiveness analysis such as the EQ-5D-3L for cost-effectiveness analyses submitted to NICE in England.
In 2012, KDIGO provided guidelines for the categorisation of patients according to GFR and albuminuria [42]. Our SR did not find any studies that reported HSU weights based on both GFR and albuminuria or albuminuria alone. A data gap exists to understand the impact of albuminuria on HRQL. Additional data gaps exist around the reporting of HSUs related to CKD stage 1, adverse events and complications in patients with CKD.
Conclusions
To our knowledge, this is the first SR examining HSU scores for patients with CKD stages 1–5 with stratification by CKD stage. This is also one of the first reviews to apply the Brazier and colleagues (2019) guidance. There were sufficient data to provide weighted mean HSU weights for most CKD stages of interest [2,3,4,5] and RRT. No data was found reporting HSUs weights according to the KDIGO 2012 GFR/albuminuria categories. The findings from the SR illustrate how HRQL is worse for patients with worse renal function. Although similar trends were seen, notable differences in absolute values were identified across instruments highlighting potential differences in sensitivity to capture changes in HRQL in patients with CKD. This could result in the estimation of different QALYs gained in cost-effectiveness models and could affect the recommendation to adopt new treatments for CKD by HTA agencies. Regression methods are an option to provide refined HSU values from longitudinal studies while meta-analytical methods could help explore differences when using aggregate data.
Availability of data and materials
Not applicable.
Abbreviations
- CKD:
-
Chronic kidney disease
- ESRD:
-
End-stage renal disease
- GFR:
-
Glomerular filtration rate
- HD:
-
Haemodialysis
- HRQL:
-
Health related quality of life
- HSU:
-
Health state utility
- HTA:
-
Health technology assessment
- HUI:
-
Health utility index
- HUI2:
-
Health Utilities Index Mark 2
- HUI3:
-
Health Utilities Index Mark 3
- IgA:
-
Immunoglobulin A
- ISPOR:
-
International Society of Pharmacoeconomics and Outcomes Research
- KDIGO:
-
Kidney Disease: Improving Global Outcomes
- NICE:
-
National Institute for Health and Care Excellence
- NR:
-
Not reported
- PD:
-
Peritoneal dialysis
- QALY:
-
Quality adjusted life year
- RAASi:
-
Renin angiotensin aldosterone system inhibitors
- RRT:
-
Renal replacement therapy
- SD:
-
Standard deviation
- SF-12:
-
Short-form 12
- SF-36:
-
Short-form 36
- SF-6D:
-
Short-form questionnaire-6 dimensions
- SR:
-
Systematic review
- T2DM:
-
Type 2 diabetes
- Trx:
-
Renal transplant
References
Hill NR, Fatoba ST, Oke JL, Hirst JA, O'Callaghan CA, Lasserson DS, et al. Global Prevalence of Chronic Kidney Disease - A Systematic Review and Meta-Analysis. PloS One. 2016;11(7):e0158765-e.
Harris RE. Epidemiology of chronic disease: global perspectives: Jones & Bartlett Learning; 2019.
Chronic Kidney Disease in the United States, 2019 Centers for Disease Control and Prevention: U.S. Departemnt of Health and Human Services; 2019 [.
Fraser SD, Blakeman T. Chronic kidney disease: identification and management in primary care. Pragmat Obs Res. 2016;7:21–32.
United States Renal Data System. 2018 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018.
Sugrue DM, Ward T, Rai S, McEwan P, van Haalen HGM. Economic Modelling of chronic kidney disease: a systematic literature review to inform conceptual model design. PharmacoEconomics. 2019;37(12):1451–68.
Wyld M, Morton RL, Hayen A, Howard K, Webster AC. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med. 2012;9(9):e1001307.
Zazzeroni L, Pasquinelli G, Nanni E, Cremonini V, Rubbi I. Comparison of quality of life in patients undergoing hemodialysis and peritoneal Dialysis: a systematic review and meta-analysis. Kidney Blood Press Res. 2017;42(4):717–27.
Glover C, Banks P, Carson A, Martin CR, Duffy T. Understanding and assessing the impact of end-stage renal disease on quality of life: a systematic review of the content validity of self-administered instruments used to assess health-related quality of life in end-stage renal disease. Patient. 2011;4(1):19–30.
Boateng EA, East L. The impact of dialysis modality on quality of life: a systematic review. J Renal Care. 2011;37(4):190–200.
Liem YS, Bosch JL, Hunink MG. Preference-based quality of life of patients on renal replacement therapy: a systematic review and meta-analysis. Value Health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2008;11(4):733–41.
Brazier J, Ara R, Azzabi I, Busschbach J, Chevrou-Séverac H, Crawford B, et al. Identification, review, and use of health state Utilities in Cost-Effectiveness Models: an ISPOR good practices for outcomes research task force report. Value Health. 2019;22(3):267–75.
Henderson LK, Craig JC, Willis NS, Tovey D, Webster AC. How to write a Cochrane systematic review. Nephrology (Carlton, Vic). 2010;15(6):617–24.
Dissemination CfRa. Systematic Reviews. CRD's guidance for undertaking reviews in healthcare. University of York: Center for Reviews and Dissemination; 2008. p. 294.
Papaioannou D, Brazier J, Paisley S. NICE Decision Support Unit Technical Support Documents. NICE DSU Technical Support Document 9: The Identification, Review and Synthesis of Health State Utility Values from the Literature. London: National Institute for Health and Care Excellence (NICE); 2010.
Jesky MD, Dutton M, Dasgupta I, Yadav P, Ng KP, Fenton A, et al. Health-related quality of life impacts mortality but not progression to end-stage renal disease in pre-Dialysis chronic kidney disease: a prospective observational study. PLoS One. 2016;11(11):e0165675.
Gorodetskaya I, Zenios S, McCulloch CE, Bostrom A, Hsu CY, Bindman AB, et al. Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int. 2005;68(6):2801–8.
Briggs AH, Parfrey PS, Khan N, Tseng S, Dehmel B, Kubo Y, et al. Analyzing health-related quality of life in the EVOLVE trial: the joint impact of treatment and clinical events. Med Decis Mak. 2016;36(8):965–72.
Lee AJ, Morgan CL, Conway P, Currie CJ. Characterisation and comparison of health-related quality of life for patients with renal failure. Curr Med Res Opin. 2005;21(11):1777–83.
Jardine MJ, Zuo L, Gray NA, de Zoysa JR, Chan CT, Gallagher MP, et al. A trial of extending hemodialysis hours and quality of life. J Am Soc Nephrol: JASN. 2017;28(6):1898–911.
Manns BJ, Johnson JA, Taub K, Mortis G, Ghali WA, Donaldson C. Dialysis adequacy and health related quality of life in hemodialysis patients. ASAIO J (American Society for Artificial Internal Organs : 1992). 2002;48(5):565–9.
Manns BJ, Walsh MW, Culleton BF, Hemmelgarn B, Tonelli M, Schorr M, et al. Nocturnal hemodialysis does not improve overall measures of quality of life compared to conventional hemodialysis. Kidney Int. 2009;75(5):542–9.
Pan CW, Wu Y, Zhou HJ, Xu BX, Wang P. Health-related quality of life and its factors of hemodialysis patients in Suzhou, China. Blood Purif. 2018;45(4):327–33.
Wong CKH, Chen JY, Fung SKS, Lo WK, Lui SL, Chan TM, et al. Health-related quality of life and health utility of Chinese patients undergoing nocturnal home haemodialysis in comparison with other modes of dialysis. Nephrology. 2019;24(6):630–7.
Davison SN, Jhangri GS, Feeny DH. Evidence on the construct validity of the health utilities index mark 2 and mark 3 in patients with chronic kidney disease. Qual Life Res: an international journal of quality of life aspects of treatment, care and rehabilitation. 2008;17(6):933–42.
Davison SN, Jhangri GS, Feeny DH. Comparing the health utilities index mark 3 (HUI3) with the short Form-36 preference-based SF-6D in chronic kidney disease. Value Health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2009;12(2):340–5.
Neri L, Dukes J, Brennan DC, Salvalaggio PR, Seelam S, Desiraju S, et al. Impaired renal function is associated with worse self-reported outcomes after kidney transplantation. Qual Life Res: an international journal of quality of life aspects of treatment, care and rehabilitation. 2011;20(10):1689–98.
Ortega F, Otero A, Crespo JF, Delgado JF, Borro JM, Cuervo J. Satisfaction and adherence with immunosuppressant treatment in renal transplant patients living with a working graft. J Nephrol. 2013;26(2):297–305.
Blakeman T, Blickem C, Kennedy A, Reeves D, Bower P, Gaffney H, et al. Effect of information and telephone-guided access to community support for people with chronic kidney disease: randomised controlled trial. PLoS One. 2014;9(10):e109135.
Manns B, Johnson JA, Taub K, Mortis G, Ghali WA, Donaldson C. Quality of life in patients treated with hemodialysis or peritoneal dialysis: what are the important determinants? Clin Nephrol. 2003;60(5):341–51.
Ortega T, Valdes C, Rebollo P, Ortega F. Evaluation of reliability and validity of Spanish version of the end-stage renal disease symptom checklist-transplantation module. Transplantation. 2007;84(11):1428–35.
Ortega T, Deulofeu R, Salamero P, Lauzurica R, Casanovas T, Cofan F, et al. Perceived state of health is worse in kidney recipients younger than 60 years vs older than 60 years. Transplant Proc. 2009;41(6):2118–21.
Brown L, Gardner G, Bonner A. A comparison of treatment options for management of end stage kidney disease in elderly patients: a systematic review. JBI Database System Rev Implement Rep. 2014;12(7):374–404.
Chung R, Howard K, Craig JC, Chapman JR, Turner R, Wong G. Economic evaluations in kidney transplantation: frequency, characteristics, and quality - a systematic review. Transplantation. 2014;97(10):1027–33.
Dale PL, Hutton J, Elgazzar H. Utility of health states in chronic kidney disease: a structured review of the literature. Curr Med Res Opin. 2008;24(1):193–206.
Ferguson T, Xu Y, Gunasekara R, Lerner B, Macdonald K, Rigatto C, et al. The cost effectiveness of erythropoietin-stimulating agents for treating anemia in patients on dialysis: a systematic review. Am J Nephrol. 2015;41(2):89–97.
Kennedy-Martin T, Paczkowski R, Rayner S. Utility values in diabetic kidney disease: a literature review. Curr Med Res Opin. 2015;31(7):1271–82.
Soni RK, Weisbord SD, Unruh ML. Health-related quality of life outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens. 2010;19(2):153–9.
McFarlane PA, Tobe SW, Culleton B. Improving outcomes in diabetes and chronic kidney disease: the basis for Canadian guidelines. Can J Cardiol. 2007;23(7):585–90.
Mujais SK, Story K, Brouillette J, Takano T, Soroka S, Franek C, et al. Health-related quality of life in CKD patients: correlates and evolution over time. Clin J Am Soc Nephrol. 2009;4(8):1293–301.
Devine EB, Smith KL, Stehman-Breen C, Patrick DL. Health-related quality of life assessment in chronic kidney disease. Expert Rev Pharmacoecon Outcomes Res. 2003;3(1):89–100.
Levin A, Stevens PE. Summary of KDIGO 2012 CKD guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85(1):49–61.
Acknowledgements
Not applicable.
Funding
This study was sponsored by AstraZeneca plc. Support for third-party writing assistance for this article, provided by Acaster Lloyd, was funded by AstraZeneca plc in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Author information
Authors and Affiliations
Contributions
Substantial contributions to study conception and design: JC, AL, AB, JJGS, ES; substantial contributions to analysis and interpretation of the data: JC, AL, AB, JJGS, ES, PM; drafting the article or revising it critically for important intellectual content: JC, AL, AB, JJGS, ES, PM; final approval of the version of the article to be published: JC, AL, AB, JJGS, ES, PM.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The study was funded by AstraZeneca plc. Support for third-party writing assistance, provided by Acaster Lloyd Consulting Ltd., was funded by AstraZeneca plc in accordance with Good Publication Practice (GPP3) guidelines. Disclosures for all authors are as follows:
• AL: Employee of Acaster Lloyd Consulting
• JC, AB: Employees of Avalon Health Economics
• JJGS, ES: Employees of AstraZeneca
• PM: Consultancy and research support from AMGEN, Astra-Zeneca, Bayer, Boehringer Ingelheim, Ilanga, Janssen, Lilly, Novartis, Otsuka, Pfizer, Sanofi-Aventis, and Takeda, as well as research support from AMGEN, Astra-Zeneca, Bayer, Boehringer Ingelheim, GSK, Janssen, Novartis, Novo-Nordisk, Ortho-Biotech, Otsuka, and Reata.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Search Strategy. PubMed search results.
Additional file 2.
Grey Literature Search. Grey literature search results.
Additional file 3.
Full Text Screening. Full text screening results with decision, HTA compatibility, bias assessment, and grade.
Additional file 4.
HSU weights from studies identified in SLR. Author and year, HRQOL Elicitation and scoring methods, subgroups, and mean utility scores for each grade one study identified in the SLR.
Additional file 5.
Quality Assessment. Bias assessment using a traffic light grading system of grade one studies identified in SLR.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cooper, J., Lloyd, A., Sanchez, J.J.G. et al. Health related quality of life utility weights for economic evaluation through different stages of chronic kidney disease: a systematic literature review. Health Qual Life Outcomes 18, 310 (2020). https://doi.org/10.1186/s12955-020-01559-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12955-020-01559-x