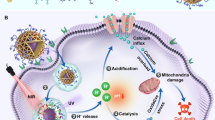
Abstract
Background
Calcium ions (Ca2+) participates in various intracellular signal cascades and especially plays a key role in pathways relevant to cancer cells. Mitochondrial metabolism stimulated by calcium overload can trigger the opening of the mitochondrial permeability transition pore (MPTP), which leads to cancer cell death.
Methods
Herein, a mitochondrial pathway for tumour growth inhibition was built via the double-activation of MPTP channel. Fe2+ doped covalent organic frameworks (COF) was synthesised and applied as template to grow CaCO3 shell. Then O2 was storaged into Fe2+ doped COF, forming O2-FeCOF@CaCO3 nanocomposite. After modification with folic acid (FA), O2-FeCOF@CaCO3@FA (OFCCF) can target breast cancer cells and realize PDT/Ca2+ overload synergistic treatment.
Results
COF can induce the production of 1O2 under 650 nm irradiation for photodynamic therapy (PDT). Low pH and hypoxia in tumour microenvironment (TME) can activate the nanocomposite to release oxygen and Ca2+. The released O2 can alleviate hypoxia in TME, thus enhancing the efficiency of COF-mediated PDT. Abundant Ca2+ were released and accumulated in cancer cells, resulting in Ca2+ overload. Notably, the reactive oxygen species (ROS) and Ca2+ overload ensure the sustained opening of MPTP, which leads to the change of mitochondria transmembrane potential, the release of cytochrome c (Cyt c) and the activation of caspases 3 for cancer cell apoptosis.
Conclusion
This multifunctional nanosystem with TME responded abilities provided a novel strategy for innovative clinical cancer therapy.
Graphical Abstract

Similar content being viewed by others

Introduction
Calcium signal plays an important role in various cancer progression processes such as proliferation, apoptosis and cell migration, which makes the regulation of calcium ions (Ca2+) in cancer cells receive increasing attention [1]. As the second messenger, Ca2+ has important effect on cell regulation, which can either induce cell survival or trigger apoptosis[2]. For instance, the fluctuations of Ca2+ content in a certain range usually promote cell proliferation and survival, whereas sustained cytosolic calcium induced Ca2+ overload can lead to cell apoptosis [3, 4]. Especially, tumor cells are more sensitive to Ca2+ overload than normal cells [5,6,7]. Therefore, it is a promising strategy for triggering intracellular Ca2+ overload to realise antitumor therapy.
As one of the intracellular Ca2+ pools, mitochondrial uptakes Ca2+ depending on the changes of transmembrane potential [8]. Mitochondrial permeability transition pore (MPTP), known as the mitochondrial megachannel, is a non-selective channel across the inner and outer layers of the mitochondrial membrane [9]. It has been reported that the continuous opening of MPTP is the direct cause of cancer cell apoptosis [10]. At physiological levels, Ca2+ can activate the transient opening of MPTP, allowing protons or positive ions to enter the mitochondrial matrix to prevent excessive accumulation in the mitochondrial intermembrane space. However, MPTP can be activated to open continuously due to high [Ca2+]m, high level of reactive oxygen species (ROS) and adenosine triphosphate (ATP) depletion, resulting in mitochondrial and cancer cells dysfunction [11,12,13,14,15]. Obviously, Ca2+ is the most important regulator and inductor for MPTP opening [16, 17]. CaCO3, a calcium-based biomineralized nanomaterial responding to the tumor acidic microenvironment with pH level as low as 6.2, has excellent degradability and good biocompatibility. As a natural Ca2+ reservoir, CaCO3 can provide sufficient Ca2+ source during the process of tumor treatment [18,19,20,21,22,23]. Moreover, when mitochondrial Ca2+ overload occurs, ROS can promote Ca2+ to stimulate sustained MPTP opening [24].
Photodynamic therapy (PDT) is a photosensitized reaction with biological effects, which involves oxygen molecules. The process is that the photosensitizer (PSs) absorbed by the tissue is excited by laser irradiation of a specific wavelength, and the excited PSs transfer energy to the surrounding oxygen to produce singlet oxygen (1O2) with strong activity. 1O2 reacts with adjacent biological macromolecules to produce cytotoxicity, resulting in cell damage and even death [25,26,27]. Compared with conventional treatments such as surgery, chemotherapy and radiotherapy, PDT has the advantages of less trauma, low toxicity and high selectivity [28,29,30]. However, PDT-mediated continuous oxygen consumption would aggravate hypoxia of cancer cells, which further blunts the therapeutic effect of PDT [31, 32]. Therefore, oxygen storage materials, including hemoglobin [33], perfluorocarbon [34] and metal organic framework (MOF) [35], are used to alleviate hypoxia in tumors.
As an emerging type of porous crystal material, covalent organic frameworks (COF) have been studied for cancer treatment in addition to their conventional applications in energy storage, catalysis, sensing and separation [36], Owe to their unique structure and characteristics such as porosity, stability and biocompatibility, COF has been widely applied for drug delivery, photodynamic therapy and photothermal therapy [37,38,39,40], Tan and his colleagues, for example, reported a porphyrin-COF for photodynamic therapy of tumors. Under near-infrared irradiation, COF nanoparticles produced abundant ROS to induce cancer cell apoptosis result in their crystalline network structure [41], Li and his colleagues synthesized a porous 8-hydroxyquinoline functionalized organic covalent framework (COF-HQ) with pH-sensitive tumor microenvironment for 5-FU loading, achieving efficient drug delivery and anti-tumor effects [42]. To the best of our knowledge, the use of COF as a smart carrier for oxygen molecules to achieve tumor-targeted transport has not been reported.
Herein, we synthesized Fe2+-doped COF by Schiff base reaction at room temperature. Then FeCOF was used as a template for the growth of FeCOF@CaCO3 by gas diffusion method. And folic acid (FA) was modified on the surface of FeCOF@CaCO3 to form FeCOF@CaCO3@FA (FCCF) for achieving targeted therapy against breast cancer. FCCF possessed highly efficient oxygen-carrying capacity owing to the affinity of doped Fe2+ with oxygen. Upon internalization into breast cancer cells, O2-FeCOF@CaCO3@FA (OFCCF) nanoparticles were disassembled, releasing O2 and Ca2+. COF, as the photosensitizer, was used to produce ROS under light exposure. The released O2 could enhance COF-mediated PDT in hypoxic tumour microenvironment (TME). Moreover, the coated CaCO3 layer exhibited excellent pH-dependent dissociation behavior, causing rapid release of Ca2+ under the acidic conditions. Large amounts of Ca2+ could accumulate in mitochondria, leading to the disruption of Ca2+ homeostasis and mitochondria dysfunction. Therefore, the high levels of ROS and intracellular accumulation of Ca2+ induced the continuous opening of MPTP, causing an influx of Ca2+ to kill cancer cells. Besides, pathological and biochemical tests confirmed that OFCCF nanoparticles could avoid the disturbance of systemic toxicity (Scheme 1).
Results
Synthesis and characterization of O2-FeCOF@CaCO3@FA NPs
The well-dispersed spherical FeCOF was simply synthesized by using acetic acid as catalyst at room temperature (Fig. 1a and Additional file 1: Figure S1). FeCOF@CaCO3 was prepared via gas diffusion method. As illustrated in Fig. 1b, in a closed vacuum, the CO2 and NH3 gases produced by the natural decomposition of NH4HCO3 would continuously diffuse into the mixed solution containing Ca2+. Simultaneously CO32− was provided into the alkaline solution to trigger the formation of CaCO3 [43]. Thin nanosheets of CaCO3 could be observed on the surface of FeCOF by transmission electron microscopy (TEM, Fig. 1c) and scanning electron microscopy (SEM, Additional file 1: Figure S2). Elemental mapping analysis showed that C, O, N, Fe and Ca elements of FCCF nanoparticles were homogeneously distributed (Fig. 1d). X-ray photoelectron spectroscopy (XPS) was used to analyse Ca element signal of FeCOF@CaCO3. The two peaks located at 347.2 eV and 350.7 eV for 2p1/2 and 2p3/2, respectively, were considered as the characteristic peaks of CaCO3 (Fig. S3) [44]. Powder X-ray diffraction (PXRD) patterns have confirmed the excellent crystallinity of FeCOF and FeCOF@CaCO3 (Additional file 1: Figure S4) [37, 45]. The surface area and pore volume of FeCOF and FeCOF@CaCO3 were 1380.170 m2/g, 1.292 cc/g and 373.731 m2/g, 0.305 cc/g. Compared with FeCOF, FeCOF@CaCO3 showed decreased Brunauer–Emmett–Teller (BET) surface area and pore volume, confirming the successful coating of CaCO3 (Additional file 1: Figure S5). Then FA was modified on the surface of FeCOF@CaCO3 to obtain the final FeCOF@CaCO3@FA (FCCF) nanoparticles (Fig. 1e). In the FTIR spectra of FeCOF@CaCO3, the peak at 1592 cm−1 (peak 1) was ascribed to the vibration of -NH2 and the characteristic peak at 712 cm−1 (peak 2) belonged to carbonate. In the FTIR spectra of FCCF, the characteristic peaks at ~ 1573 (peak 3) and 1647 cm−1 (peak 4) have been characterized as the stretching vibration mode of secondary amide bonds (C = O-NH), further demonstrating the successful synthesis of FCCF (Fig. 1f), further demonstrating the successful synthesis of FCCF [46, 47]. UV–visible (UV-vis) absorption spectrum of FCCF showed that there was a broad absorption between 400 and 700 nm, which was similar to the absorption of FeCOF nanoparticles. Meanwhile, the strong absorbance peak centered at 281 nm of FCCF validated the integration of FA into the nanosystem (Fig. 1g). TEM and Dynamic light scattering (DLS) measurements showed that the mean size of FCCF was about 230 nm (Additional file 1: Figure S6 and Table S1), slightly larger than that of FeCOF and FeCOF@CaCO3 nanoparticles. In addition, the successful synthesis of FCCF could also be proved by the zeta potential changes (Additional file 1: Figure S7). The post-modification of FA could enhance the bio-compatible and tumor targeting abilites of FCCF. As shown in Additional file 1: Figure S8, FCCF composite were uniformly dispersed in water, phosphate buffered saline (PBS) and cell culture medium (containing 10% serum) for 7 days without any aggregation at room temperature. The morphology of the nanocomposite had no change even after incubation in water for a week. These results verified the good stability of FCCF for potential clinical applications.
In vitro release study
In order to verify TME-activated bio-decomposition abilities of FCCF nanoparticles, various measurements were conducted. FCCF and FeCOF nanoparticles were dispersed in PBS with different pH to observe the morphology changes at different time points. As shown in Additional file 1: Figure S9 and S10, FCCF nanoparticles showed no change on size and structure when dispersed in PBS at pH 7.4. In contrast, FCCF nanoparticles dissociated in PBS with low pH (6.5 and 5.5), resulting in the nanosheet like morphology of the surface was largely lost after 1 h. Meanwhile, the hydrodynamic sizes of nanoparticles for time-dependent changes were monitored by DLS at different pH values. The hydrated particle of FCCF nanoparticles at pH 6.5 and 5.5 for 1 h were around 180 nm and 100 nm, respectively (Fig. 2a). Then, the time-dependent release profiles of Ca2+ were further assessed in buffers with various pH values. As shown in Fig. 2b, the low pH condition (5.5) led to a sustained release of Ca2+, with 89.2% Ca2+ being released from FeCOF carrier. However, only about 66.4% Ca2+ was released after being incubated at pH 6.5. The above results strongly proved that FCCF exhibited promising pH-responsive Ca2+ release ability.
a Time-dependent DLS-measured size changes of FCCF under different pH conditions (pH 7.4, 6.5 and 5.5). b Time-dependent release of Ca2+ from the FCCF dispersed in pH 7.4, 6.5 and 5.5 buffers. c Time-dependent dissolved oxygen generation induced by O2-COF in deoxidized PBS. d Time-dependent dissolved oxygen generation induced by OFCCF in deoxidized PBS (pH 6.5). e UV–Vis absorption of DPBF after 650 nm laser irradiation with H2O, COF (100 μg mL−1) and OFCCF (100 μg mL−1, pH 6.5). f ESR spectra of FCCF and OFCCF under 650 nm laser irradiation (0.72 W cm−2, 10 min)
The release of Ca2+ further facilitated oxygen diffusion to meet the increased oxygen demand in hypoxic tumor. O2 releasing ability of OFCCF and O2-COF in deoxidized PBS buffer was illustrated in Fig. 2c, d. The storaged O2 of OFCCF and O2-COF were released under a hypoxic environment by passive transportation. However, the released O2 from OFCCF was approximately twofold as that from O2-COF, which was attributed to the affinity of Fe2+ with O2. Moreover, the bubbles produce in the OFCCF solution also demonstrated the specific oxygen release behavior of Additional file 1: Figure S11. The ROS generation ability of OFCCF was investigated by 1,3-diphenylisobenzofuran (DPBF). Compared with free DPBF, COF showed effective time-dependent ROS production under 650 nm (0.72 W cm−2) laser irradiation, which was attributed to its strong absorption (Fig. 1g). Moreover, the released O2 could enhance the production of ROS (Fig. 2e). Next, electron spin resonance (ESR) with 2,2,6,6-tetramethyl-4-piperidinol (TEMP) as singlet 1O2 trapping agent was used for detecting 1O2 generation. 1O2 signal (1:1:1) was observed for OFCCF under 650 nm laser irradiation, which was stronger than that of FCCF (Fig. 2f). Overall, OFCCF with excellent 1O2 generation ability could effectively overcome tumor hypoxia and enhance PDT effect for breast cancer.
Cancer cell death induced by calcium overload
Next, the cellular internalization of rhodamine B (RhB)-labelled FCCF nanoparticles was examined in murine 4T1 breast cancer cells. Figure 3a indicated the time-dependent internalization process of FCCF nanoparticles, as evidenced by the colocalization of red fluorescence for RhB-labelled FCCF nanoparticles and the green fluorescence for LysoTracker. These results improved that FCCF nanoparticles could be effectively uptaken by 4T1 cells, which was beneficial to killing tumor cells. Within acidic lysosomes, OFCCF nanoparticles could release Ca2+ rapidly, which led to a direct increase of osmotic pressure and influx of Cl− and H2O molecules to result in proton spongeeffect [21, 46, 47]. Simultaneously, under 650 nm laser irradiation, a large number of 1O2 were produced by the nanoparticles, causing the destruction of lysosome membrane structure to favor the endosomal escape of nanoparticles. Then, intracellular Ca2+ concentration was monitored using the calcium indicator dye Fluo-4. Weak green fluorescence was observed in PBS group and L group of 4T1 cells. In contrast, compared with other groups, the intracellular Ca2+ concentration in the OFCCF + group was highest (Additional file 1: Figure S12). In addition, mitochondrial Ca2+ concentrations were quantified using the calcium indicator dye Rhod-2. The FCCF, COF + and CaCO3 groups exhibited weak red luminescence, while the group treated with OFCCF + exhibited strong intracellular luminescence, indicating Ca2+ influx of 4T1 cells could be better activated in the presence of ROS (Additional file 1: Figure S13). Via Calcium Colorimetric assay, the OFCCF + displayed the highest intracellular Ca2+ concentration as 19.4 μg/mL, indicating an obvious Ca2+ overloading (> 3.2 μg/mL in PBS group) (Additional file 1: Figure S14). The release of Ca2+ and the hypoxic tumor microenvironment would trigger the free diffusion of oxygen by passive-transport. The O2 probe [Ru(dpp)3]Cl2 (RDPP) which is prone to luminescence quenching by oxygen was used to monitor cellular O2-evolving. As shown in Fig. 3b, the green fluorescence intensity of OFCCF group decreased obviously under hypoxic conditions, while the green fluorescence was observed for L treated and FCCF treated groups. Then, quantitative analysis of dynamic changes of intracellular oxygen via calculating average intensity by ImageJ software. The green fluorescence intensity of 4T1 cells treated with OFCCF + was 22%, which was about three times lower than the other three groups (Additional file 1: Figure S15). Meanwhile, as the expression of HIF-1α protein is upregulated under a hypoxic condition, the degree of hypoxia can be further assessed according to the level of HIF-1α. The OFCCF treated 4T1 cells exhibited a low expression of HIF-1α by western blotting (WB) analysis (Fig. 3c and Additional file 1: Figure S16). These results suggested that OFCCF could release O2 to alleviate the hypoxic state of tumor microenvironment. The enhance of ROS generation by OFCCF-mediated O2 was proved by intracellular 1O2 test with 2ʹ,7ʹ-dichlorodihydrofluorescein diacetate (DCFH-DA) fluorescence as a probe. As shown in Additional file 1: Figure S17, a comparative intensity of green fluorescence was observed under hypoxia and normoxia conditions, suggesting the key role of oxygen release in PDT.
a The images of 4T1 cells incubated with FCCF recorded at different time points. b [Ru(dpp)3]Cl2 was used as probe to detect O2 generation after treatment with: (1) PBS + , (2) FCCF, (3) FCCF + and (4) OFCCF + . c Western blot analysis of HIF-1α expression of 4T1 cells. d Mitochondrial distribution and mitochondrial membrane potential images of 4T1 cells after different treatments. e Bio-TEM images of 4T1 cells after different treatments. Red arrows exhibit the location of destructed mitochondria in 4T1 cells
Ca2+ overload and 1O2 can cause the conformational changes of MPTP structural proteins, which allows substances with a molecular weight greater than 1500 to pass through the inner mitochondrial membrane (IMM) by non-selectively way. The entered substances result in the collapse of mitochondrial membrane potential (MMP), the uncoupling of oxidative phosphorylation process and the disturbance of ATP production, consequently causing mitochondria function impairment [16]. Therefore, we used commercial Calcein-AM or Calcein-AM + CoCl2 dye as a fluorescence indicator to evaluate whether OFCCF was able to induce the continuous activation of MPTP. Upon treatment with FCCF or COF + , green fluorescence was observed. In contrast, no green fluorescence was detected for OFCCF group. However, upon receiving simultaneous exposure of OFCCF + and 5 µM uncoupling agent (CCCP), the 4T1 cells exhibited green fluorescence signal (Additional file 1: Figure S18), suggesting the critical role of Ca2+ overloading to activate MPTP opening. Next, to reveal the degree of mitochondrial damage induced by enhanced mitochondrial Ca2+ overload, the mitochondrial membrane potential of 4T1 cells was evaluated using commercial JC-1 dye. The OFCCF + group showed strong green fluorescence signal, in marked contrast to the strong red fluorescence observed in other groups. Subsequently, intracellular mitochondrial distribution was detected by staining with Mito Tracker® Red CMXRos. As expected, the fewest mitochondria damage were detected in the OFCCF + group (Fig. 3d and Additional file 1: Figure S19). Finally, biological transmission electron microscopy (Bio-TEM) was used to visualize changes in their mitochondria (Fig. 3e). The 4T1 cells treated with FCCF, COF + or FCCF + exhibited only mild mitochondrial destruction, whereas OFCCF + caused the most obvious mitochondrial destruction, with visible swelling and cavitation of mitochondria. These results confirm that OFCCF + can cause severe mitochondrial damage through mitochondrial Ca2+ overload. In addition, the intracellular ATP content treated with OFCCF + decreased significantly in comparison to other five groups, which was partially produced via the oxidative phosphorylation inside the mitochondria (Additional file 1: Figure S20). All of the results revealed that the increased intracellular Ca2+ and production of 1O2 induced by OFCCF + could cause mitochondrial dysfunction.
The collapse of MMP induces a series of pathological changes in mitochondria, resulting in the release of Cyt c from mitochondrial matrix into cytoplasm. And Cyt c and apoptotic protein activator-1 (Apaf-1) can form a composite to trigger cell apoptosis [48]. To confirm this principle, the expression of apoptosis-related proteins was investigated by western blotting. As illustrated in the Fig. 4a, the expression levels of Cyt c and caspase 3 increased after the treatment of OFCCF + in comparison to the PBS, FCCF and FCCF + groups. By contrast, the protein levels of Bcl-2 in 4T1 cells were markedly down-regulated after OFCCF + treatment. Detailed quantitative results of WB were gathered in Fig. 4b. In addition, the release of Apaf-1 from the supernatant of 4T1 cells was measured by enzyme-linked immunosorbent assay (ELISA). As shown in Fig. 4c, the OFCCF + treatment elevated the release of Apaf-1 effectively. The above results indicated that mitochondrial-mediated apoptotic pathway was activated after the mitochondrial Ca2+ overload and production of 1O2.
a Western blot analysis Cyt c, Bcl-2 and Caspase 3 expression of 4T1 cells. b Quantitative analysis of Cyt c, Bcl-2 and Caspase 3 expression. c Apaf-1 level of cell supernatant after various treatments. P values were calculated by one-way analysis (*p < 0.05, **p < 0.01, ***p < 0.001). Data were represented as mean ± SD (n = 6). d Cytotoxicity profiles of 4T1 cells treated with: (1) PBS, (2) L, (3) FCCF, (4) COF + , (5) FCCF + and (6) OFCCF + . P values were calculated by one-way analysis (*p < 0.05, **p < 0.01, ***p < 0.001). Data were represented as mean ± SD (n = 4). e Schematic diagram of mitochondrial damage induced by bidirectional activation of MPTP opening by Ca2+ and 1O2
Then, the cytotoxic effect of COF and FCCF nanoparticles towards L929 fibroblast cells and 4T1 cells was evaluated using the standard methyl thiazolyl tetrazolium assay (MTT). L929 cells were treated with various concentrations of COF and FCCF nanoparticles within 24 and 48 h, the survival rate maintained above 80%. The proliferation ability of L929 cells treated with OFCC + and OFCCF + decreased slightly (Additional file 1: Figure S21). These results demonstrated the biosafety and biocompatibility of the nanoparticles. To further investigate the therapeutic effect of Ca2+ overload/PDT, the anti-cancer effect of OFCCF under hypoxic and normoxic environment was tested. Upon 650 nm laser irradiation, the cytotoxicity of FCCF + under normoxic environment was greatly enhanced than that of FCCF + under the hypoxic environment. Notably, the hypoxia condition had significant effect on the cancer cell killing effect of FCCF + -treated group. While the survival rate of OFCCF + treated group was almost the same as the group under hypoxia condition owing to the oxygen-carrying properties of FeCOF (Fig. 4d). Moreover, from the live/dead cell staining test, we observed that most cells remained alive after the treatment of PBS and L. By contrast, the OFCCF + treated group showed a large number of dead cells in the Additional file 1: Figure S22. This result was consistent with the flow experimental results (Additional file 1: Figure S23). Collectively, all above results reveal that OFCCF is a promising candidate to induce cancer cell death through Ca2+ overload and 1O2 co-activating MPTP opening (Fig. 4e).
In vivo therapeutic effect investigation
The in vivo therapeutic efficacy of OFCCF was further examined by a 4T1 breast tumor model. The tumor-bearing mice were randomly divided into six groups for the different treatments: (1) PBS, (2) 650 nm laser irradiation (L), (3) FCCF, (4) COF + , (5) FCCF + and (6) OFCCF + . After intravenously injection with nanocomposites (100 μg mL−1) for 12 h, the mice were treated with 650 nm laser irradiation (0.72 W cm−2) for 5 min (Fig. 5a). The obtained nanocomposites had no side effect on the body weight of mice, suggesting the good biosafety of the nanocomposites (Additional file 1: Figure S24). Tumor suppression assessments showed a considerable suppression effect on the tumors of FCCF + group and OFCCF + group in comparison with other four groups. (Fig. 5b). This result was consistent with the pictures of mice tumors and tumor weight (Fig. 5c and Additional file 1: Figure S25). Furthermore, hematoxylin and eosin (H&E) staining results were shown in Fig. 5d, obvious cell necrosis and apoptosis could be observed in OFCCF + group. From the TUNEL staining results, the green signal represented that cells apoptosis was appeared after the treatment of OFCCF + . Meanwhile, the typical morphology of apoptotic cells was detected in caspase 3 staining assay. Finally, to better assess the effect of this synergistic therapy, we next examined the lung metastasis of 4T1 tumor-bearing mice after various treatments. H&E staining and imaging of lung sections confirmed that OFCCF + effectively suppressed tumor metastasis (Additional file 1: Figure S26).
a Schematic description of the establishment of 4T1 tumor model and therapeutic outcome. b The relative tumor volume of mice in different groups after treatment. P values were calculated by one-way analysis (*p < 0.05, **p < 0.01, ***p < 0.001). Data were represented as mean ± SD (n = 6). c Tumor weight in different groups after treatment. P values were calculated by one-way analysis (*p < 0.05, **p < 0.01, ***p < 0.001). Data were represented as mean ± SD (n = 6). d H&E, TUNEL and Caspase 3 staining of tumor collected from mice after various treatments
Moreover, the therapeutic efficiency of OFCCF + was further evaluated (Fig. 6a). To study the bio-distribution of FCCF,FCCF was injected intravenously into 4T1-tumor-bearing mice and Fe content was tracked by inductively coupled plasma mass spectrometry (ICP-MS). As shown in Fig. 6b, nanoparticles preferentially accumulated at the tumor site due to the specific binding of FA with breast cancer folate receptors, showing the highest content at 12 h after injection. Apart from tumors, the nanoparticles were also accumulated in liver and kidney. As shown in Fig. 6c, the content of FCCF nanoparticles in liver and kidney decreased gradually, confirming that they could be metabolized from the body through the renal-urinary clearance system within one week. The tumor accumulation and clearance performance of FCCF provides enormous potential for effective and safe cancer treatment. Furthermore, the bio-degradation performance of nanoparticles triggered by TME was further studied. After the PBS, FCCF and OFCCF was injected intravenously into 4T1-tumor-bearing mice for 12 h, respectively, the Ca2+ released from nanoparticles accumulated in tumor owing to the acidic condition of TME. As we expected, the Ca2+ content in the tumors significantly increased after being treated with OFCCF + in comparison to FCCF and FCCF + groups. It is further revealed that the production of ROS could promote the aggregation of Ca2+, resulting in the apoptosis caused by Ca2+ overload (Fig. 6d). Next, the possible mechanism of Ca2+ overload-induced apoptosis was explored by ELISA and western blotting to determine the content of apoptosis-related proteins of tumours. Once Cyt c is released from the mitochondria, it can couple with Apaf-1 to form the apoptosome. As illustrated in Fig. 6e, the upregulation of Apaf-1 confirmed the formation of apoptosome. Besides, the expression levels of Cyt c, Bcl-2 and caspase 3 were further detected by western blotting. As illustrated in the Fig. 6f, compared with the control groups (PBS, FCCF and FCCF +), Cyt c and caspase 3 expressions levels increased significantly but the protein levels of Bcl-2 decreased after OFCCF + treatment. The quantitative analysis of western blotting was shown in Additional file 1: Figure S27. Compared with the other groups, the ATP level of OFCCF + group decreased significantly owing to Ca2+ overload in mitochondria, which would damage the energy supply of cancer cells (Additional file 1: Figure S28). To identify the ability of OFCCF to relieve tumor hypoxia, mice tumors were obtained for HIF-1α (green) staining assay after being injected with the nanoparticles for 10 h. As illustrated in the Fig. 6g, the intensity of fluorescence was hardly visible in OFCCF + group. For ROS staining assay, OFCCF + group showed the strongest DCFH-DA fluorescence signals in tumor slices, which was consistent with the HIF-1α (green) staining assay results. Meanwhile, the remission of tumor hypoxia was further verified by down-regulation of HIF-1α expression level, indicating that released oxygen could relieve intratumoral hypoxia and enhance production of ROS (Additional file 1: Figure S29).
a Schematic illustration of OFCCF-based Ca2+ release and production 1O2 activation of MPTP opening inhibits tumor growth. b The biodistribution of Fe in main tissues and tumor (c) and feces in different times after intravenously injecting with OFCCF into 4T1-tumor-bearing. Data were represented as mean ± SD (n = 3). d The biodistribution of Ca in tumor after various treatments at 12 h intravenously injecting into 4T1-tumor-bearing. Data were represented as mean ± SD (n = 3). e Apaf-1 level of tumor supernatant after various treatments. P values were calculated by one-way analysis (*p < 0.05, **p < 0.01, ***p < 0.001). Data were represented as mean ± SD (n = 3). f Western blot analysis Cyt c, Bcl-2 and Caspase 3 expression of tumor. g HIF-1α and DCFH-DA staining of tumor collected from mice after various treatments
To assess the potential therapeutic toxicity of FCCF, a systemic toxicity study was performed. The physiological pathology of main organs were analysed by H&E staining. No significant pathological changes in major organs (heart, liver, kidney lung, and spleen) were observed after different treatments (Additional file 1: Figure S30), suggesting the excellent biosafety of the nanocomposite in vivo. Meanwhile, biochemical analysis further confirmed that OFCCF + had low toxicity (Additional file 1: Figure S31).
Conclusion
In summary, we have successfully prepared an OFCCF nanocomposite with two-way activation of MPTP opening by ROS and Ca2+ overload for precise targeted therapy of breast cancer. OFCCF with tumor-targeting ability could effectively aggregate at the tumor sites. The subsequent rapid release of Ca2+ and oxygen were attributed to the hypoxia and low pH of TME. Overflowed Ca2+ rapidly accumulated inside the cytoplasm, which led to Ca2+ influx and activated MPTP opening. Upon NIR light irradiation, the released oxygen could enhance the effect of COF-mediated PDT, and the generation of ROS could promote the activation of MPTP opening. Notably, these two activation mechanisms ensured the sustained opening of MPTP, leading to the change of mitochondria transmembrane potential, release of Cyt c and activation of caspases 3, thus inducing cell apoptosis. Additionally, for the first time, we utilized FeCOF with hypoxia responsive as oxygen carrier, alleviating tumor hypoxia and enhance PDT efficiency. More importantly, OFCCF nanocomposites based treatment has been proved to be a safe and effective strategy to inhibit tumor growth and open new directions for clinical cancer therapy.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.
References
Monteith GR. Prostate cancer cells alter the nature of their calcium influx to promote growth and acquire apoptotic resistance. Cancer Cell. 2014;26(1):1–2.
Cui C, Merritt R, Fu L, Pan Z. Targeting calcium signaling in cancer therapy. Acta Pharm Sin B. 2017;7(1):3–17.
Várnai GP, Golenár T, Roy S, Purkins G, Schneider TGT, Balla G. Hajnóczky, imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface Molr. Cell. 2010;39(1):121–32.
Parkash J, Asotra K. Calcium wave signaling in cancer cells. Life Sci. 2010;87(19–22):587–95.
Lyu Y, Xie C, Chechetka SA, Miyako E, Pu K. Semi-conducting polymer nanobioconjugates for targeted photothermal activation of neurons. J Am Chem Soc. 2016;138(29):9049–52.
Ma Z, Han K, Dai X, Han H. Precisely striking tumors without adjacent normal tissue damagevia mitochondria templated accumulation. ACS Nano. 2018;12(6):6252–62.
Ma Z, Zhang J, Zhang W, Foda MF, Zhang Y, Ge L, Han H. Intracellular Ca2+ cascade guided by NIR-II photo-thermal switch for specific tumor therapy. iScience. 2020;23(5):101049.
Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria as targets for chemotherapy. Apoptosis. 2009;14(4):624–40.
Agarwal A, Wu PH, Hughes EG, Fukaya M, Tischfield MA, Langseth AJ, Wirtz D, Bergles E. Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron. 2017;93(3):587–605.
Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion-a target for cardioprotection. Cardiovascular Res. 2004;61(3):372–85.
Pérez MJ, Quintanilla RA. Development or disease: duality of the mitochondrial permeability transition pore. Biolo Dev. 2017;426(1):1–7.
Hurst S, Hoek J, Sheu SS. Mitochondrial Ca2+ and regula-tion of the permeability transition pore. J Bioenerg Biomembr. 2017;49(1):27–47.
Xu H, Cui S, Zhang Y, Ren J. Mitochondrial Ca2+ regula-tion in the etiology of heart failure: physiological and patho-physiological implications. Acta Pharmacol Sin. 2020;41(10):1301–9.
Bernardi P, Rasola A, Forte M. The mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. G Physiol Rev. 2015;95(4):1111–55.
Shanmughapriya S, Rajan S, Hoffman NE, Higgins AM, Tomar D, Nemani N, Hines KJ, Smith D, Eguchi JA, Vallem S. SPG7 is an essential and conserved component of the mito-chondrial permeability transition pore. Mol Cell. 2015;60(1):47–62.
Biasutto L, Azzolini M, Szabo I, Zoratti M. The mito chondrial permeability transition pore in AD 2016: an up-date. Acta Pharm Sin B. 2016;1863(10):2515–30.
Li Y, Sun J, Wu R, Bai J, Hou Y, Zeng Y, Zhang Y, Wang X, Wang Z, Meng X. Mitochondrial MPTP: a novel target of ethnomedicine for stroke treatment by apoptosis inhibition. Front Pharmacology. 2020;11:352.
Zhao Y, Luo Z, Li M, Qu Q, Ma X, Yu SH, Zhao Y. A preloaded amorphous calcium car-bonate/doxorubicin@silica nanoreactor for pH-responsive delivery of an anticancer drug. Angew Chem Int Ed. 2015;54(3):919–22.
Dong Z, Feng L, Hao Y, Li Q, Chen M, Yang Z, Zhao H, Liu Z. Synthesis of CaCO3-based nanomedicine for en-hanced sonodynamic therapy via amplification of tumor oxidative stress. Chem. 2020;6(6):1495–7.
Chang M, Hou Z, Jin D, Zhou J, Wang M, Shu M, Ding B, Li C, Lin J. Colorectal tumor microenvironment-activated bio-decomposable and mMetabolizable Cu2O@CaCO3 nanocomposites for synergistic oncotherapy. Adv Mater. 2020;32(43):2004647.
Dong Z, Feng L, Hao Y, Chen M, Gao M, Chao Y, Zhao H, Zhu W, Liu J, Liang C, Zhang Q, Liu Z. Synthesis of hollow biomineralized CaCO3-polydopamine nanoparticles for multimodal imaging-guided cancer photodynamic therapy with reduced skin photosensitivity. J Am Chem Soc. 2018;140(6):2165–78.
Wan X, Zhong H, Pan W, Li Y, Chen Y, Li N, Tang B. Programmed release of dihydroartemisinin for synergistic cancer therapy using a CaCO3 mineralized metal-organic framework. Angew Chem Int Ed. 2019;131(40):14272–7.
Lybaert L, Ryu KA, Nuhn L, Rycke RD, Wever OD, Chon AC, Esser-Kahn AP, Geest BG. Cancer cell lysate entrapment in CaCO3 engineered with polymeric TLR-agonists: immune-modulating microparticles in view of per-sonalized antitumor vaccination. Chem Mater. 2017;29(10):4209–17.
Zorov DB, Juhaszova MS, Sollott J. Mitochondrial ROS-induced ROS release: an update and review. Bba-Bioenergetics. 2006;1757(5–6):509–17.
Zeng Z, Zhang C, Li J, Cui D, Jiang Y, Pu K. Activatable polymer nanoenzymes for photodynamic immunometabolic cancer therapy. Adv Mater. 2021;33(4):2007247.
Li Y, Sun P, Zhao L, Yan X, Ng DKPP, Lo C. Ferric ion driven assembly of catalase-like supramolecular photosensitizing nanozymes for combating hypoxic tumors. Angew Chem Int Ed. 2020;132(51):23228–38.
Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5(8):497–508.
Singhal S, Nie S, Wang M. Nanotechnology applications in surgical oncology. Annu Rev Med. 2010;61:359–73.
Wu W, Yu L, Jiang Q, Huo M, Lin H, Wang L, Chen Y, Shi J. Enhanced tumor-specific disulfiram chemotherapy by in situ Cu2+ chelation-initiated nontoxicity-to-toxicity transition. J Am Chem Soc. 2019;141(29):11531–9.
Wang Z, Zhang Y, Ju E, Liu Z, Cao F, Chen Z, Ren J, Qu X. Biomimetic nanoflowers by self-assembly of nanozymes to induce intracellular oxidative damage against hypoxic tumors. Nat commun. 2018;9(1):3334.
Yang S, Tang Z, Hu C, Zhang D, Shen N, Yu H, Chen X. Selectively potentiating hypoxia levels by com-bretastatin A4 nanomedicine: toward highly enhanced hypoxia-activated prodrug tirapazamine therapy for metastatic tumors. Adv Mater. 2019;31(11):1805955.
Sun J, Du K, Diao J, Cai X, Feng F, Wang S. GSH and H2O2 co-activatable mitochondria-targeted photodynamic therapyunder normoxia and hypoxia. Angew Chem Int Ed. 2020;59(22):12122–8.
Liu W, Liu T, Zou M, Yu W, Li C, He Z, Zhang M, Liu M, Li Z, Feng J, Zhang X. Aggressive man-made red blood cells for hypoxia-resistant therapy. Adv Mater. 2018;30(35):802006.
Song X, Feng L, Liang C, Yang K, Liu Z. Ultrasound trig-gered tumor oxygenation with oxygen-shuttle nanoper-fluorocarbon to overcome hypoxia-associated resistance in cancer therapies. Nano lett. 2016;16(10):6145–53.
Cai X, Xie Z, Ding B, Shao S, Liang S, Pang M, Lin J. Monodispersed copper(I)-based nano metal-organic framework as a biodegradable drug carrier with enhanced photodynamic therapy efficacy. Adv Sci. 2019;6(15):1900848.
Guan Q, Zhou L, Li W, Li Y, Dong Y. Covalent organic frameworks(COFs) for cancer therapeutics. Chem Eur J. 2020;26(25):5583–91.
Liu S, Hu C, Liu Y, Zhao X, Pang M. One-pot synthesis of DOX@covalent organic framework with enhanced chemotherapeutic efficacy. J Chem Eur J. 2019;25(17):4315–9.
Hu C, Zhang Z, Liu S, Liu X, Pang M. Monodispersed CuSe sensitized covalent organic framework photosensitizer with an enhanced photodynamic and photothermal effect for cancer therapy. ACS Appl Mater pInterfaces. 2019;11(26):23072–82.
Hu C, Cai L, Liu S, Pang M. Integration of a highly mono-disperse covalent organic framework photosensitizer with cation exchange synthesized Ag2Se nanoparticles for en-hanced phototherapy. Chem Commun. 2019;55(62):9164–7.
Liu S, Zhou Y, Hu C, Cai L, Pang M. Covalent organic framework-based nanocomposite for synergetic photo-, chemodynamic-, and immunotherapies. ACS Appl Mater Interfaces. 2020;12(39):43456–65.
Wang P, Zhou F, Guan K, Wang Y, Fu X, Yang Y, Yin X, Song G, Zhang X, Tan W. In vivo therapeutic response monitoring by a selfreporting upconverting covalent organic framework nanoplatform. Chem Sci. 2020;11(5):1299–306.
Bai L, Phua SZFW, Lim Q, Jana A, Luo Z, Tham HP, Zhao L, Gao Q, Zhao Y. Nanoscale covalent organic frameworks as smart carriers for drug delivery. Chem Commun. 2016;52(22):4128–31.
Dong Z, Feng L, Zhu W, Sun X, Gao M, Zhao H, Chao Y, Liu Z. CaCO3 nanoparticles as an ultra-sensitive tumor-pH-responsive nanoplatform enabling real-time drug release monitoring and cancer combination therapy. Biomaterials. 2016;110:60–70.
Guan Q, Zhou L, Lv F, Li W, Li Y, Dong Y. A glycosyl-ated covalent organic framework equipped with BODIPY and CaCO3 for synergistic tumor therapy. Angew Chem Int Ed. 2020;59(41):18042–7.
Wang S, Ni D, Yue H, Luo N, Xi X, Wang Y, Shi M, Wei W, Ma G. Exploration of antigen induced CaCO3 nanoparticles for therapeutic vaccine. Small. 2018;14(14):1704272.
Peng J, Fumoto S, Suga T, Miyamoto H, Kuroda N, Kawakami S, Nishida K. Targeted co-delivery of protein and drug to a tumorin vivo by sophisticated RGD-modified lipid-calcium carbonate nanoparticles. J Control Release. 2019;302:42–53.
Jiang Y, Huo S, Hardie J, Liang XJ, Rotello VM. Progress and perspective of inorganic nanoparticle-based siRNA delivery systems. Expert Opin drug del. 2016;13(4):547–59.
Shaik MF, Yanamala VR, Rajanikant GK. CypD: the key to the death DOOR CnS neurological disorders-drug targets. Cns Neurol Disord-dr. 2015;14(5):654–63.
Acknowledgements
Not applicable.
Funding
This project is financially supported by the National Natural Science Foundation of China (NSFC 21471145), Science and Technology Development Planning Project of Jilin Province (20170101179JC), and “Hundred Talents Program” of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Contributions
These authors contribute equally. Correspondence: M.P, Y.Z and CZ. Conceptualization, P.M, Y.Z and CZ; writing—original draft preparation, Y.Z and S.J; experiments performing, all authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal experiment was performed strictly following the guidelines from the Animal Protection and Use Committee of Jilin University.
Consent for publication
All authors have approved the manuscript be submitted.
Competing interests
The authors declare that they have no competing interest in regard to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Size of Nanoparticles. Figure S1. The SEM images of FeCOF. Figure S2. The SEM images of FeCOF@CaCO3. Figure S3. (a) XPS spectra of FeCOF@CaCO3. (b) XPS high-resolution scans of Ca 2p. Figure S4. The PXRD of FeCOF and FeCOF@CaCO3. Figure S5. (a) N2 adsorption and desorption isotherms at 77 K of FeCOF and FeCOF@CaCO3. (b)Pore size distribution plots of FeCOF and FeCOF@CaCO3. Figure S6. The Hydrodynamic diameters of FeCOF, FeCOF@CaCO3 and FCCF. Figure S7. The zate potential of FeCOF, FeCOF@CaCO3 and FCCF. Figure S8. (a) The FCCF dispersed in water, PBS and cell culture medium (containing 10% serum) before and after 7 days incubation. (b) The TEM image of FCCF stored in cell culture medium (containing 10% serum) for a week. (c) The DLS was measured on day 0, 5 and 7 when FCCF was dispersed in water, PBS and cell culture medium (containing 10% serum). Figure S9. (a) SEM images of FCCF at pH 7.4, 6.5 and 5.5, related to Figure 2a. Data are presented as mean ± SD (n = 3). Figure S10. (a) SEM images of FeCOF at pH 7.4, 6.5 and 5.5. Data are presented as mean ± SD (n = 3). Figure S11. Oxygen release behaviour of OFCCF in solution. Figure S12. CLSM microimages of intracellular Ca2+ content in 4T1 cells. Figure S13. CLSM microimages of mitochondrial Ca2+ content in 4T1 cells. Figure S15. Quantitative analysis of the intracellular O2 generation of 4T1 cells after various treatments based on the confocal images shown in Figure 3b by using imageJ, related to Figure 3b. Figure S16. Quantitative analysis of HIF-1α protein expression, as the ratio of protein to β-actin from Western Blot results. Related to Figure 3c. P values were calculated by one-way analysis (*p<0.05, **p<0.01, ***p<0.001). Figure S17. Intracellular ROS production of 4T1 cells under normoxic and hypoxic conditions after various treatments. Figure S18. MPTP opening in mitochondria of 4T1 cells after different treatments. Figure S19. mitochondrial membrane potential images of 4T1 cells after different treatments. Figure S20. Changes of intracellular ATP contents of 4T1 cells after being treated with PBS, L, COF+, FCCF, FCCF+ and OFCCF+ for 24 h. P values were calculated by one-way analysis (*p<0.05, **p<0.01, ***p<0.001). Data were represented as mean ± SD (n = 4). Figure S21. Relative viabilities of L929 cells treated by incubation with COF and FCCF at different concentration for (a) 24 h and (b) 48 h. Data were represented as mean ± SD (n = 6). Figure S22. The images of 4T1 cells after various treatments and stained with calcein-AM (live cells: green) and PI (dead cell: red). Figure S23. Quantitative analysis of Annexin V-FITC/PI co-stained 4T1 cells at 24 h after various treatments. Figure S24. Changes of body weight of mice in different groups after treatment. Figure S25. The tumor picture of mice before and after treatment in different groups (n = 8). Figure S26. (a) The photos of lung at the end of various treatments. (b) H&E-stained lung sections at the end of various treatments. Red arrows indicate the sites of tumor metastasis. Figure S27. Quantitative analysis of caspase 3, Bcl-2 and Cyt c protein expression, as the ratio of protein to β-actin from Western Blot results. Related to Figure 6 (f). Figure S28. ATP contents of tumor after being treated with PBS, L, COF+, FCCF, FCCF+ and OFCCF+. P values were calculated by one-way analysis (*p<0.05, **p<0.01, ***p<0.001). Data are presented as mean ± SD (n = 4). Figure S29. (a) The HIF-1α protein expression of tumor via western blotting analysis. (b) Quantitative analysis of HIF-1α protein expression, as the ratio of protein to β-actin from Western Blot results. P values were calculated by one-way analysis (*p<0.05, **p<0.01, ***p<0.001). Figure S30. H&E stained images of the major organs, including heart, liver, spleen, lung, and kidney. Scale bar: 20 μm. Figure S31. BALB/c mice were sacrificed after PBS and FCCF (NPs) treatment. Serum biochemistry data including Alanine aminotransferase (ALT), Aspartate aminotransferase (AST) and Alkaline phosphatase (ALP) as hepatic function indicators and Blood urea nitrogen (BUN) as renal function indicators were measured. Blood hematological counts: Blood levels of White blood cells (WBC), Red blood cells (RBC), Hemoglobin (HGB), Hematocrit (HCT), Mean corpuscular volume (MCV), Mean corpuscular hemoglobin (MCH), Mean corpuscular hemoglobin concentration (MCHC) and Blood platelet (PLT). Data are presented as mean ± SD (n = 4).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, Y., Jing, S., Liu, S. et al. Double-activation of mitochondrial permeability transition pore opening via calcium overload and reactive oxygen species for cancer therapy. J Nanobiotechnol 20, 188 (2022). https://doi.org/10.1186/s12951-022-01392-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-022-01392-y