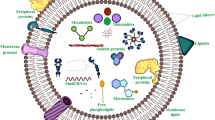
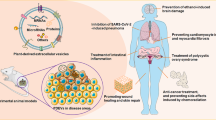
Abstract
Small extracellular vesicles (sEVs) isolated from animal sources are among the most investigated types of cell-free therapeutic tools to cure different diseases. sEVs have been isolated from a variety of sources, ranging from prokaryotes to animals and plants. Human-derived sEVs have many uses in pre- and clinical studies in medicine and drug delivery, while plant-derived EVs, also known as plant-derived nanovesicles (PDNVs), have not been widely investigated until the second decade of the 21st century. For the past five years, there has been a rapid rise in the use of plant EVs as a therapeutic tool due to the ease of massive production with high efficacy and yield of preparation. Plant EVs contain various active biomolecules such as proteins, regulatory RNAs, and secondary metabolites and play a key role in inter-kingdom communications. Many studies have already investigated the potential application of plant EVs in preventing and treating cancer, inflammation, infectious diseases, and tissue regeneration with no sign of toxicity and are therefore considered safe. However, due to a lack of universal markers, the properties of plant EVs have not been extensively studied. Concerns regarding the safety and therapeutic function of plant EVs derived from genetically modified plants have been raised. In this paper, we review the physiological role of EVs in plants. Moreover, we focus on molecular and cellular mechanisms involved in the therapeutic effects of plant EVs on various human diseases. We also provide detailed information on the methodological aspects of plant EV isolation and analysis, which could pave the way for future clinical translation.



Similar content being viewed by others
Data availability
No Data associated in the manuscript.
Abbreviations
- Small extracellular vesicle:
-
sEV
- Plant-derived nanovesicle:
-
PDNV
- Ginger-derived nanovesicle:
-
GDNV
- Citrus-derived nanovesicle:
-
CDNV
- Multivesicular bodies:
-
MVB
- Intraluminal vesicle:
-
ILV
- Tetraspanin:
-
TET
- Dextran sulfate sodium:
-
DSS
- Cabbage-derived nanovesicle:
-
CaDNV
- Strawberry-derived nanovesicle:
-
SDNV
- Reactive oxygen species:
-
ROS
- Myocardial infarction:
-
MI
- Broccoli-derived nanovesicle:
-
BDNV
- Tea leaf-derived nanovesicle:
-
TDNV
- Apple-derived nanovesicle:
-
ADNV
- 5-FU:
-
5-fluorouracil
- OSCC:
-
oral squamous cell carcinoma
- BmDNV:
-
Bitter melon-derived nanovesicles
- Grapefruit-derived nanovesicle:
-
GrDNV
- Intravenous:
-
IV
- Wheat-derived nanovesicles:
-
WDNV
References
Witkamp RF, van Norren K (2018) Let thy food be thy medicine… when possible. Eur J Pharmacol. https://doi.org/10.1016/j.ejphar.2018.06.026. 836102-14
Downer S, Berkowitz SA, Harlan TS, Olstad DL, Mozaffarian D (2020) Food is medicine: actions to integrate food and nutrition into healthcare. BMJ. https://doi.org/10.1136/bmj.m2482. 369m2482
Clem J, Barthel B (2021) A look at Plant-based diets. Mo Med. 118233–118238
Ackova DG, Smilkov K, Bosnakovski D (2019) Contemporary formulations for drug delivery of Anticancer Bioactive compounds. Recent Pat Anticancer Drug Discov. 1419–1431. https://doi.org/10.2174/1574892814666190111104834
Akao Y, Kuranaga Y, Heishima K, Sugito N, Morikawa K, Ito Y et al (2022) Plant hvu-MIR168-3p enhances expression of glucose transporter 1 (SLC2A1) in human cells by silencing genes related to mitochondrial electron transport chain complex I. J Nutr Biochem 101108922. https://doi.org/10.1016/j.jnutbio.2021.108922
Spinler JK, Karri V, Hirschi KD (2019) Planting the Microbiome. Trends Microbiol 2790–2793. https://doi.org/10.1016/j.tim.2018.12.001
Díez-Sainz E, Lorente-Cebrián S, Aranaz P, Riezu-Boj JI, Martínez JA, Milagro FI (2021) Potential mechanisms linking food-derived MicroRNAs, Gut Microbiota and Intestinal Barrier functions in the Context of Nutrition and Human Health. Front Nutr 8586564. https://doi.org/10.3389/fnut.2021.586564
Bielska E, Birch PRJ, Buck AH, Abreu-Goodger C, Innes RW, Jin H et al (2019) Highlights of the mini-symposium on extracellular vesicles in inter-organismal communication, held in Munich, Germany, August 2018. J Extracell Vesicles 81590116. https://doi.org/10.1080/20013078.2019.1590116
Christenhusz MM, Byng JW (2016) The number of known plants species in the world and its annual increase Phytotaxa. 26110.11646.
Bar-On YM, Phillips R, Milo R (2018) The biomass distribution on Earth. Proceedings of the National Academy of Sciences. 1156506-11. https://doi.org/10.1073/pnas.1711842115
Regente M, Corti-Monzón G, Maldonado AM, Pinedo M, Jorrín J, de la Canal L (2009) Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett 5833363–5833366. https://doi.org/10.1016/j.febslet.2009.09.041
Zhang M, Viennois E, Prasad M, Zhang Y, Wang L, Zhang Z et al (2016) Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 101321–101340. https://doi.org/10.1016/j.biomaterials.2016.06.018
Wang B, Zhuang X, Deng ZB, Jiang H, Mu J, Wang Q et al (2014) Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther 22522–22534. https://doi.org/10.1038/mt.2013.190
Li Z, Wang H, Yin H, Bennett C, Zhang HG, Guo P (2018) Arrowtail RNA for Ligand Display on Ginger Exosome-like nanovesicles to systemic deliver siRNA for Cancer suppression. Sci Rep 814644. https://doi.org/10.1038/s41598-018-32953-7
Dad HA, Gu T-W, Zhu A-Q, Huang L-Q, Peng L-H (2021) Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Molecular therapy: the journal of the American Society of Gene Therapy. 2913–2931. https://doi.org/10.1016/j.ymthe.2020.11.030
Teng Y, Ren Y, Sayed M, Hu X, Lei C, Kumar A et al (2018) Plant-derived exosomal MicroRNAs shape the gut microbiota. Cell Host Microbe 24637–52e8. https://doi.org/10.1016/j.chom.2018.10.001
Soleti R, Andriantsitohaina R, Martinez MC (2018) Impact of polyphenols on extracellular vesicle levels and effects and their properties as tools for drug delivery for nutrition and health. Arch Biochem Biophys 64457–64463. https://doi.org/10.1016/j.abb.2018.03.004
Ito Y, Taniguchi K, Kuranaga Y, Eid N, Inomata Y, Lee SW et al (2021) Uptake of MicroRNAs from Exosome-Like nanovesicles of Edible Plant Juice by Rat Enterocytes. Int J Mol Sci 22. https://doi.org/10.3390/ijms22073749
Rutter BD, Innes RW (2018) Extracellular vesicles as key mediators of plant–microbe interactions. Curr Opin Plant Biol 4416–4422. https://doi.org/10.1016/j.pbi.2018.01.008
Cui Y, Gao J, He Y, Jiang L (2019) Plant extracellular vesicles. https://doi.org/10.1007/s00709-019-01435-6. Protoplasma
Wang Q, Zhuang X, Mu J, Deng Z-B, Jiang H, Zhang L et al (2013) Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun 41867. https://doi.org/10.1038/ncomms2886
You JY, Kang SJ, Rhee WJ (2021) Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact Mater 64321–64332. https://doi.org/10.1016/j.bioactmat.2021.04.023
An Q, Huckelhoven R, Kogel KH, van Bel AJ (2006) Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell Microbiol 81009–81019. https://doi.org/10.1111/j.1462-5822.2006.00683.x
Pérez-Bermúdez P, Blesa J, Soriano JM, Marcilla A (2017) Extracellular vesicles in food: experimental evidence of their secretion in grape fruits. Eur J Pharm Sci 9840–9850. https://doi.org/10.1016/j.ejps.2016.09.022
Nieuwland R, Siljander PRM, Falcón-Pérez JM, Witwer KW (2022) Reproducibility of extracellular vesicle research. Eur J Cell Biol 101151226. https://doi.org/10.1016/j.ejcb.2022.151226
Pinedo M, de la Canal L, de Marcos Lousa C (2021) A call for Rigor and standardization in plant extracellular vesicle research. J Extracell Vesicles. https://doi.org/10.1002/jev2.12048. 10e12048
Woith E, Guerriero G, Hausman JF, Renaut J, Leclercq CC, Weise C et al (2021) Plant Extracellular vesicles and nanovesicles: focus on secondary metabolites, proteins and lipids with perspectives on their potential and sources. Int J Mol Sci 22. https://doi.org/10.3390/ijms22073719
Rutter BD, Innes RW (2020) Growing pains: addressing the pitfalls of plant extracellular vesicle research. New Phytol. 2281505–2281510
Kim DK, Rhee WJ (2021) Antioxidative effects of Carrot-Derived Nanovesicles in Cardiomyoblast and Neuroblastoma cells. Pharmaceutics 13. https://doi.org/10.3390/pharmaceutics13081203
Raimondo S, Naselli F, Fontana S, Monteleone F, Lo Dico A, Saieva L et al (2015) Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 619514–619527. https://doi.org/10.18632/oncotarget.4004
Teng Y, Xu F, Zhang X, Mu J, Sayed M, Hu X et al (2021) Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol Ther 292424–292440. https://doi.org/10.1016/j.ymthe.2021.05.005
Huang Y, Wang S, Cai Q, Jin H (2021) Methodological guidelines for isolation and purification of plant extracellular vesicles. bioRxiv 20210901458648. https://doi.org/10.1101/2021.09.01.458648
Woith E, Melzig MF (2019) Extracellular vesicles from fresh and dried plants-simultaneous purification and visualization using gel electrophoresis. Int J Mol Sci 20357. https://doi.org/10.3390/ijms20020357
Yang M, Liu X, Luo Q, Xu L, Chen F (2020) An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J Nanobiotechnol 18100. https://doi.org/10.1186/s12951-020-00656-9
He B, Cai Q, Qiao L, Huang CY, Wang S, Miao W et al (2021) RNA-binding proteins contribute to small RNA loading in plant extracellular vesicles. Nat Plants 7342–7352. https://doi.org/10.1038/s41477-021-00863-8
Liu G, Kang G, Wang S, Huang Y, Cai Q (2021) Extracellular vesicles: emerging players in Plant Defense against pathogens. Front Plant Sci 12. https://doi.org/10.3389/fpls.2021.757925
Doyle LM, Wang MZ (2019) Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 8. https://doi.org/10.3390/cells8070727
Tse YC, Mo B, Hillmer S, Zhao M, Lo SW, Robinson DG et al (2004) Identification of Multivesicular bodies as Prevacuolar compartments in Nicotiana tabacum BY-2 Cells[W]. Plant Cell 16672–16693. https://doi.org/10.1105/tpc.019703
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R et al (2018) a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 71535750. https://doi.org/10.1080/20013078.2018.1535750. Minimal information for studies of extracellular vesicles 2018 (MISEV2018
Alghuthaymi MA, Ahmad A, Khan Z, Khan SH, Ahmed FK, Faiz S et al (2021) Exosome/Liposome-like nanoparticles: New Carriers for CRISPR Genome editing in plants. Int J Mol Sci 22. https://doi.org/10.3390/ijms22147456
Regente M, Pinedo M, San Clemente H, Balliau T, Jamet E, de la Canal L (2017) Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J Exp Bot 685485–685495. https://doi.org/10.1093/jxb/erx355
Rutter BD, Innes RW (2017) Extracellular vesicles isolated from the Leaf Apoplast carry stress-response proteins. Plant Physiol 173728–173741. https://doi.org/10.1104/pp.16.01253
De Palma M, Ambrosone A, Leone A, Del Gaudio P, Ruocco M, Turiák L et al (2020) Plant roots release small extracellular vesicles with antifungal activity. Plants 91777
Bayat F, Afshar A, Baghban N (2021) Algal cells-derived extracellular vesicles: a review with special emphasis on their Antimicrobial effects. Front Microbiol 12785716. https://doi.org/10.3389/fmicb.2021.785716
Ciencia Dd I, Spain, (ed) Nanovesicles Are Secreted during Pollen Germination and Pollen Tube Growth: A Possible Role in Fertilization2014
Meyer D, Pajonk S, Micali C, O’Connell R, Schulze-Lefert P (2009) Extracellular transport and integration of plant secretory proteins into pathogen-induced cell wall compartments. Plant J 57986–57999. https://doi.org/10.1111/j.1365-313X.2008.03743.x
Cai Q, He B, Wang S, Fletcher S, Niu D, Mitter N et al (2021) Message in a bubble: shuttling small RNAs and proteins between cells and interacting organisms using Extracellular vesicles. Annu Rev Plant Biol 72497–72524. https://doi.org/10.1146/annurev-arplant-081720-010616
van der Grein SG, Defourny KAY, Rabouw HH, Goerdayal SS, van Herwijnen MJC, Wubbolts RW et al (2022) The encephalomyocarditis virus leader promotes the release of virions inside extracellular vesicles via the induction of secretory autophagy. Nat Commun 133625. https://doi.org/10.1038/s41467-022-31181-y
Alshehri B (2021) Plant-derived xenomiRs and cancer: cross-kingdom gene regulation. Saudi J Biol Sci 282408–282422. https://doi.org/10.1016/j.sjbs.2021.01.039
Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C et al (2019) Chronic inflammation in the etiology of disease across the life span. Nat Med 251822–251832. https://doi.org/10.1038/s41591-019-0675-0
Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB (2019) Inflammation and cancer. Ann Afr Med. https://doi.org/10.4103/aam.aam_56_18. 18121-6
Mu J, Zhuang X, Wang Q, Jiang H, Deng ZB, Wang B et al (2014) Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res 581561–581573. https://doi.org/10.1002/mnfr.201300729
Deng Z, Rong Y, Teng Y, Mu J, Zhuang X, Tseng M et al (2017) Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-Activated protein kinase. Mol Ther 251641–251654. https://doi.org/10.1016/j.ymthe.2017.01.025
Ju S, Mu J, Dokland T, Zhuang X, Wang Q, Jiang H et al (2013) Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-Induced Colitis. Mol Ther 211345–211357. https://doi.org/10.1038/mt.2013.64
Keir ME, Yi T, Lu TT, Ghilardi N (2020) The role of IL-22 in intestinal health and disease. J Exp Med 217. https://doi.org/10.1084/jem.20192195
Yan J, Yu J, Liu K, Liu Y, Mao C, Gao W (2021) The pathogenic roles of IL-22 in colitis: its transcription regulation by Musculin in T Helper subsets and Innate Lymphoid cells. Front Immunol 12. https://doi.org/10.3389/fimmu.2021.758730
Sriwastva MK, Deng ZB, Wang B, Teng Y, Kumar A, Sundaram K et al (2022) Exosome-like nanoparticles from Mulberry bark prevent DSS-induced colitis via the AhR/COPS8 pathway. EMBO Rep 23e53365. https://doi.org/10.15252/embr.202153365
Canup BS, Stetler JL, Laroui H (2016) Sa1671 targeting liver inflammation caused by Palmitic Acid Intracellular Accumulation via Plant secreted exosomes. Gastroenterology. https://doi.org/10.1016/S0016-5085(16)33685-X. 150S1091
Canup BS, Stetler JL, Qiang M, Puckett RP, Laroui H (2015) Sa1678 targeting liver cells inflammation via Plant secreted exosomes. Gastroenterology. https://doi.org/10.1016/S0016-5085(15)33446-6. 148S-1009
Raimondo S, Urzì O, Meraviglia S, Di Simone M, Corsale AM, Rabienezhad Ganji N et al (2022) Anti-inflammatory properties of lemon-derived extracellular vesicles are achieved through the inhibition of ERK/NF-κB signalling pathways. J Cell Mol Med 264195–264209. https://doi.org/10.1111/jcmm.17404
Urzì O, Cafora M, Ganji NR, Tinnirello V, Gasparro R, Raccosta S et al (2023) Lemon-derived nanovesicles achieve antioxidant and anti-inflammatory effects activating the AhR/Nrf2 signaling pathway. iScience. 26. https://doi.org/10.1016/j.isci.2023.107041
Perut F, Roncuzzi L, Avnet S, Massa A, Zini N, Sabbadini S et al (2021) Strawberry-Derived Exosome-Like nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules 11. https://doi.org/10.3390/biom11010087
Bugger H, Pfeil K (2020) Mitochondrial ROS in myocardial ischemia reperfusion and remodeling. Biochim Biophys Acta Mol Basis Dis 1866165768. https://doi.org/10.1016/j.bbadis.2020.165768
Ebadi B, Naderi N, Darbandi Azar A, Damirchi A (2021) Interval intensity Exercise improves the levels of Mitophagy-related proteins and ROS in rats with myocardial infarction. J Exerc Health Sci. https://doi.org/10.22089/jehs.2021.9615.1012. 119 – 34
Yamasaki M, Yamasaki Y, Furusho R, Kimura H, Kamei I, Sonoda H et al (2021) Onion (Allium cepa L.)-Derived nanoparticles inhibited LPS-Induced Nitrate Production, however, their intracellular incorporation by endocytosis was not involved in this effect on RAW264 cells. Molecules 26. https://doi.org/10.3390/molecules26092763
Wang Q, Ren Y, Mu J, Egilmez NK, Zhuang X, Deng Z et al (2015) Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory Tumor sites. Cancer Res 752520–752529. https://doi.org/10.1158/0008-5472.Can-14-3095
Chen X, Ji S, Yan Y, Lin S, He L, Huang X et al (2023) Engineered Plant-Derived Nanovesicles facilitate Tumor Therapy: natural bioactivity plus drug controlled release platform. Int J Nanomed 18:4779–4804. https://doi.org/10.2147/IJN.S413831
Chen Q, Zu M, Gong H, Ma Y, Sun J, Ran S et al (2023) Tea leaf-derived exosome-like nanotherapeutics retard breast tumor growth by pro-apoptosis and microbiota modulation. J Nanobiotechnol 216. https://doi.org/10.1186/s12951-022-01755-5
Sasaki D, Kusamori K, Takayama Y, Itakura S, Todo H, Nishikawa M (2021) Development of nanoparticles derived from corn as mass producible bionanoparticles with anticancer activity. Sci Rep 1122818. https://doi.org/10.1038/s41598-021-02241-y
Raimondo S, Saieva L, Cristaldi M, Monteleone F, Fontana S, Alessandro R (2018) Label-free quantitative proteomic profiling of colon cancer cells identifies acetyl-CoA carboxylase alpha as antitumor target of Citrus limon-derived nanovesicles. J Proteom 173:1–11
Schulte RR, Ho RH (2019) Organic Anion transporting polypeptides: emerging roles in Cancer Pharmacology. Mol Pharmacol 95490–95506. https://doi.org/10.1124/mol.118.114314
Fujita D, Arai T, Komori H, Shirasaki Y, Wakayama T, Nakanishi T et al (2018) Apple-Derived nanoparticles modulate expression of Organic-Anion-transporting polypeptide (OATP) 2B1 in Caco-2 cells. Mol Pharm 155772–155780. https://doi.org/10.1021/acs.molpharmaceut.8b00921
Feng X, Luo Q, Zhang H, Wang H, Chen W, Meng G et al (2017) The role of NLRP3 inflammasome in 5-fluorouracil resistance of oral squamous cell carcinoma. J Exp Clin Cancer Res 36:1–14. https://doi.org/10.1186/s13046-017-0553-x
Yang M, Luo Q, Chen X, Chen F (2021) Bitter melon derived extracellular vesicles enhance the therapeutic effects and reduce the drug resistance of 5-fluorouracil on oral squamous cell carcinoma. J Nanobiotechnol 19(1):259. https://doi.org/10.1186/s12951-021-00995-1
Ly NP, Han HS, Kim M, Park JH, Choi KY (2022) Plant-derived nanovesicles: current understanding and applications for cancer therapy. Bioact Mater 22:365–383. https://doi.org/10.1016/j.bioactmat.2022.10.005
Darvishi M, Forootan M, Nazer MR, Karimi E, Noori M (2020) Nosocomial infections, challenges and threats: a review article. Iran J Med Microbiol 14162–14181. https://doi.org/10.30699/ijmm.14.2.162
Chen Y, Zhou J, Wang L (2021) Role and mechanism of Gut Microbiota in Human Disease. Frontiers in Cellular and infection Microbiology. 11. https://doi.org/10.3389/fcimb.2021.625913
Ekundayo TC, Olasehinde TA, Okaiyeto K, Okoh AI (2021) Microbial Pathogenesis and Pathophysiology of Alzheimer’s Disease: A Systematic Assessment of Microorganisms’ Implications in the Neurodegenerative Disease. Front NeuroSci 15. https://doi.org/10.3389/fnins.2021.648484
Sundaram K, Miller DP, Kumar A, Teng Y, Sayed M, Mu J et al (2019) Plant-derived exosomal nanoparticles inhibit pathogenicity of Porphyromonas gingivalis. iScience 21308–27. https://doi.org/10.1016/j.isci.2019.10.032
Badanian A, de León EP, Rodriguez L, Bascuas T, Capo C, Battle A et al (2018) Detection of periodontal pathogens in a Uruguayan population with aggressive periodontitis using conventional and molecular methods. ODONTOESTOMATOLOGIA. 2068-77
Contaldo M, Lucchese A, Romano A, Della Vella F, Di Stasio D, Serpico R et al (2021) Oral microbiota features in subjects with Down Syndrome and Periodontal diseases: a systematic review. Int J Mol Sci. 229251
Suresh AP, Kalarikkal SP, Pullareddy B, Sundaram GM (2021) Low pH-Based method to increase the yield of plant-derived nanoparticles from Fresh Ginger rhizomes. ACS Omega 617635–617641. https://doi.org/10.1021/acsomega.1c02162
Kalarikkal SP, Sundaram GM (2021) Edible plant-derived exosomal microRNAs: exploiting a cross-kingdom regulatory mechanism for targeting SARS-CoV-2. Toxicol Appl Pharmacol 414115425. https://doi.org/10.1016/j.taap.2021.115425
Chen X, Li H, Song H, Wang J, Zhang X, Han P et al (2022) Meet changes with constancy: defence, antagonism, recovery, and immunity roles of extracellular vesicles in confronting SARS-CoV-2. J Extracell Vesicles 11(12):e12288. https://doi.org/10.1002/jev2.12288
Sahin F, Kocak P, Gunes MY, Ozkan I, Yildirim E, Kala EY (2019) In Vitro Wound Healing activity of wheat-derived nanovesicles. Appl Biochem Biotechnol 188381–188394. https://doi.org/10.1007/s12010-018-2913-1
Yang S, Lu S, Ren L, Bian S, Zhao D, Liu M et al (2023) Ginseng-derived nanoparticles induce skin cell proliferation and promote wound healing. J Ginseng Res 47133–47143. https://doi.org/10.1016/j.jgr.2022.07.005
Savcı Y, Kırbaş OK, Bozkurt BT, Abdik EA, Taşlı PN, Şahin F et al (2021) Grapefruit-derived extracellular vesicles as a promising cell-free therapeutic tool for wound healing. Food Funct 125144–125156. https://doi.org/10.1039/D0FO02953J
Yuyama K, Takahashi K, Usuki S, Mikami D, Sun H, Hanamatsu H et al (2019) Plant sphingolipids promote extracellular vesicle release and alleviate amyloid-beta pathologies in a mouse model of Alzheimer’s disease. Sci Rep 916827. https://doi.org/10.1038/s41598-019-53394-w
Murai Y, Honda T, Yuyama K, Mikami D, Eguchi K, Ukawa Y et al (2022) Evaluation of Plant Ceramide species-Induced Exosome Release from neuronal cells and Exosome Loading using Deuterium Chemistry. Int J Mol Sci. 2310751
Nemati M, Singh B, Mir RA, Nemati M, Babaei A, Ahmadi M et al (2022) Plant-derived extracellular vesicles: a novel nanomedicine approach with advantages and challenges. Cell Communication and Signaling. 2069. https://doi.org/10.1186/s12964-022-00889-1
Wang Y, Wang J, Ma J, Zhou Y, Lu R (2022) Focusing on Future Applications and Current challenges of Plant Derived Extracellular vesicles. Pharmaceuticals 15708
Liu N-J, Bao J-J, Wang L-J, Chen X-Y (2020) Arabidopsis leaf extracellular vesicles in wound-induced jasmonate accumulation. Plant Signal Behav 151833142. https://doi.org/10.1080/15592324.2020.1833142
He B, Hamby R, Jin H (2021) Plant extracellular vesicles: Trojan horses of cross-kingdom warfare. FASEB Bioadv 3657–3664. https://doi.org/10.1096/fba.2021-00040
Guo S-C, Tao S-C, Dawn H (2018) Microfluidics-based on-a-chip systems for isolating and analysing extracellular vesicles. J Extracell Vesicles 71508271. https://doi.org/10.1080/20013078.2018.1508271
Talebjedi B, Tasnim N, Hoorfar M, Mastromonaco GF, De Almeida Monteiro Melo Ferraz M (2021) Exploiting Microfluidics for Extracellular Vesicle isolation and characterization: potential use for standardized embryo Quality Assessment. Front Veterinary Sci 7. https://doi.org/10.3389/fvets.2020.620809
Raman R (2017) The impact of Genetically Modified (GM) crops in modern agriculture: A review. GM Crops Food. 8195 – 208. https://doi.org/10.1080/21645698.2017.1413522
Gworek B, Kijeńska M, Wrzosek J, Graniewska M (2021) Pharmaceuticals in the Soil and Plant Environment: a review. Water Air Soil Pollut 232145. https://doi.org/10.1007/s11270-020-04954-8
Acknowledgements
Partial financial support was received from Hamadan University of Medical Sciences [grant number: IR.UMSHA.REC.1399.198 ].
Funding
Partial financial support was received from Hamadan University of Medical Sciences [grant number: IR.UMSHA.REC.1399.198 ].
Author information
Authors and Affiliations
Contributions
Fatemeh Azizi: Visualization, Writing- original draft preparation. Salva Kazemipour-Khabbazi: Writing- original draft preparation. Stefania Raimondo: Writing- reviewing and editing. Razieh Dalirfardouei: Conceptualization, Supervision, Writing- reviewing and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azizi, F., Kazemipour-Khabbazi, S., Raimondo, S. et al. Molecular mechanisms and therapeutic application of extracellular vesicles from plants. Mol Biol Rep 51, 425 (2024). https://doi.org/10.1007/s11033-024-09379-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09379-8