Abstract
Background
Metal-organic frameworks (MOFs - MIL-101) are the most exciting, high profiled developments in nanotechnology in the last ten years, and it attracted considerable attention owing to their uniform nanoporosity, large surface area, outer-surface modification and in-pore functionality for tailoring the chemical properties of the material for anchoring specific guest moieties. MOF’s have been particularly highlighted for their excellent gas storage and separation properties. Recently biomolecules-based MOF’s were used as nanoencapsulators for antitumor and antiretroviral controlled drug delivery studies. However, usage of MOF material for removal of ionic detergent-SDS from biological samples has not been reported to date. Here, first time we demonstrate its novel applications in biological sample preparation for mass spectrometry analysis.
Methods
SDS removal using MIL-101 was assessed for proteomic analysis by mass spectrometry. We analysed removal of SDS from 0.5 % SDS solution alone, BSA mixture and HMEC cells lysate protein mixture. The removal of SDS by MIL-101 was confirmed by MALDI-TOF-MS and LC-MS techniques.
Results
In an initial demonstration, SDS has removed effectively from 0.5 % SDS solution by MIL-101via its binding attraction with SDS. Further, the experiment also confirmed that MIL-101 strongly removed the SDS from BSA and cell lysate mixtures.
Conclusions
These results suggest that SDS removal by the MIL-101 method is a practical, simple and broad applicable in proteomic sample processing for MALDI-TOF-MS and LC-MS analysis.
Similar content being viewed by others
Background
Metals organic frameworks (MOF) are nano-porous compounds that contain metal ions or clusters that are connected by organic ligands to forms two or three dimensional structures. Last 10 years, MOFs are most potential compounds in hydrogen storage [1, 2], CO2 capture [3, 4], catalysis [5, 6], sensing [7], and others [8]. It has highly porous nature that makes very attractive for catalysis applications. In addition, MOFs have large diversity in nature as compared to Zeolites due to the use of SiO4/AlO4 tetrahedral building units in the later materials. The catalytic applications of zeolites are more restricted to relatively small organic molecules (typically no larger than xylene) because due to its microporous in nature. Whereas, the size of pore, shape, dimensionality and chemical natures in MOFs have been controlled by the suitable selection of their building blocks (metal and organic linker). Apart from these features, the lower acidity of the active centers in MOFs makes these materials even very attractive compared Zeolites (highly acidic centers). Also, MOFs may be changing the interactions of adsorbing reactants and the transition states or intermediaries formed inside the framework cavity between the host and guest.
The fundamental step of global proteomics experiments, particularly involving sensitive MS technique is efficient sample preparation. Extraction of total proteins from various biological sources including tissues and cultured cells using SDS-Sodium Dodecyl Sulfate, CHAPS, and Triton is well known in several biochemical studies. SDS is widely used and considered to be very beneficial due to complete cell lysis, disaggregation and efficient solubilization of the global proteome primarily hydrophobic membrane-bound components. SDS interacted with proteins by ionic and hydrophobic bonds and dissolves proteins by changing their secondary and tertiary structures [9]. Further, it plays an important role in studies of membrane proteins or aggregated proteins, because of these proteins are not soluble in other agents. In addition, SDS continuously used in protein separations from biological samples by sodium dodecyl sulfate- polyacrylamide gel electrophoresis (SDS-PAGE) method. Unfortunately, SDS in the samples can be unfavourable due to its unwanted effects in liquid chromatography and it produces large DS− related signals and ion suppression effects [10, 11]. Therefore, SDS removal is an essential prerequisite for achieving higher peptide/protein coverage in the mass-spectrometric analysis. It can be accomplished by numerous techniques such as precipitation, strong cation exchange, protein and peptide level purification with pierce detergent removal cartridges and FASP II etc. [12]. Gu et al. 2011 [13] reported that the MOFs based material is very useful for biological applications. But, usage of MOFs material for removal of ionic detergent-SDS from biological samples has not been reported to date. Therefore, we tried to find out a novel method for removal of ionic detergent-SDS from biological samples by microdevices based MOFs material for proteomic analysis.
Results and discussion
Standard sample cleans up with different solid phase extraction (SPE) or desalting methods have not been much effective in SDS depletion to date; prompting the investigation of various methods for SDS removal will make several commercial products [14], Filter aided sample preparation (FASP) [15], high salt precipitation kits [16], and SDS specific binding spin cartridges are very famous methods for depletion of detergent from protein mixtures for proteomic sample preparation. However, a majority of techniques are hindered by low protein recovery, labour intensiveness, irreproducibility and incomplete SDS removal. To these address, we tried to find out a novel and efficient method for SDS removal. Here we used MOFs (MIL-101) as a binding material for SDS removal from the biological samples by micro- device based method. Our preliminary results were highly encouraged and demonstrated a novel biological application of MOF materials which could have significant value in sample processing i.e SDS depletion for MALDI-TOF and LC-MS analysis.
SDS removal from 0.5 % SDS solution using MIL-101
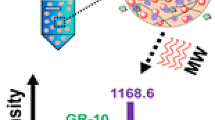
First, we tried to remove the SDS from 0.5 % SDS solution using MIL-101 as a binding material. Presently, this method has not been applied for removing SDS during proteomic analysis by the mass spectroscopy. Thus, we designed and used this method for SDS removable from biological protein mixture before MALDI-TOF-MS analysis. Initially, we removed the SDS from 0.5 % SDS solution with the help of MIL-101 material by centrifugation at 3000 rpm and the results exhibited that maximum concentration of SDS was removed as evidence the peak intensity of SDS at 287.89[M + H] was differed and clear in after removal of SDS (Fig. 1a & b). This result suggests that for the solution containing SDS can be removed by MIL-101 material.
SDS removal from BSA mixtures using MIL-101
Further, we analysed capability of MIL-101 for removal of SDS from bovine serum albumin. Different concentration of BSA was mixed with 0.5 % of SDS. Then BSA in SDS mixture was treated with MIL-101 for overnight and then centrifuged at 3000 rpm prior to MALDI-TOF-MS analysis. Generally, SDS acts as a potent surfactant that could be denatured the trypsin activity when digestion process. But, we observed good signalling intensity for BSA tryptic peptides. It indicated that MIL-101 has an ability to remove the SDS from single protein mixture with robust trypsin activity (Fig. 2a).
SDS removal from cell lysate protein mixture using MIL-101
Further, we planned to assess the weather MIL-101 have an ability to remove the SDS from cell lysate protein mixtures or not, because it is very complicated and little difficult to separate SDS from the biological samples as compared with single protein mixtures. So, we prepared protein extract from HMEC cells and mixed with 0.5 % boiling SDS. This mixture was incubated with 30 mg of MIL-101 slurry with end-over-end rotation overnight. Proteins were further separated from MIL-101 by centrifugation at 3000 rpm for 5 min. Supernatant containing protein mixture was processed for MALDI-TOF-MS analysis. This result suggests that MIL-101 has effectively removed SDS from cell lysate protein mixtures (Fig. 2b).
LC-MS analysis of tryptic peptides
Finally, the processed sample was subjected to LC-MS analysis. The result indicates that MIL-101 processed sample shows little ion suppression effect only. It may be due to trace amount of SDS was found in the samples. Overall MIL-101 effectively removed SDS from cell lysate by its binding efficiency (Fig. 3a). Figure 3b demonstrated the number of proteins with 1 or 2 unique peptide matches, Totally 750 protein were identified; among them 438 proteins having two peptide matches and 319 proteins having one peptide hits. Figure 3c demonstrated the GO (Gene Ontology) based annotations and cellular distribution of identified proteins. From our preliminary analysis, we hypothesize that MIL-101 is an attractive MOFs candidate for SDS removal from biological proteins before MS analyses.
Conclusions
The MIL-101 is an attractive MOFs candidate for removing SDS from biological samples through its electrostatic interactions. All the experimental results suggested that MIL-101 method could be a very useful method to prepare biological sample preparations for spectroscopy based proteomic analysis.
Methods
Chemical
BCA kit and HMEC cells were purchased from Thermo Fisher Scientific and American Type Culture Collection [Rockwille, MD, USA] respectively. SDS, Trypsin, BSA and DTT obtained from Sigma-Aldrich, USA. MIL-101 was from sigma product # 185361[Final concentration 100 μg/ml] and Microdevices obtained from Mobitec; product code: MobiSpin Column F (1.5 ml tubes).
SDS removal from 0.5 % SDS solution
MIL-101 (100 μg slurry) was mixed with 0.5 % SDS solution and incubated at room temperature for overnight. Then, the sample was centrifuged at 3000 rpm for 5 min and then subjected to MALDI-TOF analysis.
SDS removal from BSA mixture
Different concentrations of BSA (5, 10, 25 μg) were mixed with the freshly prepared SDS (0.5 % SDS w/v) and was further incubated overnight with the slurry prepared using 100 μg MIL-101 with one ml of freshly prepared ice cold phosphate buffer and reduced with 10 mM DTT. Later, the sample was subjected to in-solution digestion using trypsin and peptides were desalted and then loaded into MALDI-TOF analysis.
SDS removal from cell lysate mixture
HMEC cells were lysed using 0.5 % boiling SDS and sonicated to clear the chromatin. Lysate protein was estimated by BCA method and incubated with MIL-101 matrix (100 μg slurry) at room temperature for overnight to SDS attraction by the MOF and then separated by centrifugation. Protein mixture was in-solution digested, desalted and then subjected into MALDI-TOF and LCMS analysis. An outline of the work flow was illustrated in Fig. 4a-b.
MALDI-TOF analysis
Peptide mass fingerprinting (PMF) was conducted using MALDI-TOF-MS equipped with linear mode 20 KvA, laser shots 150 (337 nm, 50 H, N2 laser (Bruker Daltonics, Germany). For, each sample spectra required in the positive linear mode, and an average of 200 spectra that passed the accepted criteria of peak intensity was automatically selected and accumulated. Spectrum processing and data base searches were conducted b automatic mode with internal calibration using trypsin autolysis peaks (m/z 842.509 and m/z 2211.104). The fragmentation of selected peptide was measured using the PSD mode for MS analysis [17].
LC-MS analysis
The peptides were analysed using a reverse phase capillary column (LC system -LTQ; Thermo Scientific, San Jose, CA) prepared by slurry packing 3-μM Jupiter C18 bonded particles into a 65 cm long and 75 μM inner diameter fused silica capillary. 2.5 μg of peptides were loaded onto the column; the mobile phase was held at 100 % buffer A (0.1 % formic acid) for 20 min, followed by a linear gradient from 0 to 70 % buffer B(0.1 % formic acid in 90 % acetonitrile) for more than 85 min. Each full MS scan (m/z 400–2000) was followed by collision induced MS/MS spectra. The dynamic exclusion duration was set to 1 min; the heated capillary was maintained at 200 °C and the ESI voltage was held at 2.2 kV [18].
Abbreviations
- SDS:
-
Sodium dodecyl sulfate
- MOFs:
-
Metal organic frameworks
- HMEC:
-
Human mammary epithelial cells
- MALDI-TOF:
-
Matrix assisted laser desorption/ionization-time of flight
- BSA:
-
Bovine serum albumin
- DTT:
-
Dithiothreitol
- GO:
-
Gene ontology
- LC-MS:
-
Liquid chromatography- mass spectrometry
- FASP:
-
Filter aided sample preparation)
- SPE:
-
Solid phase extraction
- CHAPS:
-
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
References
Zhao D, Yuan D, Zhou HC. The current status of hydrogen storage in metalorganic frameworks. Energy Environ Sci. 2008;1:222–35.
Furukawa H, Michael Miller A, Omar Yaghi M. Independent verification of the saturation hydrogen uptake in mof-177 and establishment of a benchmark for hydrogen adsorption in metal-organic frameworks. J Mater Chem. 2007;17:3197–204.
Titus MP. Porous inorganic membranes for CO2 capture: present and prospects. Chem Rev. 2014;114:1413–92.
Liu J, Praveen K, Thallapally B, McGrail P, Daryl Brown R, Liu J. Progress in adsorption-based co2 capture by metal-organic frameworks. Chem Soc Rev. 2012;41:2308–22.
Farha OK, Shultz AM, Sarjeant AA, Nguyen ST, Hupp JT. Active-site-accessible, porphyrinic metal-organic framework materials. J Am Chem Soc. 2011;133:5652–5.
Lee JY, Farha OK, Roberts J, Scheidt KA, Nguyen ST, Hupp JT. Metal-organic framework materials as catalysts. Chem Soc Rev. 2009;38:1450–9.
Kreno LE, Leong K, Farha OK, Allendorf M, Van Duyne RP, Hupp JT. Metal-organic framework materials as chemical sensors. Chem Rev. 2012;112:1105–25.
Stroppa A, Barone P, Jain P, Perez-Mato JM, Picozzi S. Hybrid improper ferroelectricity in a multiferroic and magnetoelectric metal organic framework. Adv Mat. 2013;25:2284–90.
Andersen KK, Oliveira CL, Larsen KL, Poulsen FM, Callisen TH, Westh P, Pedersen JS, Otzen D. The role of decorated SDS micelles in sub-CMC protein denaturation and association. J Mol Biol. 2009;391:207–26.
Rundlett KL, Armstrong DW. Mechanism of signal suppression by anionic surfactants in capillary electrophoresis-electrospray ionization mass spectrometry. Anal Chem. 1996;68:3493–7.
Botelho D, Wall MJ, Vieira DB, Fitzsimmons S, Liu F, Doucette A. Top-down and bottom-up proteomics of SDS-containing solutions following mass-based separation. J Proteome Res. 2010;9:2863.
Yeung YG, Nieves E, Angeletti R, Stanley ES. Removal of detergents from protein digests for mass spectrometry analysis. Anal Biochem. 2008;382:135–7.
Gu ZY, Chen YJ, Jiang JQ, Yan XP. Metal-organic frameworks for efficient enrichment of peptides with simultaneous exclusion of proteins from complex biological samples. Anal Commun. 2011;47:4787–9.
Hengel SM, Floyd E, Baker ES, Zhao R, Wu S, Tolić LP. Evaluations of SDS depletion using an affinity spin column and IMS-MS detection. Proteomics. 2012;12:3138–42.
Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359–62.
Zhou JY, Dann GP, Shi T, Wang L, et al. Simple sodium dodecyl sulfate-assisted sample preparation method for LC-MS-based proteomics applications. Anal Chem. 2012;84:2862–8.
Karthik D, Ilavenil S, Kaleeswaran B, Sunil S, Ravikumar S. Proteomic analysis of plasma proteins in diabetic rats by 2D electrophoresis and MALDI-TOF-MS. Appl Biochem Biotechnol. 2012;2012(166):1507–19.
Zhou JY, Schepmoes AA, Zhang X, Moore RJ, Monroe ME, Lee JH, Camp DG, Smith RD, Qian WJ. Improved LC-MS/MS spectral counting statistics by recovering low-scoring spectra matched to confidently identified peptide sequences. J Proteome Res. 2010;9:5698–704.
Acknowledgments
This study was supported by grants from the Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ009559012016), Rural Development Administration, Korea. The authors extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding this Prolific Research Group (PRG-1437-28).
Availability of data and materials
All data and materials were given in the manuscript. We don’t have further data and materials.
Authors’ contributions
NAA-D, YOK and MVA were planned and executed the laboratory experiments. SI, MVA, PA, RB, KCC CGP, SS, and KHP participated in data analysis, results interpretation, and chemicals arrangement. All listed authors were contributed to this research work and accepted the final manuscript for publications. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Financial disclosure
The present work was supported by the Cooperative Research Program for Agriculture Science & Technology Development, Rural Development Administration, Korea (Grant No: PJ009559012016) and the authors extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding this Prolific Research Group (PRG-1437-28).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ilavenil, S., Al-Dhabi, N.A., Srigopalram, S. et al. Removal of SDS from biological protein digests for proteomic analysis by mass spectrometry. Proteome Sci 14, 11 (2016). https://doi.org/10.1186/s12953-016-0098-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12953-016-0098-5