Abstract
Background
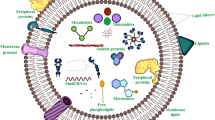
The past few years have witnessed a significant increase in research related to plant-derived extracellular vesicles (PDEVs) in biological and medical applications. Using biochemical technologies, multiple independent groups have demonstrated the important roles of PDEVs as potential mediators involved in cell-cell communication and the exchange of bio-information between species. Recently, several contents have been well identified in PDEVs, including nucleic acids, proteins, lipids, and other active substances. These cargoes carried by PDEVs could be transferred into recipient cells and remarkably influence their biological behaviors associated with human diseases, such as cancers and inflammatory diseases.
Main body of the abstract
This review summarizes the latest updates regarding PDEVs and focuses on its important role in nanomedicine applications, as well as the potential of PDEVs as drug delivery strategies to develop diagnostic and therapeutic agents for the clinical management of diseases, especially like cancers.
Conclusion
Considering its unique advantages, especially high stability, intrinsic bioactivity and easy absorption, further elaboration on molecular mechanisms and biological factors driving the function of PDEVs will provide new horizons for the treatment of human disease.
Similar content being viewed by others
Background
Although the beneficial properties of natural substances against human diseases have been recognized for several decades, clarification of the biological functions and underlying molecular mechanisms remains limited. In recent years, the use of extracellular vesicles (EVs) from natural compounds has gained huge scientific interest as a promising therapeutic strategy [1]. These nanometer-sized membrane-enclosed EVs have been extracted from many plant species [2], such as Dendropanax morbifera [3], grapefruit [4], and dried plant-derived materials [5]. These EVs are effectively uptaken by most host organs, affecting their physiological and pathological processes [4]. Nowadays, several studies have pointed out the important roles of plant-derived extracellular vesicles (PDEVs) in cell-cell communication, the exchange of bio-information between different cells, and maintaining proper tissue homeostasis and organism integrity [6, 7]. The biochemical and pharmacological studies have demonstrated the heterogeneity of PDEVs in terms of their origin, size, and content [8]. Generally, according to the size, the PDEVs are mainly divided into two categories: large microvesicles (100 to 1,000 nm) and small nanovesicles (50 to 100 nm) [9, 10].
In the 1960s, the identification of PDEVs was first attributed to Jensen’s group, who found that the multivesicular bodies were released from cotton cells [11] and carrot cells [12]. They observed that the multivesicular bodies could be released into extracellular space by the membrane fusion reaction. It was not until 2009 that Regente et al. [13] isolated the EVs from sunflower apoplastic fluids and demonstrated the presence of some proteins in these PDEVs. More importantly, in 2017, the evidence from the study by Rutter and Innes [14] indicated the promising biological activity of leaf apoplast-derived EVs in the cellular defense system. Subsequently, clarifying the biological roles and clinical applications of PDEVs has rapidly become an attractive research field [15]. Recent advances in PDEVs have demonstrated their several bioactivities, such as anti-cancer, anti-inflammatory, and anti-oxidative stress properties, in vitro and in vivo [16]. Despite this, more studies need to be performed to explore the underlying molecular mechanisms behind recipient cellular targets regulated by PDEVs. In addition, our current knowledge is not sufficient to distinguish the different biological activities and therapeutic efficacy of PDEVs from different kinds of plants.
Although the sources of PDEVs are distinct, they might have promising aspects against pathological conditions (Table 1). In this review, we mainly summarize the up-to-date pre-clinical and clinical studies to discuss the potential of PDEVs in clinical applications and therapeutic implications. This comprehensive review proposes to focus on the characterization and biomedicinal applications of PDEVs to improve human health.
Comparing PDEVs to mammalian EVs
For a long time, the EVs from animals, mainly mammals, have been gradually used in genetic, biochemical, and pharmacological fields [17,18,19,20]. In 1987, the first report of mammalian EVs was that exosomes were derived from the maturing reticulocyte [21]. After then, a large number of EVs have been constitutively identified from almost all of the mammalian cells, such as cancer cells [22, 23], immune cells [24] stem cells [25], etc. In addition, mammalian EVs have also been discovered in the biological fluids, such as urine, blood and saliva [26, 27]. Mammalian EVs contain diverse cargo molecules (proteins/nucleic acids/lipids) and could be involved in the intercellular communication [28]. Because of their satisfactory pharmacological activity and biocompatibility, mammalian EVs are widely regarded as the regulatory agents in multiple physiological and pathological processes. In myocardial infarction mice model, injection of dendritic cell-derived exosomes could promote the infiltration of Treg cells and M2 macrophages into border zoom, consequently improving the cardiac function [29]. The exosomes derived from gefitinib sensitive cancer cells could effectively reverses the gefitinib resistance by transferring microRNA-7 in non-small-cell lung cancer [30]. Besides, Cui’s group further confirmed that mammalian exosomes could transport the bioactive molecules across the cellular interface, such as blood-brain barrier (BBB), and targeting glioma cells [31]. Although the biological application of mammalian EVs is promising, there are several major issues that pose the obstacles for their clinical translation, for example low yield, difficulties to obtain high-quality EVs, and time-consuming isolation [10]. In particular, the utilization of animals as a source of vesicles frequently activates the host immune responses, likely causing side-effects [23, 32]. However, identifying PDEVs and clarifying their functional mechanisms could represent an alternative strategy to overcome these challenges.
Recently, several research groups have speculated the existence of nanovesicles in plant materials, participating in cell-to-cell communication and interspecies communication [33]. The cargoes carried by PDEVs could be transferred into the receiving cells, causing changes in the (patho)physiological functions [34] (Fig. 1). Even though the secretion mechanisms of EVs from plant or animal cells are similar to some extent, such as exocytosis-mediated release [35], a few different biological characteristics can be identified among these EVs from different species. PDEVs can be obtained in large quantities due to the low cost of various plant resources. The plant-derived nanovesicles can be produced continuously from all kinds of fruits and vegetables purchased from conveniently located local markets [15]. Moreover, the plant cells in in vitro culture media could even be used to produce sufficient PDEVs [36]. Under controlled artificial conditions, the application of food additives successfully increased PDEV production [36]. They have no significant toxicity as they are mainly extracted from naturally medicinal or edible plant materials. Additionally, PDEVs are suitable for human health management without activating the host immune responses [37]. Besides, through several proposed mechanisms, including endocytosis, phagocytosis, macropinocytosis, and membrane fusion [38], PDEVs could easily pass across different biological barriers, such as the BBB, and are subsequently absorbed by the recipient cells [39]. Considering these benefits (Table 2), we need to dedicate more in-depth investigation to better clarify the basic characteristics of PDEVs as diagnostic and therapeutic agents, which will help open new avenues for the regulation of human health.
Main pharmacological activities of PDEVs
PDEVs can transfer several cargoes to recipient cells and change the cellular phenotype, including nucleic acids, proteins, lipids, and other specific pharmacologically active substances [40] (Fig. 2). Several lipophilic secondary metabolites, such as alkaloids and curcuminoids, can be packaged into PDEVs and facilitate them to pass the membrane barrier [36]. Surprisingly, an ongoing work by Berger et al. [41] did not verify the secondary metabolites (vitamin C and naringenin) in the orange nanovesicles. These inconsistent findings may be due to the distinct scopes of nanovesicle-associated secondary metabolites in different plant species-derived EVs. Using label-free quantitative shotgun proteomics, Pocsfalvi et al. [9] found approximately 600–800 proteins in the citrus fruit juice sac cell-derived vesicles. Bioinformatic analysis revealed the important regulatory roles of these protein biocargo in multiple physiological processes, including vesicular trafficking, cellular metabolism, and cell growth. Subsequent studies supported the view that plant-derived microRNAs could be encapsulated in PDEVs, affecting the localization and stability of microRNAs [42,43,44]. The microRNAs transferred using PDEVs interfered with the pathophysiological processes of recipient cells by regulating their target genes [1]. Interestingly, these bioavailable cargoes encapsulated on PDEVs make them unique and powerful tools for health-beneficial purposes. In fact, without the need to reload other drugs, the PDEVs display natural clinical and pharmaceutical benefits [45]. For pharmaceutical application, the production of medicinal PDEVs did not exhibit any immunogenic or toxic effects on the host cells [46].
Besides the inherent biological activities, PDEVs can act as excellent nanovectors for the intercellular delivery of poorly soluble agents or therapeutic compounds as they may interfere with or potentialize their pharmacological activity. In addition, PDEVs are a potential source of desirable morphologies, feasible for large-scale production, inexpensive, and made of environmentally-friendly materials [47]. Man et al. (2021) extracted ginger-derived EVs (GDEVs) using ultrahigh-speed centrifugation. They found that the loading capacity of GDEVs strongly improved the lipid solubility of gingerol compounds, facilitating the intestinal absorption and transportation of gingerols [48]. Folic acid (FA)-positive ginger-derived EVs were used for the targeted delivery of survivin siRNA to human oral epidermoid carcinoma KB cells, leading to the downregulation of survivin and suppression of cell growth in vitro and in vivo [49]. The doxorubicin-containing PDEVs efficiently strengthened the cytotoxic effects of doxorubicin on the colon cancer cells, SW480. Meanwhile, other therapeutic agents, such as antisense oligonucleotides, were also encapsulated into PDEVs and successfully transported into human cells [50]. Collectively, these findings prove the value and potential of PDEVs as nanoscale vehicles for the delivery of therapeutic cargoes and offer a meaningful indication for the further use of PDEVs as a drug delivery system [51]. However, more investigations are still needed to exactly determine the potential of these nanoscale drug carriers to the next level for the benefit of patients in the future.
Several studies focus on modification techniques to improve the drug delivery efficiency of PDEVs (Fig. 3). The pharmacological particles are directedly encapsulated into the PDEVs by internalization processes, such as phagocytosis or endocytosis, to be selectively taken up by the recipient cells [52]. Once inside the PDEVs, the therapeutic drugs are significantly transported to the inflammatory tumor sites, leading to the inhibition of tumor growth [53]. Tian et al. [54] developed another surface modification strategy, named the bio-orthogonal copper-free azide alkyne cyclo-addition. Using this heterobifunctional click chemistry, the functional ligands were effectively loaded onto the surfaces of EVs. For example, the cyclo(CRGDKGPDC), an integrin-specific iRGD peptide, promoted the conjugation efficiency of integrin αvβ3 on the EV membrane [55]. Recently, Sato et al. [56] fabricated an engineered hybrid EV by membrane fusion with various liposomes. Functional studies have demonstrated that the liposome-mediated membrane-fusion approach facilitates the design of advanced engineered EVs to deliver exogenous hydrophobic compounds across the tissue barriers [57]. Lastly, a review published in 2022 [58] indicated that the metal-organic frameworks (MOF), a synthetic porous functional material with excellent biocompatibility, could be regarded as a promising alternative for disease management. The bioactive compound-loaded MOF encapsulated into EVs prompted the delivery of cargoes into the targeted recipient cells [35, 59]. Thus, these modification methods provide novel opportunities for the significant advancement of PDEVs as appropriate nanodelivery systems.
Modification techniques to facilitate PDEVs as the potential therapeutic nanocarriers. (A) Pharmacological particles directly encapsulated into the PDEVs. (B) Functional ligands effectively loaded onto the surfaces of EVs using a surface modification strategy. (C) Liposome-mediated membrane-fusion approach facilitating the design of advanced engineered PDEVs. (D) Bioactive agent-loaded MOF encapsulated into PDEVs, consequently prompting the targeted delivery of cargoes into recipient cells
Properties of PDEVs for cancer research and treatment
Over the last few decades, studies exploring novel strategies for improving therapeutic efficacy have been a dominant topic in clinical cancer research. Nowadays, EVs derived from plants have been demonstrated to participate in regulating different biological functions and broadening cancer treatment strategies with several benefits [47].
The EVs isolated from Kaempferia parviflora (KPEVs) had the attractive ability to antagonize tumorigenesis. The engineered KPEVs were stably absorbed into the gastric adenocarcinoma (AGS) cells, leading to the suppression of cell viability in a dose-dependent manner [60]. Other interesting findings were the identification of exosome-like nanotherapeutics from tea flowers [61] and tea leaves [62]. The cellular experiments indicated strong cytotoxicity effects of these exosome-like nanotherapeutics against breast cancer cells in vivo and in vitro. Administration of exosome-like nanotherapeutics significantly stimulated the mitochondrial damage and reactive oxygen species (ROS) amplification, consequently triggering the apoptotic effect in 4T1 cells. Similarly, Yang and colleagues confirmed the functional roles of lemon juice-derived EVs (LDEVs) on ROS generation in cancer cells [63]. Using an innovative method, the electrophoretic technique combined with a 300 kDa cut-off dialysis bag, Yang’s group successfully isolated the LDEVs, and found that LDEVs were effectively internalized into the gastric cancer cells (AGS, BGC-823, and SGC-7901), causing improved ROS concentration and induction of cell apoptosis. In addition, the paper on “kinase-targeted cancer therapies” reviewed various protein kinases driving cancer progression, such as protein kinase AKT and extracellular-signal-regulated kinase (ERK) [64]. To further confirm these points, Stanly et al. [65] evaluated the effects of grapefruit-derived EVs (GFDEVs) on the AKT-ERK signaling pathway. They found that the administration of GFDEVs remarkably downregulated the activation of the AKT-ERK axis in different cancer cell lines, consequently causing cell cycle arrest and cell apoptosis. Overall, the data presented here strongly support the biomedical toxicity of PDEVs on cancer cells.
In addition, PDEVs are highlighted as novel agents that can be used to overcome the defection of conventional anti-cancer therapy. The potential utilization of these bioactive PDEVs in combination with conventional methods can open a new gateway for the treatment of cancer patients. Some conventional anti-cancer methods, such as radiotherapy, frequently cause excessive ROS generation, resulting in oxidative stress and side-effects in cancer patients [66]. Administration of the bitter melon-derived EVs (BMDEVs) scavenged the elevated mitochondrial ROS and maintained mitochondrial homeostasis, dominantly preventing radiation-induced cardiomyocyte injury and myocardial fibrosis [67]. Moreover, these BMDEVs exerted synergistic anti-cancer effects of 5-fluorouracil against oral squamous cell carcinoma [68, 69]. In vitro and in vivo analyses further demonstrated the sensitizing effect of BMDEVs on human cancer therapy [70]. The above findings demonstrate the clinical potential of PDEV-based strategies to enhance therapeutic efficacy.
Although a detailed understanding of PDEVs is not present, their applications in cancer research and treatment have attracted a great deal of attention. Notably, the PDEVs display good safety and biocompatibility for future clinical applications [71, 72]. Several groups consistently revealed that PDEVs displayed remarkable killing effects on cancer cells without significantly affecting normal cell growth [73, 74]. This view was further confirmed by Özkan and colleagues [75], who proved that the dermal fibroblast cells from a healthy person could remain unaffected upon treatment with garlic-derived EVs. Subsequently, the experimental animal models showed no significant weight loss in the mice after oral treatment with PDEVs [49]. In summary, these unique characteristics make PDEV-based strategies a profound candidate against cancer pathogenesis.
Biomedical applications of PDEVs in anti-inflammatory response
Generally, the inflammatory response has been regarded as a phenomenon induced by imbalanced immune signaling. The improper control of this immune dysregulation can cause persistent inflammation, which may contribute to chronic or acute inflammatory diseases, threatening human health [76, 77]. To date, a series of experimental evidence has shown the great potential of PDEVs in the regulation of immune function and inflammatory responses in in vitro and in vivo models [78, 79]. The bioactive materials loaded on PDEVs, such as microRNAs, could be transferred into recipient cells and inhibit the SARS-CoV-2-induced inflammatory responses in the lung [80]. The EVs isolated from Petasites japonicus (PJ-EVs) showed increased activation of MAPK and NF-κB signaling, considerably inducing the maturation of murine dendritic cells and strengthening their antigen-presenting ability. Furthermore, treatment with PJ-EVs boosted the activation of Th1 T cells and cytotoxic T cells in inflammatory responses [81]. Another recent finding showed that nanovesicles derived from Pueraria lobata, an edible and medicinal herb, were well-taken up by mouse macrophages and facilitated M2 macrophage polarization. Through shifting M1 macrophages toward M2-like phenotypes, Pueraria lobata-derived EVs function as an attractive anti-inflammatory therapeutic biomaterial [82]. In addition, the roles of PDEVs in alleviating inflammatory-related diseases were directly demonstrated through the mulberry bark-derived EVs-mediated protection effect on intestinal epithelial cells in a mouse colitis model [83].
It is conceivable that the interaction between PDEVs and targeted cells can generate beneficial effects by mediating the secretion of multiple inflammatory factors. In an alcohol-induced chronic brain inflammation model, oat nanovesicles crossed the BBB and were preferentially taken up by the microglial cells. Oral administration of these oat nanovesicles decreased the secretion of pro-inflammatory cytokines, such as IL-6, IL-1β, and TNFα, from microglial cells and contributed to the prevention of ethanol-induced brain damage [84]. Similarly, the downregulated pro-inflammatory transcripts of IL-6, IL-1β, and COX-2 induced by pre-treatment with cabbage nanovesicles exhibited promising anti-inflammatory activities in human keratinocytes and fibroblasts [50]. In addition, PDEVs can be used to avoid inflammatory damage by promoting the upregulation of anti-inflammatory molecules, such as IL-9 and IL-10 [85]. The EVs from several fruits and vegetables, including grapes, grapefruit, ginger, and carrot, increased Wnt activation-mediated IL-10 secretion, providing beneficial effects for maintaining host cell homeostasis [45].
Oxidation-mediated inflammation has recently become an emerging research topic. Aging and other diseases are fundamentally caused by the imbalance between oxidation and anti-oxidation, which results in inflammatory injury and disease risks [86]. Using specific ELISA colorimetric assays, Logozzi’s group [87] identified high antioxidative content inside the PDEVs from fruit mixes, indicating their high level of antioxidant capacity. The strawberry-derived EVs were internalized by human mesenchymal stromal cells and prevented oxidative stress induced by hydrogen peroxide (H2O2) in a dose-dependent manner [88]. Similarly, the EVs from pomegranate juice showed an obvious effect to alleviate H2O2-induced oxidative damage [89]. The anti-inflammatory ability and health-promoting activity against oxidative stress of PDEVs have been proven to be mediated by up-regulating the expression of antioxidative molecules in the recipient cells, including nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase 1 (HO-1), and NAD(P)H quinone dehydrogenase 1 (NQO1) [90]. Through inhibiting the reduction of antioxidative molecules, PDEVs function as the prospective scavengers of free radicals, impairing their harmful cellular effects [91]. Taken together, this information may provide the basis for using PDEVs as biological antioxidant agents. In addition, it would be interesting to explore the underlying anti-oxidation mechanisms for more broad therapeutic applications in inflammatory diseases.
Current findings and importance of PDEVs for skin-based therapy
Due to their highlighted therapeutic properties, the roles of PDEVs in dermatological conditions are of intense interest and raise some concern. Accumulating evidence has described the applications of PDEVs in the treatment of dermal diseases, such as cutaneous lesions, skin regeneration, and skin cancer [92, 93]. Thus, it is important to consider PDEVs as novel biotechnological skin protectants for maintaining normal skin function.
Every year, millions of individuals suffer from mutilating scarring and serious wounds that take much time to cure and impose a high infection risk. PDEV-based signaling has been recently proven to participate in the wound healing progress [94], supporting their attractive properties to facilitate wound-closure induction and acceleration. Savci et al. [95] demonstrated PDEVs as the prospective cell-free biomaterials for wound healing. In human epidermal keratinocyte HaCaT cells, the administration of GFDEVs remarkably promoted cell viability and reduced cellular ROS generation in a dose-dependent manner [95]. Moreover, the tube formation capabilities of human umbilical vein endothelial cells (HUVEC) were significantly increased after treatment with Aloe saponaria-derived EVs (AS-EVs) [96], suggesting the effective pro-angiogenesis activity of AS-EVs within the wound healing. After the injury, the ginseng-derived EVs (G-EVs) functioned as nanoplatforms for effective delivery of plant microRNAs into bone marrow-derived mesenchymal stem cells (BMSCs), consequently facilitating the BMSC neural differentiation and neural restoration surrounding the wound sites. G-EVs were also shown to stimulate neovascularization by increasing angiogenic factors, such as vascular endothelial growth factor [97]. All these biological progresses are significant for tissue regeneration and wound healing.
In addition, because of a few side-effects and high skin penetration, the natural sources could function as an alternative to chemotherapeutic agents [98, 99]. The spectrophotometric and biochemical approaches indicated that G-EVs could act as the immunopotentiator for altering the polarization of M2 macrophages, finally improving the anti-tumor immune response in melanoma-bearing mice [100]. The EVs extracted from Dendropanax morbifera were uptaken by B16BL6 melanoma cells, resulting in reduced cellular melanin concentration and increased whitening effect by impairing tyrosinase-related signaling. Notably, treatment with Dendropanax morbifer-derived EVs induced nonsignificant cytotoxicity to healthy human skin tissues [101].
For better bio-therapeutic purposes, PDEVs can be engineered to contain specific pharmaceutical compounds or used for targeted transport by labeling with certain surface biomarkers [102, 103]. For example, in 2020, Yepes-Molina et al. [104] explored the biological potential of broccoli-derived EVs (BDEVs) as agent delivery nanoplatforms. The membrane vesicle-encapsulated fluorescent products were notably detected in keratinocyte skin cells, suggesting BDEVs as nanosized technology for the transdermal delivery of drugs. Furthermore, to enhance skin permeation of plasmid DNA (pDNA) into melanoma tissues, Niu’s group established the cell-penetrating peptide and cationic poly(ethyleneimine) conjugated pDNA-loaded PDEVs. They found that these functional peptide-conjugated PDEVs were highly efficient in facilitating transdermal pDNA delivery into skin melanomas [105].
PDEV-based therapy in clinical trials
In the last couple of decades, many studies have extended the understanding of the scientific community about the functional advantages of PDEVs, highlighting possible novel insights in biomedicine for the treatment of human diseases. In this regard, several clinical studies on the beneficial activities of PDEVs for clinical management have been currently registered in the ClinicalTrials.gov database [106] (Table 3). In particular, the properties of EVs isolated from many kinds of fruits and their ability as a delivery vehicle to increase the bioavailability of oral curcumin are being tested on human colon cancer patients (NCT01294072) [107]. In a subsequent study, Zhang et al. [108] aimed to evaluate the great prospects of GDEVs as natural anti-inflammatory agents for irritable bowel disease (NCT04879810). This trial concluded that GDEVs were likely reliable therapeutic nanoparticles for effectively preventing inflammatory bowel disease. Another completed trial confirmed the anti-inflammatory activity of grape-derived EVs (NCT01668849) [107]. Additionally, they proved the important roles of grape-derived EVs in preventing some side-effects induced by chemoradiation treatment in head and neck cancer patients, such as oral mucositis pain. Finally, an exploratory trial was designed to evaluate the effectiveness of EVs isolated from ginger or aloe plants in the treatment of patients with polycystic ovarian syndrome (NCT03493984); however, this meaningful study has not been approved so far. Therefore, considering the above-mentioned promising advantages [109, 110], the researchers should conduct more preclinical and clinical trials to determine the bioactivities of PDEVs in humans and define their minimum dosage in further studies.
Critical thinking and future outlooks
It is well established that PDEVs could act as important information conveyers between donor and recipient cells by transporting various bioactive cargoes, including proteins, nucleic acids, and other therapeutic agents. Although multiple omics techniques exploring their contents are increasing, the lack of specific biomarkers still results in the poor characterization of PDEVs and remains a major challenge [111]. The development of new computational algorithms to analyze the PDEV-associated omics data is a significant requirement. Recently, the characteristics of nanoparticles from plants have been annotated in FoodEVs from FAO/INFOODS database (https://www.fao.org/infoods/infoods/tables-and-databases/faoinfoods-databases/en/) [37]. Beyond doubt, the emerging studies on PDEVs have already revolutionized our knowledge of intercellular communication. Furthermore, PDEVs display the capacity to re-engineer themselves with specific biomarkers or compounds for precise therapy. However, there are still several unresolved concerns governing the extraction and function of PDEVs.
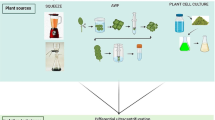
Currently, technological advancements have allowed researchers to isolate and characterize EVs. Several methods, such as filtration plus centrifugation, polymer-based precipitation, microfluidics technologies and immunoaffinity capture-based isolation, are frequently utilized for mammal-derived EVs but not for PDEVs [33, 103]. These techniques require different sample pre-processing procedures, and produce mammalian EVs of varying quality and purity. The EVs from cell culture media or body fluids are frequently subjected to multiple centrifugal procedures [112]. It’s worth noting that these centrifugation steps may be different depending on the experimental design, sample properties, and downstream analysis [113]. The polymer-based precipitation is a strategy employed to isolate EVs from the biofluids by altering their solubility. Using the commercial ExoQuick-TC kit, polymer-based precipitation could yield higher quantities of exosomal cargoes than the centrifugation [114]. Alternative method for isolation of mammalian EVs is a microfluidic exosome isolation and detection system (EXID system), which could incorporate the exosome capture and biomarker labelling on a microfluidic chip [115]. This integrated system enables one-stop capture, isolation and detection of exosomes from cancer cells and peripheral blood samples. However, the purification methods for PDEVs are still waiting to be fully tapped. To date, the most traditionally used techniques for PDEV extraction are the different centrifugal-based methods. In 2009, the Regente lab initially developed the vacuum infiltration-centrifugation procedure and applied this technology to successfully separate the sunflower seed-derived EVs [13]. Later, multiple research groups used differential ultracentrifugation to separate and purify EVs from plant tissues [116,117,118]. A recent review by Urzì O, Raimondo S, and Alessandro R succinctly summarized the representative steps of ultracentrifugal methods for PDEV extraction [111] (Fig. 4). In brief, after the tissues are manually squeezed by juicers, low-speed centrifugation at 500–3000 g for about 30 min was initially used to remove large particles and plant fibers. Next, intermediate-speed centrifugation, about 2000–10,000 g for 30 min, was used to remove the cell fragments and organelles. At last, high-speed centrifugation at 100,000–150,000 g for about 2 h was used to acquire the EV pellets. Although specialized equipment and much time are required, these ultracentrifugation-based strategies have been defined by different studies as the well-established gold standard for PDEV preparation [119]. In addition, some handy and rapid isolation methods have been established to facilitate future biochemical studies and downstream applications of PDEVs. The project of Jackson’s group was to evaluate a rapid hydrophobic interaction chromatography (HIC)-based capillary-channeled polymer (C-CP) tip isolation strategy for PDEV isolation. This HIC-based C-CP tip successfully obtains the desirable integrity and yield of PDEVs and is less time-consuming and low-cost [120]. Another efficient method for PDEV preparation, electrophoresis combined with a 300 kDa cut-off dialysis bag, has also been demonstrated to be time-saving [63]. Şahin et al. [121] utilized a commercial kit, Exo-spin™ Exosome Purification Kit, to isolate the homogenous and stable PDEVs from wheat grass juice. Although efficacious, most of these processes depend on specialized instruments or lack standard protocols, consequently representing a limitation for their clinical applications [106]. The method using polyethylene glycol (PEG) could effectively prevent Nicotiana tabacum-derived vesicles from forming the aggregates [122]. In the future, there is an urgent need to develop the standardization of qualitative and quantitative procedures to better achieve the successful commercialization of PDEVs in the field of translational medicine.
It should be noted that environmental conditions critically affect the stability and biological activities of PDEVs. Moreover, the intrinsic properties of isolated PDEVs are subject to change with some physical parameters, including pH values, temperature, and other processing factors [3, 15]. Under a simulated stomach condition, the PDEVs have the potential to resist gastric digestion and maintain high stability [60]. Unfortunately, at the moment, few studies have specifically investigated the effect of the environment on the structural integrity and bioactivity of PDEVs [109]. A recent study by Berger’s group showed that the altered morphology of PDEVs was found in the unpasteurized juice prepared by industrial processes. Furthermore, no PDEVs were detected in the concentrated orange juice [41]. By comparing the quantity and quality of PDEVs isolated from organic farms or conventional farms, Logozzi et al. [87] found that the former materials resulted in greater yield and higher anti-oxidant capacity. Thus, various environmental factors should be well considered for the plant sample collection and PDEV extraction.
The pathogen invasion generally induces infection and damages plant tissues. Phytoviruses are widely observed in animals, humans, as well as other environmental substances [123]. Independent scholars successfully confirmed the existence of phytovirus infection-associated molecules in the plant resource-derived nanovesicles [124, 125]. Moreover, they recognized several similar physical features between virus particles and PDEVs, such as particle size. In addition, plant viruses present in plant materials were frequently co-extracted with PDEVs [126], suggesting viral contamination in plant nanovesicle samples. Thus, it is imperative to develop novel platforms and strategies to remove virus particles from PDEV isolates and overcome virus contamination. Accordingly, with the help of the sucrose- or iodixanol-based density gradient ultracentrifugation technique (DGUC), Mammadova and colleagues effectively separated PDEVs from viral particles in tomato homogenate [126]. This has motivated the exploration of developing more promising technologies to purify PDEVs without possible virus contamination.
Conclusions
In conclusion, emerging evidence from preclinical and clinical studies has shed light on the biomedical application potential of natural PDEV-based strategies for pharmacogenetic discovery and validation. The molecular, biological, genetic, and pharmacological technologies suggest that natural and endogenic PDEVs have many attractive advantages, especially high stability, intrinsic bioactivity, and easy absorption by the recipient cells. Furthermore, numerous bioactive cargoes with pharmaceutical interest were demonstrated on PDEV isolates. As a promising drug delivery system, PDEVs can increase the sensitivity of drugs against numerous pathologies and concomitantly reduce their toxic side-effects. However, the underlying molecular mechanisms and biological factors driving the functions of PDEVs in diseases remain to be defined. The already-existing information offers fascinating and promising insights into the potential benefits of PDEVs for their medical translation in the future.
Data Availability
The data used to support this review are included within the article.
Abbreviations
- PDEVs:
-
Plant-derived extracellular vesicles
- EVs:
-
Extracellular vesicles
- BBB:
-
Blood-brain barrier
- GDEVs:
-
Ginger-derived EVs
- FA:
-
Folic acid
- MOF:
-
Metal-organic frameworks
- KPEVs:
-
EVs isolated from Kaempferia parviflora
- ROS:
-
Reactive oxygen species
- LDEVs:
-
Lemon juice-derived EVs
- ERK:
-
Extracellular-signal-regulated kinase
- GFDEVs:
-
Grapefruit-derived EVs
- BMDEVs:
-
Bitter melon-derived EVs
- PJ-EVs:
-
EVs isolated from Petasites japonicus
- H2O2 :
-
Hydrogen peroxide
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- HO-1:
-
Heme oxygenase 1
- NQO1:
-
NAD(P)H quinone dehydrogenase 1
- BMSCs:
-
Bone marrow-derived mesenchymal stem cells
- AS-EVs:
-
Aloe saponaria-derived EVs
- G-EVs:
-
Ginseng-derived EVs
- HUVEC:
-
Human umbilical vein endothelial cells
- BDEVs:
-
Broccoli-derived EVs
- pDNA:
-
Plasmid DNA
- HIC:
-
Hydrophobic interaction chromatography
- C-CP:
-
Capillary-channeled polymer
- DGUC:
-
Density gradient ultracentrifugation technique
References
Li D, Yao X, Yue J, Fang Y, Cao G, Midgley AC, et al. Advances in Bioactivity of MicroRNAs of Plant-Derived Exosome-Like Nanoparticles and milk-derived extracellular vesicles. J Agric Food Chem. 2022;70(21):6285–99.
Soleti R, Andriantsitohaina R, Martinez MC. Impact of polyphenols on extracellular vesicle levels and effects and their properties as tools for drug delivery for nutrition and health. Arch Biochem Biophys. 2018;644:57–63.
Kim K, Park J, Sohn Y, Oh CE, Park JH, Yuk JM, et al. Stability of Plant Leaf-Derived Extracellular Vesicles according to Preservative and Storage temperature. Pharmaceutics. 2022;14(2):457.
Garaeva L, Kamyshinsky R, Kil Y, Varfolomeeva E, Verlov N, Komarova E, et al. Delivery of functional exogenous proteins by plant-derived vesicles to human cells in vitro. Sci Rep. 2021;11(1):6489.
Aquilano K, Ceci V, Gismondi A, De Stefano S, Iacovelli F, Faraonio R, et al. Adipocyte metabolism is improved by TNF receptor-targeting small RNAs identified from dried nuts. Commun Biol. 2019;2:317.
Chen C, Wang J, Sun M, Li J, Wang HD. Toward the next-generation phyto-nanomedicines: cell-derived nanovesicles (CDNs) for natural product delivery. Volume 145. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie; 2022. p. 112416.
Kameli N, Dragojlovic-Kerkache A, Savelkoul P, Stassen FR. Plant-Derived Extracellular vesicles: current Findings, Challenges, and future applications. Membranes. 2021;11(6):411.
Ali NB, Abdull Razis AF, Ooi J, Chan KW, Ismail N, Foo JB. Theragnostic applications of Mammal and Plant-Derived Extracellular vesicles: latest findings, Current Technologies, and prospects. Molecules. 2022;27(12):3941.
Pocsfalvi G, Turiak L, Ambrosone A, Del Gaudio P, Puska G, Fiume I, et al. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J Plant Physiol. 2018;229:111–21.
Kim J, Li S, Zhang S, Wang J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J Pharm Sci. 2022;17(1):53–69.
Jensen WA. The composition and ultrastructure of the Nucellus in Cotton. J Ultrastruct Res. 1965;13:112–28.
Halperin W, Jensen WA. Ultrastructural changes during growth and embryogenesis in carrot cell cultures. J Ultrastruct Res. 1967;18(3):428–43.
Regente M, Corti-Monzon G, Maldonado AM, Pinedo M, Jorrin J, de la Canal L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 2009;583(20):3363–6.
Rutter BD, Innes RW. Extracellular vesicles isolated from the Leaf Apoplast carry stress-response proteins. Plant Physiol. 2017;173(1):728–41.
Kim SQ, Kim KH. Emergence of Edible Plant-Derived Nanovesicles as functional Food components and nanocarriers for therapeutics delivery: potentials in Human Health and Disease. Cells. 2022;11(14):2232.
Urzi O, Gasparro R, Ganji NR, Alessandro R, Raimondo S. Plant-RNA in Extracellular vesicles: the secret of Cross-Kingdom Communication. Membranes. 2022;12(4):352.
Roy M, Yang YP, Bosquet O, Deo SK, Daunert S. Understanding the role and clinical applications of Exosomes in Gynecologic Malignancies: a review of the current literature. Cancers. 2021;14(1):158.
Mai Z, Chen H, Ye Y, Hu Z, Sun W, Cui L, et al. Translational and clinical applications of Dental Stem Cell-Derived Exosomes. Front Genet. 2021;12:750990.
Du S, Ling H, Guo Z, Cao Q, Song C. Roles of exosomal miRNA in vascular aging. Pharmacol Res. 2021;165:105278.
Qu H, Fan C, Chen M, Zhang X, Yan Q, Wang Y, et al. Recent advances of fluorescent biosensors based on cyclic signal amplification technology in biomedical detection. J Nanobiotechnol. 2021;19(1):403.
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–20.
Huang C, Liu Z, Chen M, Du L, Liu C, Wang S, et al. Tumor-derived biomimetic nanozyme with immune evasion ability for synergistically enhanced low dose radiotherapy. J Nanobiotechnol. 2021;19(1):457.
Zhang H, Yang M, Wu X, Li Q, Li X, Zhao Y, et al. The distinct roles of exosomes in tumor-stroma crosstalk within gastric tumor microenvironment. Pharmacol Res. 2021;171:105785.
Xu Z, Zeng S, Gong Z, Yan Y. Exosome-based immunotherapy: a promising approach for cancer treatment. Mol Cancer. 2020;19(1):160.
Wang N, Li X, Zhong Z, Qiu Y, Liu S, Wu H, et al. 3D hESC exosomes enriched with mir-6766-3p ameliorates liver fibrosis by attenuating activated stellate cells through targeting the TGFbetaRII-SMADS pathway. J Nanobiotechnol. 2021;19(1):437.
Li L, Zhang L, Montgomery KC, Jiang L, Lyon CJ, Hu TY. Advanced technologies for molecular diagnosis of cancer: state of pre-clinical tumor-derived exosome liquid biopsies. Mater today Bio. 2023;18:100538.
Barreiro K, Lay AC, Leparc G, Tran VDT, Rosler M, Dayalan L, et al. An in vitro approach to understand contribution of kidney cells to human urinary extracellular vesicles. J Extracell vesicles. 2023;12(2):e12304.
Thakur A, Johnson A, Jacobs E, Zhang K, Chen J, Wei Z et al. Energy Sources for Exosome Communication in a Cancer Microenvironment.Cancers. 2022;14(7).
Zhang Y, Cai Z, Shen Y, Lu Q, Gao W, Zhong X, et al. Hydrogel-load exosomes derived from dendritic cells improve cardiac function via Treg cells and the polarization of macrophages following myocardial infarction. J Nanobiotechnol. 2021;19(1):271.
Chen R, Qian Z, Xu X, Zhang C, Niu Y, Wang Z, et al. Exosomes-transmitted miR-7 reverses gefitinib resistance by targeting YAP in non-small-cell lung cancer. Pharmacol Res. 2021;165:105442.
Cui J, Wang X, Li J, Zhu A, Du Y, Zeng W et al. Immune Exosomes Loading Self-Assembled Nanomicelles Traverse the Blood-Brain Barrier for Chemo-immunotherapy against Glioblastoma.ACS nano. 2023.
Li YJ, Wu JY, Liu J, Xu W, Qiu X, Huang S, et al. Artificial exosomes for translational nanomedicine. J Nanobiotechnol. 2021;19(1):242.
Cong M, Tan S, Li S, Gao L, Huang L, Zhang HG, et al. Technology insight: plant-derived vesicles-how far from the clinical biotherapeutics and therapeutic drug carriers? Adv Drug Deliv Rev. 2022;182:114108.
Zhang M, Viennois E, Xu C, Merlin D. Plant derived edible nanoparticles as a new therapeutic approach against diseases. Tissue Barriers. 2016;4(2):e1134415.
Karamanidou T, Tsouknidas A. Plant-derived extracellular vesicles as therapeutic nanocarriers. Int J Mol Sci. 2021;23(1):191.
Woith E, Guerriero G, Hausman JF, Renaut J, Leclercq CC, Weise C, et al. Plant Extracellular vesicles and nanovesicles: focus on secondary metabolites, proteins and lipids with perspectives on their potential and sources. Int J Mol Sci. 2021;22(7):3719.
Logozzi M, Di Raimo R, Mizzoni D, Fais S. The potentiality of plant-derived nanovesicles in Human Health-A comparison with human Exosomes and Artificial Nanoparticles. Int J Mol Sci. 2022;23(9):4919.
Liu Y, Wu S, Koo Y, Yang A, Dai Y, Khant H, et al. Characterization of and isolation methods for plant leaf nanovesicles and small extracellular vesicles. Nanomedicine. 2020;29:102271.
Brotherton HUS, Inal D. Communication is key: extracellular vesicles as mediators of infection and defence during host-microbe interactions in animals and plants. FEMS Microbiol Rev. 2022;46(1):fuab044.
Wang Y, Wang J, Ma J, Zhou Y, Lu R. Focusing on Future Applications and Current Challenges of Plant Derived Extracellular vesicles. Pharmaceuticals. 2022;15(6):708.
Berger E, Colosetti P, Jalabert A, Meugnier E, Wiklander OPB, Jouhet J, et al. Use of nanovesicles from Orange Juice to Reverse Diet-Induced gut modifications in Diet-Induced obese mice. Mol Ther Methods Clin Dev. 2020;18:880–92.
Xiao J, Feng S, Wang X, Long K, Luo Y, Wang Y, et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. PeerJ. 2018;6:e5186.
Kalarikkal SP, Sundaram GM. Edible plant-derived exosomal microRNAs: exploiting a cross-kingdom regulatory mechanism for targeting SARS-CoV-2. Toxicol Appl Pharmacol. 2021;414:115425.
Yang J, Hotz T, Broadnax L, Yarmarkovich M, Elbaz-Younes I, Hirschi KD. Anomalous uptake and circulatory characteristics of the plant-based small RNA MIR2911. Sci Rep. 2016;6:26834.
Mu J, Zhuang X, Wang Q, Jiang H, Deng ZB, Wang B, et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58(7):1561–73.
Rome S. Biological properties of plant-derived extracellular vesicles. Food Funct. 2019;10(2):529–38.
Raimondo S, Giavaresi G, Lorico A, Alessandro R. Extracellular vesicles as Biological Shuttles for targeted therapies. Int J Mol Sci. 2019;20(8):1848.
Man F, Meng C, Liu Y, Wang Y, Zhou Y, Ma J, et al. The study of ginger-derived extracellular vesicles as a natural Nanoscale drug carrier and their intestinal absorption in rats. AAPS PharmSciTech. 2021;22(6):206.
Li Z, Wang H, Yin H, Bennett C, Zhang HG, Guo P. Arrowtail RNA for ligand display on Ginger Exosome-like nanovesicles to systemic deliver siRNA for Cancer suppression. Sci Rep. 2018;8(1):14644.
You JY, Kang SJ, Rhee WJ. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact Mater. 2021;6(12):4321–32.
Nemati M, Singh B, Mir RA, Nemati M, Babaei A, Ahmadi M, et al. Plant-derived extracellular vesicles: a novel nanomedicine approach with advantages and challenges. Cell Commun Signal. 2022;20(1):69.
Wang B, Zhuang X, Deng ZB, Jiang H, Mu J, Wang Q, et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther. 2014;22(3):522–34.
Wang Q, Ren Y, Mu J, Egilmez NK, Zhuang X, Deng Z, et al. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory Tumor Sites. Cancer Res. 2015;75(12):2520–9.
Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137–49.
Liu Q, Dai G, Wu Y, Zhang M, Yang M, Wang X, et al. iRGD-modified exosomes-delivered BCL6 siRNA inhibit the progression of diffuse large B-cell lymphoma. Front Oncol. 2022;12:822805.
Sato YT, Umezaki K, Sawada S, Mukai SA, Sasaki Y, Harada N, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6:21933.
Lee J, Kim J, Jeong M, Lee H, Goh U, Kim H, et al. Liposome-based engineering of cells to package hydrophobic compounds in membrane vesicles for tumor penetration. Nano Lett. 2015;15(5):2938–44.
Gong W, Chen Z, Dong J, Liu Y, Cui Y. Chiral Metal-Organic Frameworks. Chem Rev. 2022;122(9):9078–144.
Illes B, Hirschle P, Barnert S, Cauda V, Wuttke S, Engelke H. Exosome-coated Metal–Organic Framework Nanoparticles: an efficient drug delivery platform. Chem Mater. 2017;29(19):8042–6.
Nemidkanam V, Chaichanawongsaroj N. Characterizing Kaempferia parviflora extracellular vesicles, a nanomedicine candidate. PLoS ONE. 2022;17(1):e0262884.
Chen Q, Li Q, Liang Y, Zu M, Chen N, Canup BSB, et al. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm Sin B. 2022;12(2):907–23.
Chen Q, Zu M, Gong H, Ma Y, Sun J, Ran S, et al. Tea leaf-derived exosome-like nanotherapeutics retard breast tumor growth by pro-apoptosis and microbiota modulation. J Nanobiotechnol. 2023;21(1):6.
Yang M, Liu X, Luo Q, Xu L, Chen F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J Nanobiotechnol. 2020;18(1):100.
Bhullar KS, Lagaron NO, McGowan EM, Parmar I, Jha A, Hubbard BP, et al. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol Cancer. 2018;17(1):48.
Stanly C, Alfieri M, Ambrosone A, Leone A, Fiume I, Pocsfalvi G. Grapefruit-Derived Micro and Nanovesicles Show distinct metabolome profiles and anticancer activities in the A375 Human Melanoma Cell line. Cells. 2020;9(12):2722.
Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12(12):931–47.
Cui WW, Ye C, Wang KX, Yang X, Zhu PY, Hu K, et al. Momordica. Charantia-derived extracellular vesicles-like nanovesicles protect Cardiomyocytes against Radiation Injury via attenuating DNA damage and Mitochondria Dysfunction. Front Cardiovasc Med. 2022;9:864188.
Tan ZL, Li JF, Luo HM, Liu YY, Jin Y. Plant extracellular vesicles: a novel bioactive nanoparticle for tumor therapy. Front Pharmacol. 2022;13:1006299.
Ly NP, Han HS, Kim M, Park JH, Choi KY. Plant-derived nanovesicles: current understanding and applications for cancer therapy. Bioact Mater. 2023;22:365–83.
Yang M, Luo Q, Chen X, Chen F. Bitter melon derived extracellular vesicles enhance the therapeutic effects and reduce the drug resistance of 5-fluorouracil on oral squamous cell carcinoma. J Nanobiotechnol. 2021;19(1):259.
Li DF, Yang MF, Xu HM, Zhu MZ, Zhang Y, Tian CM, et al. Nanoparticles for oral delivery: targeted therapy for inflammatory bowel disease. J Mater Chem B. 2022;10(31):5853–72.
Fang Z, Liu K. Plant-derived extracellular vesicles as oral drug delivery carriers. J Control Release. 2022;350:389–400.
Kim K, Yoo HJ, Jung JH, Lee R, Hyun JK, Park JH, et al. Cytotoxic Effects of Plant Sap-Derived Extracellular vesicles on various Tumor cell types. J Funct biomaterials. 2020;11(2):22.
Tajik T, Baghaei K, Moghadam VE, Farrokhi N, Salami SA. Extracellular vesicles of cannabis with high CBD content induce anticancer signaling in human hepatocellular carcinoma. Volume 152. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie; 2022. p. 113209.
Ozkan I, Kocak P, Yildirim M, Unsal N, Yilmaz H, Telci D, et al. Garlic (Allium sativum)-derived SEVs inhibit cancer cell proliferation and induce caspase mediated apoptosis. Sci Rep. 2021;11(1):14773.
Giraud J, Saleh M. Host-microbiota interactions in liver inflammation and Cancer. Cancers. 2021;13(17):4342.
Almasabi S, Ahmed AU, Boyd R, Williams BRG. A potential role for integrin-linked kinase in Colorectal Cancer Growth and Progression via regulating senescence and immunity. Front Genet. 2021;12:638558.
Zhang Z, Yu Y, Zhu G, Zeng L, Xu S, Cheng H, et al. The emerging role of plant-derived Exosomes-Like Nanoparticles in Immune Regulation and Periodontitis Treatment. Front Immunol. 2022;13:896745.
Kim WS, Ha JH, Jeong SH, Lee JI, Lee BW, Jeong YJ, et al. Immunological Effects of Aster yomena Callus-Derived Extracellular vesicles as potential therapeutic agents against allergic asthma. Cells. 2022;11:18.
Teng Y, Xu F, Zhang X, Mu J, Sayed M, Hu X, et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol Ther. 2021;29(8):2424–40.
Han JM, Song HY, Lim ST, Kim KI, Seo HS, Byun EB. Immunostimulatory potential of Extracellular vesicles isolated from an Edible Plant, Petasites japonicus, via the induction of murine dendritic cell maturation. Int J Mol Sci. 2021;22(19):10634.
Wu J, Ma X, Lu Y, Zhang T, Du Z, Xu J, et al. Edible Pueraria lobata-derived Exosomes promote M2 macrophage polarization. Molecules. 2022;27(23):8184.
Sriwastva MK, Deng ZB, Wang B, Teng Y, Kumar A, Sundaram K, et al. Exosome-like nanoparticles from Mulberry bark prevent DSS-induced colitis via the AhR/COPS8 pathway. EMBO Rep. 2022;23(3):e53365.
Xu F, Mu J, Teng Y, Zhang X, Sundaram K, Sriwastva MK, et al. Restoring oat nanoparticles mediated brain memory function of mice Fed Alcohol by sorting inflammatory Dectin-1 Complex into Microglial Exosomes. Small. 2022;18(6):e2105385.
Raimondo S, Urzi O, Meraviglia S, Di Simone M, Corsale AM, Rabienezhad Ganji N, et al. Anti-inflammatory properties of lemon-derived extracellular vesicles are achieved through the inhibition of ERK/NF-kappaB signalling pathways. J Cell Mol Med. 2022;26(15):4195–209.
Raimondo S, Nikolic D, Conigliaro A, Giavaresi G, Lo Sasso B, Giglio RV, et al. Preliminary results of CitraVes Effects on low density lipoprotein cholesterol and Waist circumference in healthy subjects after 12 weeks: a pilot open-label study. Metabolites. 2021;11(5):276.
Logozzi M, Di Raimo R, Mizzoni D, Fais S. Nanovesicles from Organic Agriculture-Derived fruits and vegetables: characterization and functional antioxidant content. Int J Mol Sci. 2021;22(15):8170.
Perut F, Roncuzzi L, Avnet S, Massa A, Zini N, Sabbadini S, et al. Strawberry-Derived Exosome-Like Nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules. 2021;11(1):87.
Sanchez-Lopez CM, Manzaneque-Lopez MC, Perez-Bermudez P, Soler C, Marcilla A. Characterization and bioactivity of extracellular vesicles isolated from pomegranate. Food Funct. 2022;13(24):12870–82.
Kim DK, Rhee WJ. Antioxidative Effects of Carrot-Derived Nanovesicles in Cardiomyoblast and Neuroblastoma cells. Pharmaceutics. 2021;13(8):1203.
Mahdipour E. Beta vulgaris juice contains biologically active exosome-like nanoparticles. Tissue Cell. 2022;76:101800.
Xu Z, Peng B, Liang Q, Chen X, Cai Y, Zeng S, et al. Construction of a ferroptosis-related Nine-lncRNA signature for Predicting Prognosis and Immune Response in Hepatocellular Carcinoma. Front Immunol. 2021;12:719175.
Ganesan P, Choi DK. Current application of phytocompound-based nanocosmeceuticals for beauty and skin therapy. Int J Nanomedicine. 2016;11:1987–2007.
Zhou X, Brown BA, Siegel AP, El Masry MS, Zeng X, Song W, et al. Exosome-mediated crosstalk between Keratinocytes and Macrophages in Cutaneous Wound Healing. ACS Nano. 2020;14(10):12732–48.
Savci Y, Kirbas OK, Bozkurt BT, Abdik EA, Tasli PN, Sahin F, et al. Grapefruit-derived extracellular vesicles as a promising cell-free therapeutic tool for wound healing. Food Funct. 2021;12(11):5144–56.
Kim M, Park JH. Isolation of Aloe saponaria-Derived Extracellular Vesicles and Investigation of Their Potential for Chronic Wound Healing.Pharmaceutics. 2022;14(9).
Xu XH, Yuan TJ, Dad HA, Shi MY, Huang YY, Jiang ZH, et al. Plant exosomes as novel nanoplatforms for MicroRNA transfer stimulate neural differentiation of stem cells in Vitro and in vivo. Nano Lett. 2021;21(19):8151–9.
AlQathama A, Prieto JM. Natural products with therapeutic potential in melanoma metastasis. Nat Prod Rep. 2015;32(8):1170–82.
Danciu C, Soica C, Antal D, Alexa E, Pavel IZ, Ghiulai R, et al. Natural Compounds in the Chemoprevention of Malignant Melanoma. Anticancer Agents Med Chem. 2018;18(5):631–44.
Cao M, Yan H, Han X, Weng L, Wei Q, Sun X, et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J Immunother Cancer. 2019;7(1):326.
Lee R, Ko HJ, Kim K, Sohn Y, Min SY, Kim JA, et al. Anti-melanogenic effects of extracellular vesicles derived from plant leaves and stems in mouse melanoma cells and human healthy skin. J Extracell Vesicles. 2020;9(1):1703480.
Narauskaite D, Vydmantaite G, Rusteikaite J, Sampath R, Rudaityte A, Stasyte G, et al. Extracellular vesicles in skin Wound Healing. Pharmaceuticals. 2021;14(8):811.
Dad HA, Gu TW, Zhu AQ, Huang LQ, Peng LH. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol therapy: J Am Soc Gene Therapy. 2021;29(1):13–31.
Yepes-Molina L, Martinez-Ballesta MC, Carvajal M. Plant plasma membrane vesicles interaction with keratinocytes reveals their potential as carriers. J Adv Res. 2020;23:101–11.
Niu J, Chu Y, Huang YF, Chong YS, Jiang ZH, Mao ZW, et al. Transdermal gene delivery by functional peptide-conjugated Cationic Gold Nanoparticle reverses the progression and metastasis of cutaneous melanoma. ACS Appl Mater Interfaces. 2017;9(11):9388–401.
Alfieri M, Leone A, Ambrosone A. Plant-Derived Nano and Microvesicles for Human Health and therapeutic potential in Nanomedicine. Pharmaceutics. 2021;13(4):498.
Wu K, Xing F, Wu SY, Watabe K. Extracellular vesicles as emerging targets in cancer: recent development from bench to bedside. Biochim Biophys Acta Rev Cancer. 2017;1868(2):538–63.
Zhang M, Viennois E, Prasad M, Zhang Y, Wang L, Zhang Z, et al. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–40.
Suharta S, Barlian A, Hidajah AC, Notobroto HB, Ana ID, Indariani S, et al. Plant-derived exosome-like nanoparticles: a concise review on its extraction methods, content, bioactivities, and potential as functional food ingredient. J Food Sci. 2021;86(7):2838–50.
Chen YS, Lin EY, Chiou TW, Harn HJ. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Ci Ji Yi Xue Za Zhi. 2020;32(2):113–20.
Urzi O, Raimondo S, Alessandro R. Extracellular vesicles from plants: current knowledge and open questions. Int J Mol Sci. 2021;22(10):5366.
Xu WM, Li A, Chen JJ, Sun EJ. Research Development on exosome separation technology. J Membr Biol. 2023;256(1):25–34.
Martins TS, Vaz M, Henriques AG. A review on comparative studies addressing exosome isolation methods from body fluids. Anal Bioanal Chem. 2023;415(7):1239–63.
Gao M, Cai J, Zitkovsky HS, Chen B, Guo L. Comparison of yield, purity, and Functional Properties of large-volume exosome isolation using Ultrafiltration and Polymer-Based precipitation. Plast Reconstr Surg. 2022;149(3):638–49.
Lu Y, Ye L, Jian X, Yang D, Zhang H, Tong Z, et al. Integrated microfluidic system for isolating exosome and analyzing protein marker PD-L1. Biosens Bioelectron. 2022;204:113879.
Baldini N, Torreggiani E, Roncuzzi L, Perut F, Zini N, Avnet S. Exosome-like nanovesicles isolated from Citrus limon L. Exert Antioxidative Effect. Curr Pharm Biotechnol. 2018;19(11):877–85.
Stanly C, Fiume I, Capasso G, Pocsfalvi G. Isolation of Exosome-Like vesicles from plants by Ultracentrifugation on Sucrose/Deuterium oxide (D2O) density cushions. Methods Mol Biol. 2016;1459:259–69.
Zhang W, Yuan Y, Li X, Luo J, Zhou Z, Yu L, et al. Orange-derived and dexamethasone-encapsulated extracellular vesicles reduced proteinuria and alleviated pathological lesions in IgA nephropathy by targeting intestinal lymphocytes. Front Immunol. 2022;13:900963.
Subudhi PD, Bihari C, Sarin SK, Baweja S. Emerging role of Edible Exosomes-Like Nanoparticles (ELNs) as Hepatoprotective Agents. Nanotheranostics. 2022;6(4):365–75.
Jackson KK, Mata C, Marcus RK. A rapid capillary-channeled polymer (C-CP) fiber spin-down tip approach for the isolation of plant-derived extracellular vesicles (PDEVs) from 20 common fruit and vegetable sources. Talanta. 2023;252:123779.
Sahin F, Kocak P, Gunes MY, Ozkan I, Yildirim E, Kala EY. In Vitro Wound Healing activity of wheat-derived nanovesicles. Appl Biochem Biotechnol. 2019;188(2):381–94.
Kocholata M, Prusova M, Auer Malinska H, Maly J, Janouskova O. Comparison of two isolation methods of tobacco-derived extracellular vesicles, their characterization and uptake by plant and rat cells. Sci Rep. 2022;12(1):19896.
Kim JS, Yoon SJ, Park YJ, Kim SY, Ryu CM. Crossing the kingdom border: human diseases caused by plant pathogens. Environ Microbiol. 2020;22(7):2485–95.
Stanly C, Moubarak M, Fiume I, Turiak L, Pocsfalvi G. Membrane transporters in Citrus clementina Fruit Juice-Derived nanovesicles. Int J Mol Sci. 2019;20(24):6205.
Quesenberry PJ, Aliotta J, Camussi G, Abdel-Mageed AB, Wen S, Goldberg L, et al. Potential functional applications of extracellular vesicles: a report by the NIH Common Fund Extracellular RNA Communication Consortium. J Extracell vesicles. 2015;4:27575.
Mammadova R, Fiume I, Bokka R, Kralj-Iglic V, Bozic D, Kisovec M, et al. Identification of Tomato infecting viruses that co-isolate with nanovesicles using a combined Proteomics and Electron-Microscopic Approach. Nanomaterials. 2021;11(8):1922.
Acknowledgements
Not applicable.
Funding
This study is supported by grants from the Science and Technology Innovation Program of Hunan Province (2022RC1210, 2021RC3029), the National Natural Science Foundation of China (82272659) and the China Postdoctoral Science Foundation (2021T140754, 2020M672521).
Author information
Authors and Affiliations
Contributions
YY and KZ: conception and design. YL, QL, AT and WL: data curation. ZX and YX: writing the manuscript and revision of the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that there are no conflicts of interest.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, Z., Xu, Y., Zhang, K. et al. Plant-derived extracellular vesicles (PDEVs) in nanomedicine for human disease and therapeutic modalities. J Nanobiotechnol 21, 114 (2023). https://doi.org/10.1186/s12951-023-01858-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-023-01858-7